Abstract
Significance: Mitochondria are currently believed to play an important role in the neurodysfunction and neurodegeneration that underlie Parkinson's disease (PD). Recent Advances: While it increasingly appears that mitochondrial dysfunction in PD can have different causes, it has been proposed that mitochondrial DNA (mtDNA) may account for or drive mitochondrial dysfunction in the majority of the cases. If correct, the responsible mtDNA signatures could represent acquired mutations, inherited mutations, or population-distributed polymorphisms. Critical Issues and Future Directions: This review discusses the case for mtDNA as a key mediator of PD, and especially focuses on data from studies of PD cytoplasmic hybrid (cybrid) cell lines. Antioxid. Redox Signal. 16, 950–964.
Introduction
Most investigators in the Parkinson's disease (PD) research field realize that for decades mitochondrial dysfunction has been considered a common feature, if not general cause, of PD (6). The field's interest in mitochondria began in earnest in the mid-1980s, after the parkinsonism-inducing toxin identified by Langston et al., N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), was shown by Nicklas et al. to inhibit complex I in its 1-methyl-4-phenyl-pyridine (MPP+) form (68, 88). Since then, other complex I inhibitors, such as rotenone, have been shown to have the peculiar ability to preferentially kill dopaminergic neurons of the substantia nigra while sparing the integrity of other cell types (10).
The mechanistic basis for the anatomically specific destruction induced by complex I inhibitors is not entirely clear. In the case of MPTP, evidence indicates that after astrocytes take up MPTP, convert it to MPP+, and release it to the extracellular space that neuron dopamine transporters preferentially absorb the toxin (105). This should of course ensure that MPP+ concentrations in dopaminergic neurons exceed those of other neuron types, which could lead to a disproportionally greater complex I inhibition and hence toxicity in dopaminergic neurons. Still, after MPTP administration it is found distributed throughout the brain for at least a brief period and the degree of complex I inhibition that results throughout the brain can reach easily detectable levels (43). Another relevant point is that rotenone does not appear to show preferential uptake by dopaminergic neurons (10). Therefore, an argument can be made that for unknown reasons dopaminergic neurons are less able than other cell types to tolerate complex I dysfunction (97).
In 1989 several studies reported evidence of altered complex I function in persons with idiopathic PD (11, 83, 94, 112). From a methodologic perspective these studies used either spectrophotometric Vmax assays or immunochemistry. Although in some respect their essential observations were consistent and could have unified the PD research community, what resulted was a long-running controversy over the complex I defect's anatomic distribution. The reasons for this controversy arose from the fact that one early report claimed in brain the complex I defect was restricted to the substantia nigra (113). Two of the other reports, though, identified deficient complex I activities in both PD subject platelets and muscle (11, 94). This was a debate with important implications. If the PD subject complex I activity reduction was in fact restricted to the substantia nigra, it would reduce the likelihood that the phenomenon was genetically mediated. If the PD complex I defect was systemic, it would conversely suggest a genetic basis. Two decades later, the controversy over the anatomic distribution of the PD complex I defect seems to have been settled (128, 131). It has repeatedly been demonstrated in PD subject platelets and muscle, and has also been demonstrated in fibroblasts and lymphocytes (7, 9, 12, 13, 18, 34, 42, 65, 85, 87, 94, 119, 162). More recently, it was shown that the original controversy likely arose entirely as a consequence of methodological issues (128) (Fig. 1). Because it is difficult to prepare enriched mitochondrial fractions from small brain regions, the study of Schapira et al. necessarily determined complex I Vmax activities in tissue homogenates (113). Unfortunately, determining electron transport chain (ETC) Vmax activities in brain homogenates provides a substantially reduced reaction signal, which makes it a somewhat insensitive way to detect inter-group ETC Vmax differences. This limitation would predictably be exacerbated by the use of autopsy brain tissue, which is subject to postmortem artifacts that further reduce assay sensitivity. Indeed, a 2008 study demonstrated that when complex I activity was determined in frontal cortex homogenates of PD subject autopsy brains, no difference was detected compared with the activity in control brains, but that with increasing enrichment of the assayed mitochondrial fractions a clear difference emerged (96).
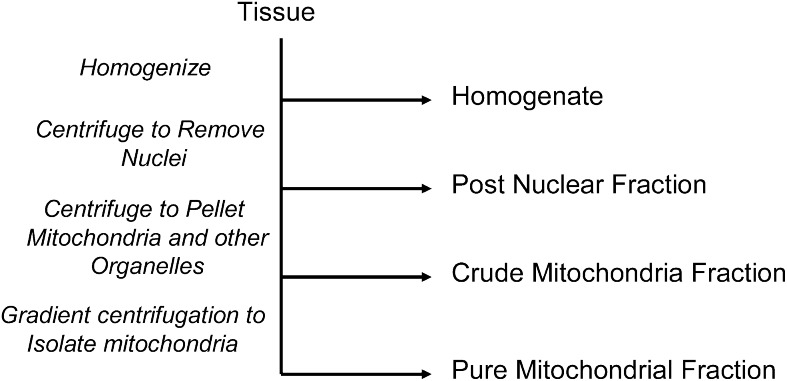
FIG. 1.
Stages of mitochondrial enrichment. When measuring mitochondrial ETC enzyme activities from a tissue section the first step is homogenization. There is no mitochondrial enrichment in the homogenate, and most of the protein in the homogenate is not mitochondrial. The first enrichment step involves the removal of nuclei to produce a postnuclear fraction. The supernatant from the postnuclear fraction can be centrifuged to pellet mitochondria and other similar-sized organelles. This stage is called a crude mitochondria fraction. The highest degree of enrichment results after the mitochondria have been separated from other organelles via density gradient centrifugation. With each enrichment step the assayed signal is greater and therefore more sensitive to subtle differences between samples.
During the 1990s investigators, nevertheless, began to test for a potential genetic contribution to the PD complex I defect. Complex I functions as a large multimeric holoenzyme that contains 46 known subunits all of which are encoded by different genes, thus complicating a comprehensive evaluation of this possibility (37) (Fig. 2). Most initial attempts to demonstrate a genetic basis for the PD complex I defect were sequence based and either negative or, if positive, not supported through replication (62, 118, 122, 123, 139). The first durable data to strongly make a case for mitochondrial DNA (mtDNA) as an important idiopathic PD mediator come not from sequence based studies, but rather from studies of cytoplasmic hybrids, or “cybrids”(130).
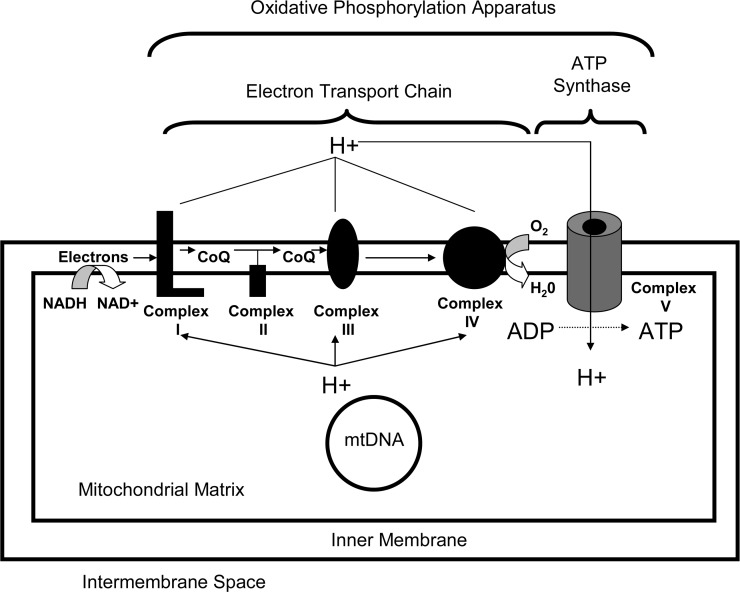
FIG. 2.
Complex I is part of the ETC. Complex I, also known as NADH:ubiquinone oxidoreductase, accepts electrons from NADH and delivers those electrons to coenzyme Q. Electrons eventually reach complex IV, also called cytochrome oxidase, where they are used to reduce oxygen and form water. As electrons are passed from higher to lower energy states, the energy that is released is used to pump protons from the mitochondrial matrix and into the mitochondrial intermembrane space. These protons re-access the matrix through a non-ETC enzyme called complex V, or the ATP synthase, and energy from that proton flux is used to generate ATP. Complexes I through V are all multimeric enzymes, and complexes I, III, IV, and V are bi-genomically encoded and contain subunits encoded on both mtDNA and nuclear DNA. Complex I contains 7 mtDNA-encoded subunits and at least 39 nuclear DNA-encoded subunits. ETC, electron transport chain; mtDNA, mitochondrial DNA.
What Are Cybrids?
Decades ago investigators succeeded in fusing two nucleated cells together to generate binucleated hybrid cells. Cybrid cells differ from hybrid cells because they are generated by mixing the contents of a non-nucleated cell, or cytoplast, with a nucleated cell (101) (Fig. 3). Cybrid cells, therefore, have just one nucleus but contain the cytosolic contents of two different cells. This approach was developed during the 1970s to explore the functional consequences of unique mtDNA species (16). In one early cybrid study, enucleated cytoplasts from a chloramphenicol-resistant HeLa cell strain were mixed with the intact cells of a chloramphenicol-sensitive HeLa strain. The resulting cybrid cell line showed stable chloramphenicol resistance and illustrated in cultured cells choramphenicol sensitivity versus tolerance is determined by mtDNA (157).
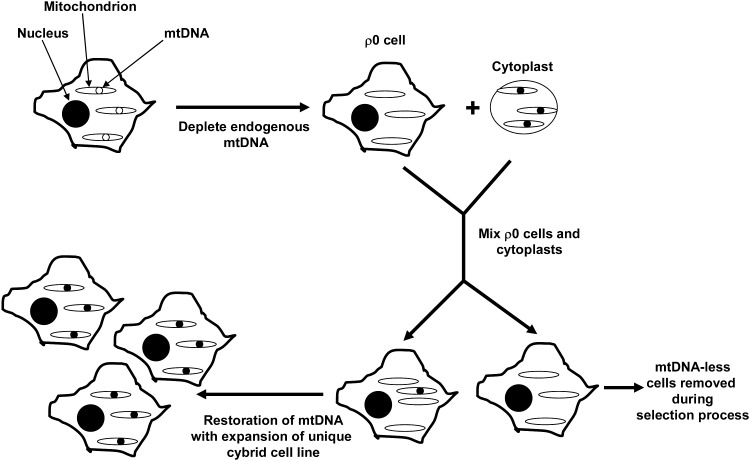
FIG. 3.
Cybrid cell lines are created by mixing the cytosolic components of a nucleated cell with the cytosolic contents of a non-nucleated cell or cytoplast. When control over a cybrid cell line's mtDNA content is desired, an mtDNA-containing cytoplast can be mixed with a ρ0 cell line that was previously depleted of its endogenous mtDNA. In this figure the cell line's original endogenous mtDNA is shown as open circles within mitochondria, and after conversion to a ρ0 cell the mtDNA-less mitochondria are shown to lack mtDNA. The cytoplast's mtDNA is shown as filled circles. During a transfer process some of the cytoplast's mtDNA-containing mitochondria mix with the cytosolic contents of some of the ρ0 cells. Because ρ0 cells lack 13 key components of the ETC and ATP synthase that are normally encoded by mtDNA, placing the cells in a selection medium that will not support ρ0 cell survival removes nontransformed cells. Cells that incorporated mtDNA from the cytoplast, though, are restored to aerobic competency and expand during the selection process. The result is a unique cell line that has the nucleus of the original ρ0 cell line and the mtDNA from the cytoplast donor.
The creation of mtDNA-depleted cell lines facilitated adaptations of this basic approach. During the 1970s and 1980s it was shown that chronic exposure to cationic DNA mutagens, such as ethidium bromide, can selectively block mtDNA replication (84, 160). Selective mtDNA toxicity in this case results from the fact that cationic mutagens concentrate within negatively charged mitochondrial matrices where they can reach concentrations capable of disrupting mtDNA replication while sparing nuclear DNA replication.
Through a series of experiments it was found that with proper metabolic support it was possible to completely eliminate the endogenous mtDNA from some cell lines. Because before its identification as mtDNA cytoplasmic DNA was referred to as ρ DNA (30), cell lines completely depleted of endogenous mtDNA are typically called ρ0 cells. First, it was shown that mtDNA-depleted cells require uridine (84). The accepted reason for this is that one step of the pyrimidine de novo pathway, the dihydorotate dehydrogenase-mediated dehydrogenation of dihydroorotate to orotate, is coupled to the reduction of ubiquinone to ubiquinol (51, 84, 150, 163). Cells that lack a functional ETC theoretically cannot regenerate ubiquinone, which subsequently blocks de novo pyrimidine synthesis. Uridine, a nucleoside created through the N1-glycosidic linkage of uracil, a pyrimidine base, to a ribose sugar increases flux through the pyrimidine salvage pathway and compensates for the loss of de novo pyrimidine synthesis. Pyruvate appears to further promote the expansion of mtDNA-depleted cell lines, and it has been proposed to accomplish this by shifting the cell redox balance to a more oxidized state (58). This would theoretically increase glycolysis pathway flux, which is relevant because without a functional ETC cell ATP demand must be met entirely through glycolysis. It is worth noting that most successfully generated ρ0 cell lines are tumor cell lines (130), which have already shifted to some degree their energy production from mitochondrial respiration to glycolysis, a phenomenon widely known as the Warburg Effect (152, 158).
In 1989, King and Attardi reported the creation of an osteosarcoma ρ0 cell line that expanded when its medium was supplemented with uridine and pyruvate, but which was not viable in their absence (58). Uridine and pyruvate auxotrophy was eliminated following either the “fusion” or injection of these osteosarcoma ρ0 cells with enucleated fibroblast cytoplasts. Both approaches restored aerobic competency by repopulating the osteosarcoma cells with cytoplast mitochondria and hence mtDNA. The ability to generate cybrid cell lines from ρ0 cells allowed investigators to better control the mtDNA content of the cybrid lines they were generating, and the technique was soon adapted for studies of mtDNA mutational threshold (Fig. 4). For example, osteosarcoma ρ0 cell lines were fused with fibroblast cytoplasts or platelets obtained from individuals with heteroplasmic point mutations responsible for causing the mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome and the myoclonic epilepsy and ragged red fiber syndrome (22, 59, 76).
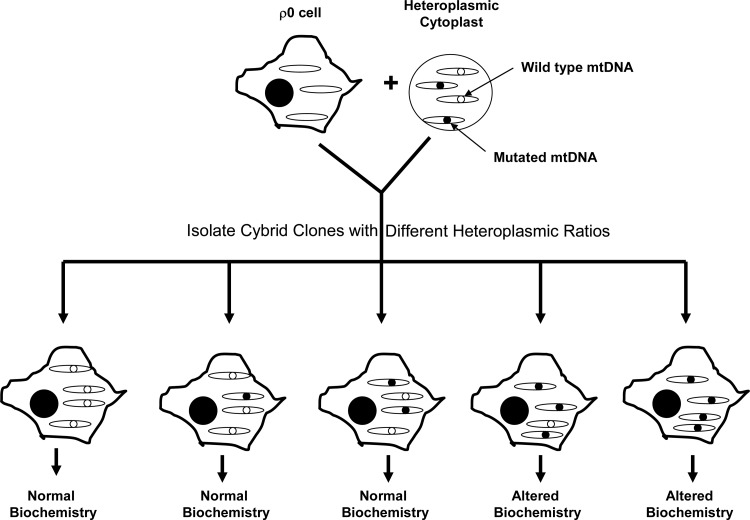
FIG. 4.
Cybrid clonal selection facilitates testing of threshold effects. After ρ0 cells are mixed with cytoplasts that contain an mtDNA heteroplasmy, it is possible to isolate the individual colonies that arise through the expansion of cells that have incorporated cytoplast mtDNA. The fusion process can therefore lead to cybrid sub-cell lines that have different heteroplasmic ratios. Biochemical assays of each sub-cell line can reveal the mutational burden, or threshold, that is required to cause biochemical consequences. Results must be carefully interpreted since mutations may have greater effects in primary tissues than they do in cell culture.
In 1994, a variation of the cybrid approach was described in which platelets were substituted for fibroblast cytoplasts (21). Platelets lack nuclei and are easy to isolate, since they can readily be separated from whole blood samples via centrifugation. Several milliliters of whole blood can generate enough platelets to generate a cybrid cell line. The ability to use platelets as a source of mitochondrial and therefore mtDNA transfer meant that virtually any desired subject at any time could serve as an mtDNA donor when creating a cybrid cell line. In 1996 the first human neuronal ρ0 cell line, the SH-SY5Y neuroblastoma cell line, was reported (81).
Why Were Cybrids Used to Study PD?
By the mid-1990s several groups had reported that PD subject platelet mitochondria have reduced complex I activity, but the explanation for this phenomenon was unknown (9, 42, 65, 94, 162). It was proposed that environmental or endogenously generated toxins could account for this, and several candidate toxins were considered (28, 44, 47, 73, 77, 86, 89, 104). No potential toxic agent, though, seemed able to generate sustained enthusiasm.
Although initial efforts to identify a genetic explanation for the PD complex I defect were unsuccessful, a compelling rationale for a genetic and especially an mtDNA-related genetic basis remained. Part of this rationale was based on the epidemiology of PD, which revealed that although individuals with a family history of PD generally have an increased risk of developing PD, only in rare cases does this increased risk demonstrate recognizable autosomal dominant or autosomal recessive Mendelian inheritance patterns (97, 140). Monozygotic twin studies also reveal clinical discordance, although PD sub-clinical endophenotypes may develop in the otherwise asymptomatic monozygotic twin siblings of PD subjects (46, 67, 103, 141, 159). For these reasons most idiopathic PD is said to show “sporadic” epidemiology. Sporadic epidemiology for a disease, though, does not mean that genes do not contribute to its risk or pathophysiology. It simply suggests that autosomal dominant or autosomal recessive inheritance is unlikely to account for the majority of the cases. Under such circumstances it is worth considering the relevance of other genetic mechanisms that can influence disease risk yet not produce recognizable Mendelian inheritance patterns.
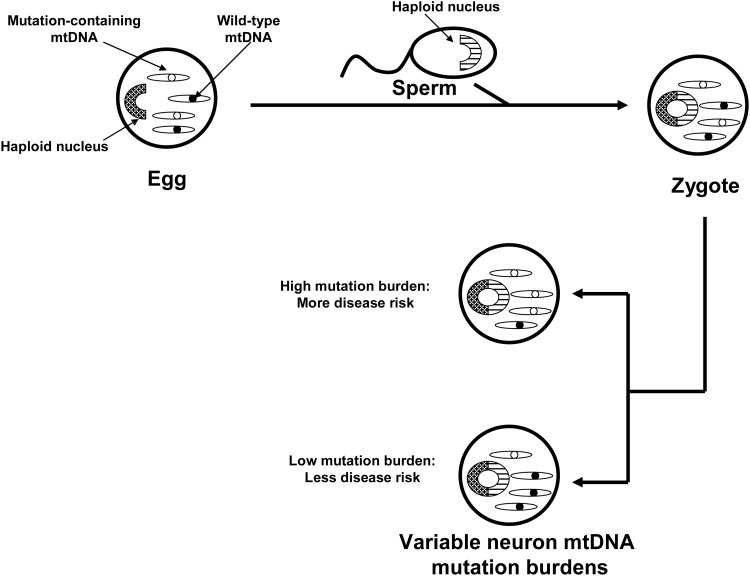
In the 1980s mtDNA was demonstrated to show almost exclusive maternal inheritance (39). During that decade primary mtDNA disorders were expected to exclusively show recognizable maternal inheritance that is distinguishable from Mendelian inheritance. This assumption was questioned by Parker, who hypothesized that because mtDNA shows heteroplasmy, threshold effects and mitotic segregation even maternally inherited mtDNA disorders would present in a sporadic fashion (93) (Fig. 5). According to this hypothesis inherited mtDNA signatures could confer disease risk within an obvious matrilineal context, within a context in which maternal inheritance is reflected only by a subtle maternal inheritance bias, or in a completely sporadic fashion. Based on this, Parker et al. proposed that mtDNA could specifically explain the finding that complex I activity is systemically lower in PD subjects than it is in non-PD subjects (94). Parker et al. further postulated that because most PD is sporadic, low abundance mtDNA heteroplasmic mutations were of particular interest.
FIG. 5.
Model via which mtDNA heteroplasmy can produce sporadic-appearing phenotypes through threshold effects and mitotic segregation. An ova with a heteroplasmic mtDNA mutation is fertilized to produce a zygote. As the zygote gives rise to a multicellular organism, its mitochondria are distributed randomly so that different tissues end up containing unequal amounts of the mutation. This process is called “mitotic segregation.” The individual's subsequent risk for developing a central nervous system mitochondriopathy disease depends on the burden and severity of the mutation in the central nervous system, a concept known as “threshold.” In this model individuals with low burdens of nondeleterious mutations would avoid disease, whereas individuals with higher burdens of the same mutation or lower burdens of more deleterious mutations would manifest a disease phenotype.
The Parker hypothesis was somewhat radical. By the mid-1990s, low abundance mtDNA heteroplasmies had not been shown to exist, but this was mostly due to the fact that low abundance heteroplasmies were not detected by the routine dideoxynucleotide sequencing protocols used at the time (52). Of course, since then many of the key technical limitations responsible for this have been resolved, and it has been clearly demonstrated that low abundance heteroplasmies not only exist but are in fact commonplace (17, 23, 69, 80, 121, 125).
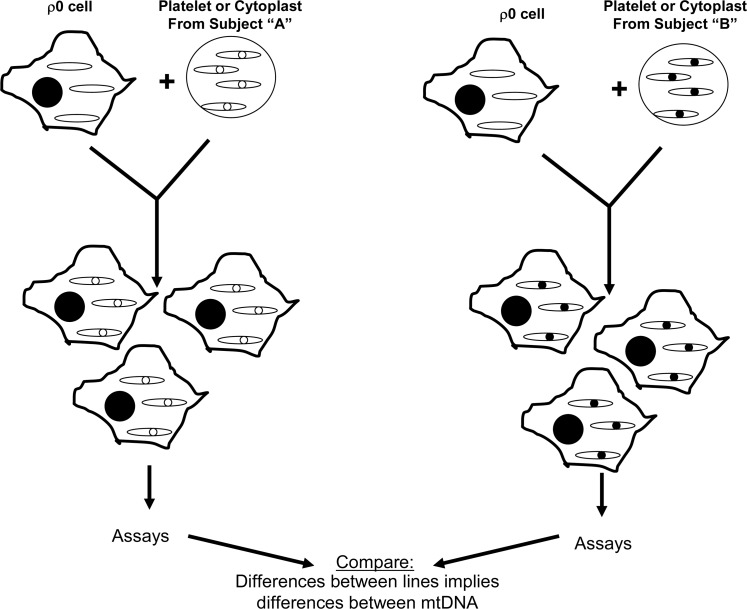
In the mid-1990s, though, the cybrid technique was felt to represent a reasonable approach for testing whether mtDNA might account for the PD complex I defect. The idea of using cybrids to study this question was, nevertheless, greeted with some skepticism as it represented a departure from the way cybrids were currently being used at the time, which was mostly to study threshold and functional effects of already-defined mtDNA mutations (130). Rather, the use of cybrids to study PD mtDNA was more reminiscent of the chloramphenicol resistance experiments of two decades prior, in which cybrid cell lines were generated to simply address the question of whether mtDNA could induce a particular cell biology, physiologic, or biochemical phenotype (16, 157) (Fig. 6). This type of application can infer a distinct mtDNA signature exists, but cannot identify the actual sequence signature. The results of what has now become an extensive body of PD cybrid work strongly argue that sporadic PD subject mtDNA differs in some way or ways from the mtDNA of control individuals. This body of work is summarized in the following sections.
FIG. 6.
Inferences about a subject's mtDNA can be made by comparing a cybrid cell line made with that subject's mtDNA to cybrid cell lines containing mtDNA from different subjects. Because the nuclear genetic composition and the environment are equivalent between the different cybrid lines, differences in function or structure most likely arise from differences between mtDNA. This type of cybrid approach can be used to screen for functional mtDNA differences between individuals.
Complex I Activity Is Reduced in PD Cybrids
The first PD cybrid study was reported in 1996 (138). The SH-SY5Y ρ0 cell line was used to generate a total of 52 distinct cybrid cell lines. mtDNA reconstitution for each cell line was accomplished by mixing platelets obtained from a different individual with an aliquot of ρ0 cells. In this study, 24 platelet samples were obtained from sporadic PD subjects, and 28 platelet samples were obtained from age-matched control subjects. The mixing was performed in a polypropylene tube using gentle agitation and polyethylene glycol to enhance the exchange of cytosolic contents between platelets and ρ0 cells. Following the mixing procedure, tube contents were plated in a cell culture flask and placed in medium that was supplemented with neither uridine nor pyruvate. Over the next several weeks nontransformed cells detached and died, whereas transformed cells grew initially as visible colonies that were eventually dispersed by routine passaging of the flask contents. The selection for transformed cells was completed over a 6-week period, during which time the cells underwent multiple cycles of cell replication. Therefore, although the initial cytoplasmic mixing between platelets and ρ0 cells likely involved more than just platelet mitochondria, over the many weeks of cell selection and many cycles of cell division the potential effects of any nonperpetuating, nonreplicating platelet components were theoretically eliminated through degradation and dilution. Theoretically, the only perpetuating, replicating component transferred from platelets was platelet mtDNA. While this mtDNA was incorporated as a component of the whole platelet mitochondria that were transferred from the platelets, by the end of the selection period the only unique mitochondrial feature carried over from the transfer event was ideally the mtDNA. By the end of the selection process, virtually all mitochondrial membrane was newly synthesized, and all nuclear-encoded mitochondrial proteins, whether constituting part of the ETC, an outer or inner mitochondrial membrane protein, or an inter-membrane or matrix space protein was a product of the SH-SY5Y cell line's nuclear DNA. On the other hand, the 13 mtDNA-encoded ETC components were translated according to the sequence specifications of the platelet donor subject's mtDNA. Therefore, the cybrid cell lines that were generated differed from each other only in the origin of their mtDNA, and hence their mtDNA sequence.
At the conclusion of the selection process enriched mitochondrial fractions were prepared from each individual cybrid line and complex I and complex IV Vmax activities were spectrophotometrically determined. The mean complex I activity of the PD cybrid group was only 80% of that of the control cybrid group. The mean complex IV activities between the groups were comparable. This study concluded mtDNA at least partly accounts for the low systemic complex I activity observed in PD subjects.
Since this initial report, other PD and control cybrid series have been generated using SH-SY5Y ρ0 cells and obtained the same outcome. Shults and Miller reported the results of their SH-SY5Y cybrid study in 1998 (120). Also in 1998, Swerdlow et al. prepared SH-SY5Y cybrid cell lines from members of an extended family in which PD appeared to pass through maternal lines of inheritance (137). In that study, cybrid cell lines prepared using platelet mitochondria from PD-affected kindred members and the young, asymptomatic descendents of PD mothers had lower complex I activities than cybrid cell lines prepared from platelet mitochondria obtained from asymptomatic kindred members descended through paternal lines.
In 1998, Gu et al. reported a study of PD cybrid cell lines that were produced on an A549 lung adenocarcinoma epithelial cell background (41). In this cybrid series the mean complex I activity was 75% of the control mean complex I activity. This study also directly measured the platelet mitochondria complex I activities from the subjects it studied, and concretely established that low complex I activities in PD subject platelet mitochondria are carried over into and persist within cybrid cell lines created through mitochondrial and mtDNA transfer from the platelets of those subjects.
In 2008, Esteves et al. reported a study of PD cybrid cell lines that were produced on an NT2 teratocarcinoma cell background (34). The mean complex I activity in the PD cybrid lines was approximately half of what it was in the control cybrid lines. As was the case with the Gu et al. study, complex I activities were also directly measured in platelet mitochondria from subjects whose platelet mitochondria were used to accomplish mtDNA reconstitution of the ρ0 cells. Again it was found that low complex I activities in PD subject platelet mitochondria are carried over into and persist within cybrid cell lines. Indeed, the magnitude of the complex I defect seen in the PD cybrid cell lines was equivalent to what was seen in the direct platelet mitochondria measurements. In 2010, Esteves et al. reported data from a separate series of NT2-based PD cybrids in which the complex I activity was ∼60% that of the control cybrid group (35).
Challenges to the fundamental finding of these studies, which is that platelet mtDNA transfer from PD subjects to ρ0 cells restores complex I activity in the resulting cybrid cells to levels below those seen when mtDNA is transferred from control subjects, have been relatively minor. After the first PD cybrid study was published the primary criticism was that perhaps there was some unique, unrecognized property of the SH-SY5Y ρ0 cell line that produced an inaccurate result. This possibility has been addressed by the fact that the essential finding has now been seen in cybrid cell lines with three different nuclear backgrounds. Another early criticism was that the cell colonies, or clones, that resulted from a particular cybrid fusion were grown in the same flask and not separated from each other during the selection process (114). At the time, clonal isolation was a standard approach that was used to generate cell lines with different heteroplasmic burdens of mtDNA mutation. The studies described above, though, were not designed to study strict questions of heteroplasmy although the results certainly do not rule out heteroplasmy. The Gu et al. study also considered whether clonal cybrid populations might behave differently than mixed cybrid populations, and found no evidence to support that possibility (41).
In 2000, a study of PD cybrids produced on a HeLa cell background failed to find reduced complex I activity (3). The authors concluded that mtDNA must not contribute to the PD complex I defect. In light of the fact that multiple studies by multiple groups using other ρ0 cell lines have obtained different results suggests this is an over-generalization. This possibility is further supported by the fact that in studies of other diseases HeLa-based cybrid cell lines also yield results that diverge from those of other investigators who use different ρ0 cell lines (50, 115, 130, 136).
Concerns have also been raised by the fact that in the positive cybrid studies mtDNA reconstitution of the ρ0 cell lines was accomplished via transfer of intact platelet mitochondria, and other components of the platelet cytosol could have also undergone transfer as well. This is certainly likely and does imply a conceptual concern, although the likelihood that mtDNA does not account for differences in ETC activities between cybrid cell lines that have different mtDNA sequences would seem to be minor. To date, no alternative explanation for the PD cybrid results has been demonstrated, and in fact a study in which PD-affected members of the Contursi kindred provided platelet mitochondria did not reveal a cybrid complex I defect (135). Contursi kindred members have an autosomal dominant version of PD that is caused by a mutation in the α-synuclein gene, and therefore have a distinct and identifiable etiology that does not need to implicate mtDNA (100). The fact that a persistent complex I defect was not seen in the Contursi kindred cybrids indirectly argues against the possibility that a nongenetic, cytosolic factor transferred during the fusion process accounts for the complex I defect that is seen when cybrids are generated from sporadic PD subject platelet mitochondria. Regardless, this criticism does lead to the biggest remaining question about the cybrid studies discussed above, which is if mtDNA does account at least in part for the PD complex I defect, what specifically is different about the mtDNA of sporadic PD subjects?
PD Cybrid Modeling of Other PD-Associated Pathologies
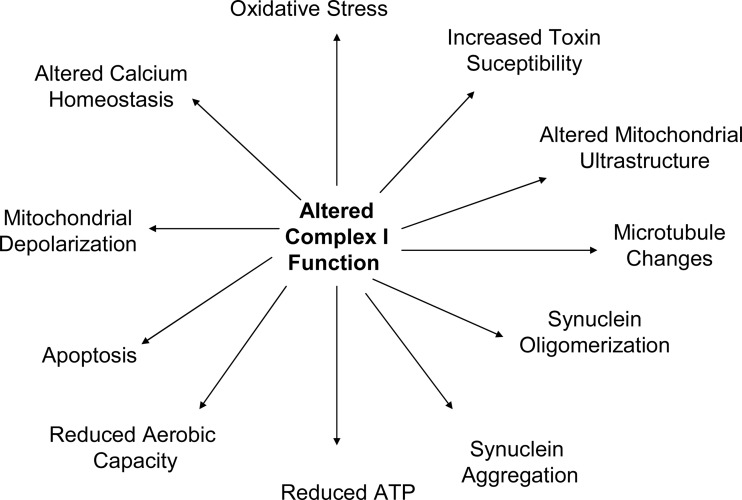
The PD and control cybrids produced by Swerdlow et al., Gu et al., Esteves et al., Esteves et al., and others have been used to study a variety of physiologic phenomena (34, 35, 41, 138) and have shown that a number of pathologies observed in PD subject brains are actually recapitulated by PD cybrids. When considering these pathologies, it is important to keep several basic tenets in mind. First, the only recognized difference between PD and control cell lines is the origin of their mtDNA and their mtDNA sequence. Second, mtDNA only encodes only 13 proteins, 22 tRNAs, and 2 rRNAs. Since all mtDNA-encoded proteins are ETC constituents, mtDNA-mediated cell differences must arise through differences in ETC function. Third, as is the case with PD subjects, PD cybrids have altered complex I function. Therefore, all unique physiologic phenomena observed within PD cybrids must result from their apparent mtDNA-dependent ETC peturbations and especially to perturbation of the complex I holoenzyme (Fig. 7).
FIG. 7.
PD cybrid cell lines demonstrate numerous functional and structural perturbations. Many of these perturbations recapitulate phenomena observed in persons with PD. Documenting these functional phenomena in PD cybrid cell lines suggests that the complex I defect seen in sporadic, idiopathic PD subjects is able to cause the phenomena. PD, Parkinson's disease.
PD cybrid mitochondrial ultrastructure is altered (34, 137, 148). Mitochondria tend to be larger and are more likely to show disruption of cristae than mitochondria in control cybrids. PD cybrid mitochondria show more frequent inclusions.
PD cybrid viability is different from that of control cybrids. Under basal conditions PD cybrid cultures show increased LDH release, as well as an increased activation state of the intrinsic apoptosis pathway, altered levels of apoptosis-relevant proteins such as the bcl proteins, PARP activation, and condensed nuclei (34, 90, 91, 153). PD cybrids are also more likely than control cybrids to show apoptotic changes when exposed to equivalent concentrations of the MPP+toxin (34, 138). Related findings include the fact that PD cybrid mitochondria are relatively depolarized (34). This probably accounts for the reduced ability of their mitochondria to buffer cytosolic calcium (32, 116).
PD cybrid cell line ATP levels are reduced compared to those of control cybrids (31, 33, 34). The status of pathways influenced by cell bioenergetics is also altered. PD cybrids show reduced SIRT1 phosphorylation, reduced peroxisome proliferator-activated receptor-gamma coactivator-1alpha levels, and increased NF-kB activation (20, 35). Some of these redox-dependent changes may also arise in part as a downstream consequence of increased oxidative stress, as PD cybrids show enhanced rates of peroxide formation, decreased glutathione, and increased protein oxidation despite the presence of a presumably compensatory increase in antioxidant enzyme activities (19, 31, 91, 138). Recapitulation of oxidative stress in PD cybrid lines is of particular interest as oxidative stress occurs in the brains and peripheral tissues of PD subjects (25, 27, 53, 75, 110, 111). The PD cybrid model therefore implicates a specific potential source for the oxidative stress that is observed in this disease. Cell mtDNA content is reduced in PD cybrids, which is perhaps consistent with their lower proliferator-activated receptor-gamma coactivator-1alpha levels (14, 55).
PD cybrids appear to have ATP-dependent cytoskeletal changes, which manifest as microtubule depolymerization (31, 33). α-synuclein protein is also altered in PD cybrids. α-synuclein oligomer levels are increased (31–33). When neuronal PD cybrids are differentiated the resultant neurites show detectable increases in α-synuclein (145). α-synuclein aggregates reminiscent of Lewy bodies are also commonly observed in PD cybrids, while they are far less commonly detected in control cybrids (31, 146). In conjunction with altered mitochondrial function, cytoskeletal changes may account for reductions in mitochondrial movement that are seen in PD cybrids (147).
Recently, improvements in respirometer technology have facilitated oxygen consumption measurements in whole cells. In a study of NT2 PD cybrids, basal oxygen consumption between PD and control cybrid lines was comparable. However, when cell mitochondria were pushed via chemical-induced uncoupling into a state of maximum oxygen consumption, the control cybrid lines showed a greater increase (35). PD cybrids, therefore, have a reduced respiratory reserve capacity. It would be interesting to know if this is also the case in the brains of PD subjects, but of course this parameter would be difficult to test.
Are Specific mtDNA Signatures Typical of PD?
A standard mtDNA sequence called the Cambridge Sequence was agreed upon several decades ago (1, 2). During the 1990s investigators reported homoplasmic Cambridge Sequence deviations are common in PD subjects, although homoplasmic Cambridge Sequence deviations are also common in control populations (49, 61, 155). Association studies have occasionally concluded particular homoplasmic Cambridge Sequence deviations may be more frequent or less frequent in a particular PD cohort as compared to a particular control cohort. In most cases, though, replication of such reported associations is inconsistent or awaits confirmation. For example, an A4336G transition in the mtDNA tRNA (Gln) gene has been found to be over-represented in PD subjects in some but not other studies (78, 118, 122, 139). More recently, one large study found that a common nt10398G change is underrepresented in PD subjects, but a second study did not (123, 151). A G5460A transition was found in one study to associate with PD, but this was not seen in other cohorts (62, 122, 139). The ND1 gene T4216C polymorphism has reportedly associated with an elevated risk of PD (60, 107).
Certain mtDNA variations are typical of particular geographical regions or populations (108, 109). Some variations tend to occur in combination and are used to define mtDNA haplogroups. The possibility that haplogroups might influence risk for particular conditions or diseases has been raised (74, 106). One study reported that among the classic European mtDNA haplogroups, as compared to persons with haplogroup H persons with haplogroups J and K have reduced PD risk (151). Additional haplogroup association studies also conclude that particular mtDNA haplogroups or haplogroup clusters may influence PD risk although risk attributions between the different populations analyzed do vary (5, 38, 102, 151). The question of whether mtDNA haplogroups are functionally relevant has also been raised, and one recent cybrid study did find differences in mitochondrial respiratory capacity in cells containing haplogroup H versus haplogroup Uk (40). Other cybrid studies have similarly reported that common mtDNA polymorphisms may influence mitochondrial function (54, 99).
Although individual case or family reports do suggest that particular homoplasmic or high-abundance mtDNA mutations can cause parkinsonism (124, 143), the association studies thus far mentioned in this section do not persuasively argue that any particular homoplasmic mtDNA sequence deviation is an outright cause of PD in a large percentage of the cases. While common variations may turn out to influence risk, with particular mtDNA and nuclear complex I gene polymorphism combinations having additive or subtractive effects on someone's overall risk (139), common variations do not appear to be PD-deterministic.
If actual mtDNA mutations are deterministic in a large percentage of PD cases, then those mutations would, therefore, have to exist in the form of low-abundance heteroplasmies. Although recent advances are making the detection of low abundance heteroplasmies more practical, to this point the sensitive and accurate identification of low abundance mtDNA heteroplasmies has been technically challenging. Regardless, several relevant studies have found increased numbers of mtDNA deletions in the substantia nigra neurons of deceased PD subjects (8, 63). Surveys of low abundance heteroplasmic point mutations have also been performed. In these studies methodologic approaches and in some cases interpretations have varied. These studies have generally concluded that low abundance heteroplasmic point mutations are common in the brains of aging individuals, and between PD and control subjects point mutation burdens are equivalent (4, 95, 121, 125). In terms of mutation patterns or localizations, some have found no differences between PD and control subjects (4, 121), whereas others have claimed characteristic mtDNA signatures that distinguish PD subject brains from those of control subjects. For example, Smigrodzki et al. and Parker et al. have reported low-abundance heteroplasmic mutations in a defined region of the ND5 gene are found in most PD subject brains but rarely in the brains of persons who died without PD (95, 125).
Are PD-Relevant mtDNA Signatures Inherited or Acquired?
MtDNA mutations accumulate with advancing age. It has been postulated that these mutations are acquired or “somatic” mutations, and that they may contribute to common age-related disorders such as Alzheimer's disease, PD, and type II diabetes (156). Empiric evidence further supports the possibility that age-associated increases in mtDNA mutation may drive aging-related phenotypes (66, 144). Whether the responsible mtDNA mutations are point mutations or deletions is a topic of debate (29, 64, 154). Interestingly, humans with mtDNA polymerase gamma variants or mutations that could potentially accelerate mtDNA mutation accumulation can present with parkinsonism or Parkinsons's disease (26, 48, 71, 72).
Transgenic mouse models have leveraged defective mtDNA polymerase gamma proofreading to accelerate formation of mtDNA somatic mutations (66, 144). Free radical species are also known to mutagenize DNA, with mtDNA being affected more than nuclear DNA (79). Somatic DNA mutation rates are presumably higher in the mitochondria than the nucleus because mitochondria are the main source of oxygen radical production in eukaryotic cells (117). In one common mutagenic reaction, a cytosine nucleotide is oxidatively deaminated, and the lost amino group is replaced by an oxygen double bond. This reaction converts the cytosine to uracil. When DNA replication occurs, the uracil of the replicating strand is read as a thymine, and an adenine is placed in the daughter strand at a position where there previously was a guanine. In the next replication cycle, the uracil is replaced by thymine. The end result is substitution of an A-T pair for a G-C pair (82, 92, 98). Imbalances in mitochondrial purine and pyrimidine pools also appear to facilitate the relatively high rate of both point and deletion-type mtDNA somatic mutations (126, 127).
As was discussed in the previous section, mtDNA point mutations and deletions do accumulate in the aging substantia nigra. Deletion accumulation in PD subjects exceeds that of non-PD subjects (8, 63). Point mutation burden is comparable between groups, although certain point mutations seem to be found only in PD subjects (95, 121, 125). It could be argued these findings imply a role for somatic mtDNA mutation in PD, but this is a somewhat complicated subject. MtDNA heteroplasmic ratios can drift over time, so the fact that mtDNA mutational burdens increase during aging does not prove that those mutations were not inherited. Also, it has been shown that mtDNA point mutations may themselves induce mtDNA depletion and potentially, therefore, deletion (45, 149). This raises the possibility that increased levels of substantia nigra mtDNA deletions in PD subjects could actually serve as a marker for potentially inherited mtDNA point mutations. Also, it is important to keep in mind that the PD complex I defect is seen in multiple tissues throughout the body, which would mean an acquired mutation would likely have to arise very early during development, and probably during the oogonia gamete stage (24, 36).
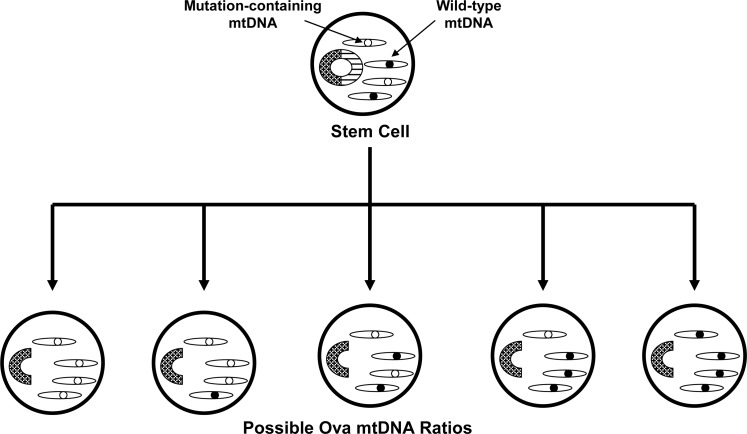
Parker et al. hypothesized that inherited mtDNA mutations are potentially more relevant to sporadic PD than acquired mutations (94, 97). This view is perhaps more consistent with the multi-tissue complex I defect seen in sporadic subjects. Parker has further hypothesized that, in general, the inheritance of low abundance heteroplasmic mutations could play a role in a variety of sporadic diseases because diseases arising from the inheritance of low abundance, heteroplasmic mutations would be expected to show neither Mendelian nor consistent maternal epidemiology (93) (Fig. 8).
FIG. 8.
Heteroplasmy may cause disease-free mothers to transmit a high risk of disease to their offspring, and cause disease-affected mothers to transmit a low risk of disease to their offspring. During early development a stem cell gives rise to a female embryo's oogonia. A stem cell with heteroplasmic mtDNA could produce oogonia with varying amounts of a heteroplasmic mutation. The disease risk of that female's offspring would in turn depend on the amount of mtDNA mutation in the eggs that are fertilized. The disease risk of the mother herself, meanwhile, depends not on the mutational burden in her oogonia but rather on the mutational burden in her central nervous system (see Fig. 5). Mechanisms such as this would enable mtDNA-inherited diseases to appear as sporadic diseases as opposed to matrilineal diseases, and diseases that are influenced by mtDNA inheritance to show non-Mendelian epidemiology.
It is increasingly recognized that gene inheritance may contribute to the development of sporadic diseases. In sporadic PD, this is reflected by the finding that even though Mendelian inheritance patterns are not clearly demonstrated sporadic PD subjects are more likely to have a PD-affected parent than are non-PD subjects (97, 140). When data on such parent–child relationships are closely examined subtle evidence of maternal inheritance bias does emerge. Wooten et al. found that among PD clusters in which there are two affected siblings and an affected parent, the affected parent is more likely to be the mother than the father (161). Further, an analysis of multiple PD subject databases found sex ratio differences between PD probands and the PD-affected parents of those probands (134). While the proband sex ratio showed more men than women, the PD-affected parental generation showed more women than men. This finding is consistent with a maternal inheritance bias.
Lastly, inherited homoplasmic mtDNA polymorphisms either in isolation or as part of haplogroups may influence PD risk (5, 38, 102, 151). It increasingly appears these polymorphisms may subtly influence mitochondrial function (40, 54, 99). This may apply to even extremely common synonymous mtDNA polymorphisms that do not cause amino acid substitutions (70). It has been observed that inherited mtDNA sequence variations may influence the acquisition of somatic mutations (45). It is therefore worth considering the possibility that both inherited and acquired mtDNA variation contributes to the development of sporadic PD.
Implications and Conclusions
The perceived relevance of mitochondria to PD has certainly evolved over the past three decades. During the 1980s and early 1990s, the MPTP story and subsequent demonstration of complex I dysfunction in idiopathic PD subjects catapulted mitochondria to the forefront of the PD research community. During the late 1990s this interest waned as investigators began to identify mutations in families with Mendelian PD subtypes. As the last decade progressed, though, an emerging impression that many genes implicated in Mendelian PD influence or are influenced by mitochondrial function restored the mitochondria to a position of research prominence (15, 142).
Advances in transgenic technology now make the development of disease models in which histological, biochemical, and clinical phenotypes are induced by nuclear gene mutations increasingly commonplace. On the other hand, modeling sporadic diseases that do not have identifiable Mendelian causes is conceptually challenging. Because of this, it is extremely tempting to want to extrapolate findings from Mendelian PD model studies to sporadic PD. While in some ways this will no doubt advance our knowledge of PD in general and especially our understanding of the Mendelian forms, over-generalizations could prove dangerous and an over-reliance on Mendelian models could impede PD therapeutic development as it has in other fields (129, 132, 133). In this respect PD cybrids currently provide one of the most straightforward ways to model the PD complex I defect. While their past contributions to the PD research field are well recognized, an active role for PD cybrids in ongoing PD research and especially PD therapeutic development requires consideration. In the meantime, some groups are currently developing new technologies that would facilitate the direct transfer of exogenous mtDNA to cells (56, 57). This could enable new iterations of the cybrid technique, such as the creation of PD cybrid cell lines that contain mtDNA from PD subject substantia nigra neurons.
Abbreviations Used
- cybrid
cytoplasmic hybrid
- ETC
electron transport chain
- MPP+
1-methyl-4-phenyl-pyridine
- MPTP
N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtDNA
mitochondrial DNA
- PD
Parkinson's disease
Acknowledgments
This work was supported by grants from the Morgan Family Foundation and the PD Foundation of the Heartland.
References
- 1.Anderson S. Bankier AT. Barrell BG. de Bruijn MH. Coulson AR. Drouin J. Eperon IC. Nierlich DP. Roe BA. Sanger F. Schreier PH. Smith AJ. Staden R. Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Andrews RM. Kubacka I. Chinnery PF. Lightowlers RN. Turnbull DM. Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 3.Aomi Y. Chen CS. Nakada K. Ito S. Isobe K. Murakami H. Kuno SY. Tawata M. Matsuoka R. Mizusawa H. Hayashi JI. Cytoplasmic transfer of platelet mtDNA from elderly patients with Parkinson's disease to mtDNA-less HeLa cells restores complete mitochondrial respiratory function. Biochem Biophys Res Commun. 2001;280:265–273. doi: 10.1006/bbrc.2000.4113. [DOI] [PubMed] [Google Scholar]
- 4.Arthur CR. Morton SL. Dunham LD. Keeney PM. Bennett JP., Jr. Parkinson's disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance. Mol Neurodegener. 2009;4:37. doi: 10.1186/1750-1326-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autere J. Moilanen JS. Finnila S. Soininen H. Mannermaa A. Hartikainen P. Hallikainen M. Majamaa K. Mitochondrial DNA polymorphisms as risk factors for Parkinson's disease and Parkinson's disease dementia. Hum Genet. 2004;115:29–35. doi: 10.1007/s00439-004-1123-9. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee R. Starkov AA. Beal MF. Thomas B. Mitochondrial dysfunction in the limelight of Parkinson's disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso N. Campos Y. Huertas R. Esteban J. Molina JA. Alonso A. Gutierrez-Rivas E. Arenas J. Respiratory chain enzyme activities in lymphocytes from untreated patients with Parkinson disease. Clin Chem. 1993;39:667–669. [PubMed] [Google Scholar]
- 8.Bender A. Krishnan KJ. Morris CM. Taylor GA. Reeve AK. Perry RH. Jaros E. Hersheson JS. Betts J. Klopstock T. Taylor RW. Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 9.Benecke R. Strumper P. Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson's disease but normal in Parkinson-plus syndromes. Brain. 1993:1451–1463. doi: 10.1093/brain/116.6.1451. 116 (Pt 6) [DOI] [PubMed] [Google Scholar]
- 10.Betarbet R. Sherer TB. MacKenzie G. Garcia-Osuna M. Panov AV. Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 11.Bindoff LA. Birch-Machin M. Cartlidge NE. Parker WD., Jr. Turnbull DM. Mitochondrial function in Parkinson's disease. Lancet. 1989;2:49. doi: 10.1016/s0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 12.Bindoff LA. Birch-Machin MA. Cartlidge NE. Parker WD., Jr. Turnbull DM. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson's disease. J Neurol Sci. 1991;104:203–208. doi: 10.1016/0022-510x(91)90311-t. [DOI] [PubMed] [Google Scholar]
- 13.Blin O. Desnuelle C. Rascol O. Borg M. Peyro Saint Paul H. Azulay JP. Bille F. Figarella D. Coulom F. Pellissier JF, et al. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson's disease and multiple system atrophy. J Neurol Sci. 1994;125:95–101. doi: 10.1016/0022-510x(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 14.Borland MK. Mohanakumar KP. Rubinstein JD. Keeney PM. Xie J. Capaldi R. Dunham LD. Trimmer PA. Bennett JP., Jr. Relationships among molecular genetic and respiratory properties of Parkinson's disease cybrid cells show similarities to Parkinson's brain tissues. Biochim Biophys Acta. 2009;1792:68–74. doi: 10.1016/j.bbadis.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueler H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Exp Neurol. 2009;218:235–246. doi: 10.1016/j.expneurol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Bunn CL. Wallace DC. Eisenstadt JM. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci USA. 1974;71:1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantuti-Castelvetri I. Lin MT. Zheng K. Keller-McGandy CE. Betensky RA. Johns DR. Beal MF. Standaert DG. Simon DK. Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiol Aging. 2005;26:1343–1355. doi: 10.1016/j.neurobiolaging.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Cardellach F. Marti MJ. Fernandez-Sola J. Marin C. Hoek JB. Tolosa E. Urbano-Marquez A. Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson's disease. Neurology. 1993;43:2258–2262. doi: 10.1212/wnl.43.11.2258. [DOI] [PubMed] [Google Scholar]
- 19.Cassarino DS. Fall CP. Swerdlow RH. Smith TS. Halvorsen EM. Miller SW. Parks JP. Parker WD., Jr. Bennett JP., Jr. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 20.Cassarino DS. Halvorsen EM. Swerdlow RH. Abramova NN. Parker WD., Jr. Sturgill TW. Bennett JP., Jr. Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson's disease. J Neurochem. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- 21.Chomyn A. Lai ST. Shakeley R. Bresolin N. Scarlato G. Attardi G. Platelet-mediated transformation of mtDNA-less human cells: analysis of phenotypic variability among clones from normal individuals—and complementation behavior of the tRNALys mutation causing myoclonic epilepsy and ragged red fibers. Am J Hum Genet. 1994;54:966–974. [PMC free article] [PubMed] [Google Scholar]
- 22.Chomyn A. Martinuzzi A. Yoneda M. Daga A. Hurko O. Johns D. Lai ST. Nonaka I. Angelini C. Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci USA. 1992;89:4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coskun PE. Beal MF. Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coskun PE. Wyrembak J. Derbereva O. Melkonian G. Doran E. Lott IT. Head E. Cotman CW. Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer's disease and down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl 2):S293–S310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damier P. Hirsch EC. Zhang P. Agid Y. Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 26.Davidzon G. Greene P. Mancuso M. Klos KJ. Ahlskog JE. Hirano M. DiMauro S. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59:859–862. doi: 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 27.Dexter DT. Holley AE. Flitter WD. Slater TF. Wells FR. Daniel SE. Lees AJ. Jenner P. Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 28.Drucker G. Raikoff K. Neafsey EJ. Collins MA. Dopamine uptake inhibitory capacities of beta-carboline and 3,4-dihydro-beta-carboline analogs of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) oxidation products. Brain Res. 1990;509:125–133. doi: 10.1016/0006-8993(90)90318-6. [DOI] [PubMed] [Google Scholar]
- 29.Edgar D. Shabalina I. Camara Y. Wredenberg A. Calvaruso MA. Nijtmans L. Nedergaard J. Cannon B. Larsson NG. Trifunovic A. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–138. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Ephrussi B. Hottinger H. Chimenes A. Action de l'acriflavine sur les levures, I: la mutation “petite clonie.”. Ann Inst Pasteur. 1949;76:531. [Google Scholar]
- 31.Esteves AR. Arduino DM. Swerdlow RH. Oliveira CR. Cardoso SM. Oxidative stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2009;11:439–448. doi: 10.1089/ars.2008.2247. [DOI] [PubMed] [Google Scholar]
- 32.Esteves AR. Arduino DM. Swerdlow RH. Oliveira CR. Cardoso SM. Dysfunctional mitochondria uphold calpain activation: contribution to Parkinson's disease pathology. Neurobiol Dis. 2010;37:723–730. doi: 10.1016/j.nbd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Esteves AR. Arduino DM. Swerdlow RH. Oliveira CR. Cardoso SM. Microtubule depolymerization potentiates alpha-synuclein oligomerization. Front Aging Neurosci. 2010;1:5. doi: 10.3389/neuro.24.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteves AR. Domingues AF. Ferreira IL. Januario C. Swerdlow RH. Oliveira CR. Cardoso SM. Mitochondrial function in Parkinson's disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Esteves AR. Lu J. Rodova M. Onyango I. Lezi E. Dubinsky R. Lyons KE. Pahwa R. Burns JM. Cardoso SM. Swerdlow RH. Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson's subject mitochondrial transfer. J Neurochem. 2010;113:674–682. doi: 10.1111/j.1471-4159.2010.06631.x. [DOI] [PubMed] [Google Scholar]
- 36.Fan W. Waymire KG. Narula N. Li P. Rocher C. Coskun PE. Vannan MA. Narula J. Macgregor GR. Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008;319:958–962. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fearnley IM. Carroll J. Walker JE. Proteomic analysis of the subunit composition of complex I (NADH:ubiquinone oxidoreductase) from bovine heart mitochondria. Methods Mol Biol. 2007;357:103–125. doi: 10.1385/1-59745-214-9:103. [DOI] [PubMed] [Google Scholar]
- 38.Ghezzi D. Marelli C. Achilli A. Goldwurm S. Pezzoli G. Barone P. Pellecchia MT. Stanzione P. Brusa L. Bentivoglio AR. Bonuccelli U. Petrozzi L. Abbruzzese G. Marchese R. Cortelli P. Grimaldi D. Martinelli P. Ferrarese C. Garavaglia B. Sangiorgi S. Carelli V. Torroni A. Albanese A. Zeviani M. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson's disease in Italians. Eur J Hum Genet. 2005;13:748–752. doi: 10.1038/sj.ejhg.5201425. [DOI] [PubMed] [Google Scholar]
- 39.Giles RE. Blanc H. Cann HM. Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Duran A. Pacheu-Grau D. Lopez-Gallardo E. Diez-Sanchez C. Montoya J. Lopez-Perez MJ. Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 41.Gu M. Cooper JM. Taanman JW. Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 42.Haas RH. Nasirian F. Nakano K. Ward D. Pay M. Hill R. Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 43.Herkenham M. Little MD. Bankiewicz K. Yang SC. Markey SP. Johannessen JN. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40:133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- 44.Hirata Y. Sugimura H. Takei H. Nagatsu T. The effects of pyridinium salts, structurally related compounds of 1-methyl-4-phenylpyridinium ion (MPP+), on tyrosine hydroxylation in rat striatal tissue slices. Brain Res. 1986;397:341–344. doi: 10.1016/0006-8993(86)90636-0. [DOI] [PubMed] [Google Scholar]
- 45.Holt IJ. Zen and the art of mitochondrial DNA maintenance. Trends Genet. 2010;26:103–109. doi: 10.1016/j.tig.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Holthoff VA. Vieregge P. Kessler J. Pietrzyk U. Herholz K. Bonner J. Wagner R. Wienhard K. Pawlik G. Heiss WD. Discordant twins with Parkinson's disease: positron emission tomography and early signs of impaired cognitive circuits. Ann Neurol. 1994;36:176–182. doi: 10.1002/ana.410360209. [DOI] [PubMed] [Google Scholar]
- 47.Hubble JP. Cao T. Hassanein RE. Neuberger JS. Koller WC. Risk factors for Parkinson's disease. Neurology. 1993;43:1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- 48.Hudson G. Schaefer AM. Taylor RW. Tiangyou W. Gibson A. Venables G. Griffiths P. Burn DJ. Turnbull DM. Chinnery PF. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- 49.Ikebe S. Tanaka M. Ozawa T. Point mutations of mitochondrial genome in Parkinson's disease. Brain Res Mol Brain Res. 1995;28:281–295. doi: 10.1016/0169-328x(94)00209-w. [DOI] [PubMed] [Google Scholar]
- 50.Ito S. Ohta S. Nishimaki K. Kagawa Y. Soma R. Kuno SY. Komatsuzaki Y. Mizusawa H. Hayashi J. Functional integrity of mitochondrial genomes in human platelets and autopsied brain tissues from elderly patients with Alzheimer's disease. Proc Natl Acad Sci USA. 1999;96:2099–2103. doi: 10.1073/pnas.96.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ittarat I. Asawamahasakda W. Bartlett MS. Smith JW. Meshnick SR. Effects of atovaquone and other inhibitors on Pneumocystis carinii dihydroorotate dehydrogenase. Antimicrob Agents Chemother. 1995;39:325–328. doi: 10.1128/aac.39.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakupciak JP. Wang W. Markowitz ME. Ally D. Coble M. Srivastava S. Maitra A. Barker PE. Sidransky D. O'Connell CD. Mitochondrial DNA as a cancer biomarker. J Mol Diagn. 2005;7:258–267. doi: 10.1016/S1525-1578(10)60553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalra J. Rajput AH. Mantha SV. Prasad K. Serum antioxidant enzyme activity in Parkinson's disease. Mol Cell Biochem. 1992;110:165–168. doi: 10.1007/BF02454194. [DOI] [PubMed] [Google Scholar]
- 54.Kazuno AA. Munakata K. Nagai T. Shimozono S. Tanaka M. Yoneda M. Kato N. Miyawaki A. Kato T. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet. 2006;2:e128. doi: 10.1371/journal.pgen.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keeney PM. Dunham LD. Quigley CK. Morton SL. Bergquist KE. Bennett JP., Jr. Cybrid models of Parkinson's disease show variable mitochondrial biogenesis and genotype-respiration relationships. Exp Neurol. 2009;220:374–382. doi: 10.1016/j.expneurol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keeney PM. Quigley CK. Dunham LD. Papageorge CM. Iyer S. Thomas RR. Schwarz KM. Trimmer PA. Khan SM. Portell FR. Bergquist KE. Bennett JP., Jr. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther. 2009;20:897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan SM. Bennett JP., Jr. Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004;36:387–393. doi: 10.1023/B:JOBB.0000041773.20072.9e. [DOI] [PubMed] [Google Scholar]
- 58.King MP. Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 59.King MP. Koga Y. Davidson M. Schon EA. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992;12:480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirchner SC. Hallagan SE. Farin FM. Dilley J. Costa-Mallen P. Smith-Weller T. Franklin GM. Swanson PD. Checkoway H. Mitochondrial ND1 sequence analysis and association of the T4216C mutation with Parkinson's disease. Neurotoxicology. 2000;21:441–445. [PubMed] [Google Scholar]
- 61.Kosel S. Grasbon-Frodl EM. Mautsch U. Egensperger R. von Eitzen U. Frishman D. Hofmann S. Gerbitz KD. Mehraein P. Graeber MB. Novel mutations of mitochondrial complex I in pathologically proven Parkinson disease. Neurogenetics. 1998;1:197–204. doi: 10.1007/s100480050029. [DOI] [PubMed] [Google Scholar]
- 62.Kosel S. Lucking CB. Egensperger R. Mehraein P. Graeber MB. Mitochondrial NADH dehydrogenase and CYP2D6 genotypes in Lewy-body parkinsonism. J Neurosci Res. 1996;44:174–183. doi: 10.1002/(SICI)1097-4547(19960415)44:2<174::AID-JNR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Kraytsberg Y. Kudryavtseva E. McKee AC. Geula C. Kowall NW. Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 64.Kraytsberg Y. Simon DK. Turnbull DM. Khrapko K. Do mtDNA deletions drive premature aging in mtDNA mutator mice? Aging Cell. 2009;8:502–506. doi: 10.1111/j.1474-9726.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krige D. Carroll MT. Cooper JM. Marsden CD. Schapira AH. Platelet mitochondrial function in Parkinson's disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- 66.Kujoth GC. Hiona A. Pugh TD. Someya S. Panzer K. Wohlgemuth SE. Hofer T. Seo AY. Sullivan R. Jobling WA. Morrow JD. Van Remmen H. Sedivy JM. Yamasoba T. Tanokura M. Weindruch R. Leeuwenburgh C. Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 67.Laihinen A. Ruottinen H. Rinne JO. Haaparanta M. Bergman J. Solin O. Koskenvuo M. Marttila R. Rinne UK. Risk for Parkinson's disease: twin studies for the detection of asymptomatic subjects using [18F]6-fluorodopa PET. J Neurol. 2000;247(Suppl 2):II110–II113. doi: 10.1007/pl00022911. [DOI] [PubMed] [Google Scholar]
- 68.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 69.Lin MT. Simon DK. Ahn CH. Kim LM. Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 70.Lu J. Wang K. Rodova M. Esteves R. Berry D E L. Crafter A. Barrett M. Cardoso SM. Onyango I. Parker WD. Fontes J. Burns JM. Swerdlow RH. Polymorphic variation in cytochrome oxidase subunit genes. J Alzheimers Dis. 2010;21:141–154. doi: 10.3233/JAD-2010-100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luoma P. Melberg A. Rinne JO. Kaukonen JA. Nupponen NN. Chalmers RM. Oldfors A. Rautakorpi I. Peltonen L. Majamaa K. Somer H. Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 72.Luoma PT. Eerola J. Ahola S. Hakonen AH. Hellstrom O. Kivisto KT. Tienari PJ. Suomalainen A. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- 73.Makino Y. Ohta S. Tachikawa O. Hirobe M. Presence of tetrahydroisoquinoline and 1-methyl-tetrahydro-isoquinoline in foods: compounds related to Parkinson's disease. Life Sci. 1988;43:373–378. doi: 10.1016/0024-3205(88)90115-4. [DOI] [PubMed] [Google Scholar]
- 74.Mancuso M. Filosto M. Orsucci D. Siciliano G. Mitochondrial DNA sequence variation and neurodegeneration. Hum Genomics. 2008;3:71–78. doi: 10.1186/1479-7364-3-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marttila RJ. Lorentz H. Rinne UK. Oxygen toxicity protecting enzymes in Parkinson's disease. Increase of superoxide dismutase-like activity in the substantia nigra and basal nucleus. J Neurol Sci. 1988;86:321–331. doi: 10.1016/0022-510x(88)90108-6. [DOI] [PubMed] [Google Scholar]
- 76.Masucci JP. Davidson M. Koga Y. Schon EA. King MP. In vitro analysis of mutations causing myoclonus epilepsy with ragged-red fibers in the mitochondrial tRNA(Lys)gene: two genotypes produce similar phenotypes. Mol Cell Biol. 1995;15:2872–2881. doi: 10.1128/mcb.15.5.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsubara K. Kobayashi S. Kobayashi Y. Yamashita K. Koide H. Hatta M. Iwamoto K. Tanaka O. Kimura K. beta-Carbolinium cations, endogenous MPP+ analogs, in the lumbar cerebrospinal fluid of patients with Parkinson's disease. Neurology. 1995;45:2240–2245. doi: 10.1212/wnl.45.12.2240. [DOI] [PubMed] [Google Scholar]
- 78.Mayr-Wohlfart U. Rodel G. Henneberg A. Mitochondrial tRNA(Gln) and tRNA(Thr) gene variants in Parkinson's disease. Eur J Med Res. 1997;2:111–113. [PubMed] [Google Scholar]
- 79.Mecocci P. MacGarvey U. Kaufman AE. Koontz D. Shoffner JM. Wallace DC. Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 80.Michikawa Y. Mazzucchelli F. Bresolin N. Scarlato G. Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 81.Miller SW. Trimmer PA. Parker WD., Jr. Davis RE. Creation and characterization of mitochondrial DNA-depleted cell lines with “neuronal-like” properties. J Neurochem. 1996;67:1897–1907. doi: 10.1046/j.1471-4159.1996.67051897.x. [DOI] [PubMed] [Google Scholar]
- 82.Minnick DT. Pavlov YI. Kunkel TA. The fidelity of the human leading and lagging strand DNA replication apparatus with 8-oxodeoxyguanosine triphosphate. Nucleic Acids Res. 1994;22:5658–5664. doi: 10.1093/nar/22.25.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mizuno Y. Ohta S. Tanaka M. Takamiya S. Suzuki K. Sato T. Oya H. Ozawa T. Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989;163:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- 84.Morais R. Desjardins P. Turmel C. Zinkewich-Peotti K. Development and characterization of continuous avian cell lines depleted of mitochondrial DNA. In Vitro Cell Dev Biol. 1988;24:649–658. doi: 10.1007/BF02623602. [DOI] [PubMed] [Google Scholar]
- 85.Mytilineou C. Werner P. Molinari S. Di Rocco A. Cohen G. Yahr MD. Impaired oxidative decarboxylation of pyruvate in fibroblasts from patients with Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1994;8:223–228. doi: 10.1007/BF02260943. [DOI] [PubMed] [Google Scholar]
- 86.Nagatsu T. Yoshida M. An endogenous substance of the brain, tetrahydroisoquinoline, produces parkinsonism in primates with decreased dopamine, tyrosine hydroxylase and biopterin in the nigrostriatal regions. Neurosci Lett. 1988;87:178–182. doi: 10.1016/0304-3940(88)90166-8. [DOI] [PubMed] [Google Scholar]
- 87.Nakagawa-Hattori Y. Yoshino H. Kondo T. Mizuno Y. Horai S. Is Parkinson's disease a mitochondrial disorder? J Neurol Sci. 1992;107:29–33. doi: 10.1016/0022-510x(92)90205-y. [DOI] [PubMed] [Google Scholar]
- 88.Nicklas WJ. Vyas I. Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 89.Niwa T. Takeda N. Kaneda N. Hashizume Y. Nagatsu T. Presence of tetrahydroisoquinoline and 2-methyl-tetrahydroquinoline in parkinsonian and normal human brains. Biochem Biophys Res Commun. 1987;144:1084–1089. doi: 10.1016/s0006-291x(87)80075-x. [DOI] [PubMed] [Google Scholar]
- 90.Onyango IG. Tuttle JB. Bennett JP., Jr. Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson's disease cybrids. Mol Cell Neurosci. 2005;28:452–461. doi: 10.1016/j.mcn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Onyango IG. Tuttle JB. Bennett JP., Jr. Brain-derived growth factor and glial cell line-derived growth factor use distinct intracellular signaling pathways to protect PD cybrids from H2O2-induced neuronal death. Neurobiol Dis. 2005;20:141–154. doi: 10.1016/j.nbd.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 93.Parker WD. Sporadic neurologic disease and the electron transport chain: a hypothesis. In: Pascuzzi RM, editor. Proceedings of the 1989 Scientfic Meeting of the American Society for Neurological Investigation: New Developments in Neuromuscular Disease. Bloomington, Indiana: Indiana University Printing Services; 1990. [Google Scholar]
- 94.Parker WD., Jr. Boyson SJ. Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 95.Parker WD., Jr. Parks JK. Mitochondrial ND5 mutations in idiopathic Parkinson's disease. Biochem Biophys Res Commun. 2005;326:667–669. doi: 10.1016/j.bbrc.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 96.Parker WD., Jr. Parks JK. Swerdlow RH. Complex I deficiency in Parkinson's disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parker WD., Jr. Swerdlow RH. Mitochondrial dysfunction in idiopathic Parkinson disease. Am J Hum Genet. 1998;62:758–762. doi: 10.1086/301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavlov YI. Minnick DT. Izuta S. Kunkel TA. DNA replication fidelity with 8-oxodeoxyguanosine triphosphate. Biochemistry. 1994;33:4695–4701. doi: 10.1021/bi00181a029. [DOI] [PubMed] [Google Scholar]
- 99.Pello R. Martin MA. Carelli V. Nijtmans LG. Achilli A. Pala M. Torroni A. Gomez-Duran A. Ruiz-Pesini E. Martinuzzi A. Smeitink JA. Arenas J. Ugalde C. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Hum Mol Genet. 2008;17:4001–4011. doi: 10.1093/hmg/ddn303. [DOI] [PubMed] [Google Scholar]
- 100.Polymeropoulos MH. Lavedan C. Leroy E. Ide SE. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos ES. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson WG. Lazzarini AM. Duvoisin RC. Di Iorio G. Golbe LI. Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 101.Poste G. Reeve P. Enucleation of mammalian cells by cytochalasin B. II. Formation of hybrid cells and heterokaryons by fusion of anucleate and nucleated cells. Exp Cell Res. 1972;73:287–294. doi: 10.1016/0014-4827(72)90050-x. [DOI] [PubMed] [Google Scholar]
- 102.Pyle A. Foltynie T. Tiangyou W. Lambert C. Keers SM. Allcock LM. Davison J. Lewis SJ. Perry RH. Barker R. Burn DJ. Chinnery PF. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol. 2005;57:564–567. doi: 10.1002/ana.20417. [DOI] [PubMed] [Google Scholar]
- 103.Rajput AH. Birdi S. Epidemiology of Parkinson's disease. Parkinsonism Relat Disord. 1997;3:175–186. doi: 10.1016/s1353-8020(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 104.Rajput AH. Uitti RJ. Stern W. Laverty W. O'Donnell K. O'Donnell D. Yuen WK. Dua A. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson's disease. Can J Neurol Sci. 1987;14:414–418. doi: 10.1017/s0317167100037823. [DOI] [PubMed] [Google Scholar]
- 105.Ramsay RR. Salach JI. Singer TP. Uptake of the neurotoxin 1-methyl-4-phenylpyridine (MPP+) by mitochondria and its relation to the inhibition of the mitochondrial oxidation of NAD+-linked substrates by MPP+ Biochem Biophys Res Commun. 1986;134:743–748. doi: 10.1016/s0006-291x(86)80483-1. [DOI] [PubMed] [Google Scholar]
- 106.Raule N. Sevini F. Santoro A. Altilia S. Franceschi C. Association studies on human mitochondrial DNA: methodological aspects and results in the most common age-related diseases. Mitochondrion. 2007;7:29–38. doi: 10.1016/j.mito.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Ross OA. McCormack R. Maxwell LD. Duguid RA. Quinn DJ. Barnett YA. Rea IM. El-Agnaf OM. Gibson JM. Wallace A. Middleton D. Curran MD. mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson's disease in the Irish. Exp Gerontol. 2003;38:397–405. doi: 10.1016/s0531-5565(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 108.Ruiz-Pesini E. Lott MT. Procaccio V. Poole JC. Brandon MC. Mishmar D. Yi C. Kreuziger J. Baldi P. Wallace DC. An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Res. 2007;35:D823–D828. doi: 10.1093/nar/gkl927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruiz-Pesini E. Mishmar D. Brandon M. Procaccio V. Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 110.Saggu H. Cooksey J. Dexter D. Wells FR. Lees A. Jenner P. Marsden CD. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem. 1989;53:692–697. doi: 10.1111/j.1471-4159.1989.tb11759.x. [DOI] [PubMed] [Google Scholar]
- 111.Sanchez J. Overvik E. Ames BN. A marker of oxyradical-mediated DNA damage (8-hydroxy-2′deoxyguanosine) is increased in nigro-striatum of Parkinson's disease brain. Neurodegeneration. 1994;3:197–204. [Google Scholar]
- 112.Schapira AH. Cooper JM. Dexter D. Jenner P. Clark JB. Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 113.Schapira AH. Mann VM. Cooper JM. Dexter D. Daniel SE. Jenner P. Clark JB. Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- 114.Schon EA. Shoubridge EA. Moraes CT. Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology. 1998;51:326–327. doi: 10.1212/wnl.51.1.326. [DOI] [PubMed] [Google Scholar]
- 115.Sheehan JP. Swerdlow RH. Miller SW. Davis RE. Parks JK. Parker WD. Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheehan JP. Swerdlow RH. Parker WD. Miller SW. Davis RE. Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. J Neurochem. 1997;68:1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- 117.Shigenaga MK. Hagen TM. Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shoffner JM. Brown MD. Torroni A. Lott MT. Cabell MF. Mirra SS. Beal MF. Yang CC. Gearing M. Salvo R, et al. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 1993;17:171–184. doi: 10.1006/geno.1993.1299. [DOI] [PubMed] [Google Scholar]
- 119.Shoffner JM. Watts RL. Juncos JL. Torroni A. Wallace DC. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991;30:332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- 120.Shults CW. Miller SW. Reduced complex I activity in parkinsonian cybrids. Mov Disord. 1998;13(suppl. 2):217. [Google Scholar]
- 121.Simon DK. Lin MT. Zheng L. Liu GJ. Ahn CH. Kim LM. Mauck WM. Twu F. Beal MF. Johns DR. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson's disease. Neurobiol Aging. 2004;25:71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 122.Simon DK. Mayeux R. Marder K. Kowall NW. Beal MF. Johns DR. Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson's disease. Neurology. 2000;54:703–709. doi: 10.1212/wnl.54.3.703. [DOI] [PubMed] [Google Scholar]
- 123.Simon DK. Pankratz N. Kissell DK. Pauciulo MW. Halter CA. Rudolph A. Pfeiffer RF. Nichols WC. Foroud T. Maternal inheritance and mitochondrial DNA variants in familial Parkinson's disease. BMC Med Genet. 2010;11:53. doi: 10.1186/1471-2350-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simon DK. Pulst SM. Sutton JP. Browne SE. Beal MF. Johns DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53:1787–1793. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- 125.Smigrodzki R. Parks J. Parker WD. High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 126.Song S. Pursell ZF. Copeland WC. Longley MJ. Kunkel TA. Mathews CK. DNA precursor asymmetries in mammalian tissue mitochondria and possible contribution to mutagenesis through reduced replication fidelity. Proc Natl Acad Sci USA. 2005;102:4990–4995. doi: 10.1073/pnas.0500253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song S. Wheeler LJ. Mathews CK. Deoxyribonucleotide pool imbalance stimulates deletions in HeLa cell mitochondrial DNA. J Biol Chem. 2003;278:43893–43896. doi: 10.1074/jbc.C300401200. [DOI] [PubMed] [Google Scholar]
- 128.Swerdlow RH. Mitochondria and Parkinson's disease. In: Chesselet M-F, editor. Molecular Mechanisms of Neurodegenerative Diseases. Totowa, NJ: Humana Press; 2000. pp. 233–270. [Google Scholar]
- 129.Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging. 2007;28:1465–1480. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- 131.Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Swerdlow RH. Burns JM. Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;2(20 Suppl):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swerdlow RH. Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swerdlow RH. Parker WD. Currie LJ. Bennett JP. Harrison MB. Trugman JM. Wooten GF. Gender ratio differences between Parkinson's disease patients and their affected relatives. Parkinsonism Relat Disord. 2001;7:129–133. doi: 10.1016/s1353-8020(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 135.Swerdlow RH. Parks JK. Cassarino DS. Binder DR. Bennett JP., Jr. Di Iorio G. Golbe LI. Parker WD., Jr. Biochemical analysis of cybrids expressing mitochondrial DNA from Contursi kindred Parkinson's subjects. Exp Neurol. 2001;169:479–485. doi: 10.1006/exnr.2001.7674. [DOI] [PubMed] [Google Scholar]
- 136.Swerdlow RH. Parks JK. Cassarino DS. Maguire DJ. Maguire RS. Bennett JP., Jr. Davis RE. Parker WD., Jr. Cybrids in Alzheimer's disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 137.Swerdlow RH. Parks JK. Davis JN. Cassarino DS. Trimmer PA. Currie LJ. Dougherty J. Bridges WS. Bennett JP., Jr. Wooten GF. Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson's disease family. Ann Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- 138.Swerdlow RH. Parks JK. Miller SW. Tuttle JB. Trimmer PA. Sheehan JP. Bennett JP., Jr. Davis RE. Parker WD., Jr. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 139.Swerdlow RH. Weaver B. Grawey A. Wenger C. Freed E. Worrall BB. Complex I polymorphisms, bigenomic heterogeneity, and family history in Virginians with Parkinson's disease. J Neurol Sci. 2006;247:224–230. doi: 10.1016/j.jns.2006.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanner CM. Goldman SM. Epidemiology of Parkinson's disease. Neurol Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanner CM. Ottman R. Goldman SM. Ellenberg J. Chan P. Mayeux R. Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 142.Thomas B. Beal MF. Parkinson's disease. Hum Mol Genet. 2007;2:R183–R194. doi: 10.1093/hmg/ddm159. 16 Spec No. [DOI] [PubMed] [Google Scholar]
- 143.Thyagarajan D. Bressman S. Bruno C. Przedborski S. Shanske S. Lynch T. Fahn S. DiMauro S. A novel mitochondrial 12SrRNA point mutation in parkinsonism, deafness, and neuropathy. Ann Neurol. 2000;48:730–736. [PubMed] [Google Scholar]
- 144.Trifunovic A. Wredenberg A. Falkenberg M. Spelbrink JN. Rovio AT. Bruder CE. Bohlooly YM. Gidlof S. Oldfors A. Wibom R. Tornell J. Jacobs HT. Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 145.Trimmer PA. Bennett JP., Jr. The cybrid model of sporadic Parkinson's disease. Exp Neurol. 2009;218:320–325. doi: 10.1016/j.expneurol.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trimmer PA. Borland MK. Keeney PM. Bennett JP., Jr. Parker WD., Jr. Parkinson's disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem. 2004;88:800–812. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- 147.Trimmer PA. Schwartz KM. Borland MK. De Taboada L. Streeter J. Oron U. Reduced axonal transport in Parkinson's disease cybrid neurites is restored by light therapy. Mol Neurodegener. 2009;4:26. doi: 10.1186/1750-1326-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trimmer PA. Swerdlow RH. Parks JK. Keeney P. Bennett JP., Jr. Miller SW. Davis RE. Parker WD., Jr. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 149.Turner CJ. Granycome C. Hurst R. Pohler E. Juhola MK. Juhola MI. Jacobs HT. Sutherland L. Holt IJ. Systematic segregation to mutant mitochondrial DNA and accompanying loss of mitochondrial DNA in human NT2 teratocarcinoma Cybrids. Genetics. 2005;170:1879–1885. doi: 10.1534/genetics.105.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ullrich A. Knecht W. Fries M. Loffler M. Recombinant expression of N-terminal truncated mutants of the membrane bound mouse, rat and human flavoenzyme dihydroorotate dehydrogenase. A versatile tool to rate inhibitor effects? Eur J Biochem. 2001;268:1861–1868. [PubMed] [Google Scholar]
- 151.van der Walt JM. Nicodemus KK. Martin ER. Scott WK. Nance MA. Watts RL. Hubble JP. Haines JL. Koller WC. Lyons K. Pahwa R. Stern MB. Colcher A. Hiner BC. Jankovic J. Ondo WG. Allen FH., Jr. Goetz CG. Small GW. Mastaglia F. Stajich JM. McLaurin AC. Middleton LT. Scott BL. Schmechel DE. Pericak-Vance MA. Vance JM. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vander Heiden MG. Cantley LC. Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Veech GA. Dennis J. Keeney PM. Fall CP. Swerdlow RH. Parker WD., Jr. Bennett JP., Jr. Disrupted mitochondrial electron transport function increases expression of anti-apoptotic bcl-2 and bcl-X(L) proteins in SH-SY5Y neuroblastoma and in Parkinson disease cybrid cells through oxidative stress. J Neurosci Res. 2000;61:693–700. doi: 10.1002/1097-4547(20000915)61:6<693::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 154.Vermulst M. Wanagat J. Kujoth GC. Bielas JH. Rabinovitch PS. Prolla TA. Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 155.Vives-Bauza C. Andreu AL. Manfredi G. Beal MF. Janetzky B. Gruenewald TH. Lin MT. Sequence analysis of the entire mitochondrial genome in Parkinson's disease. Biochem Biophys Res Commun. 2002;290:1593–1601. doi: 10.1006/bbrc.2002.6388. [DOI] [PubMed] [Google Scholar]
- 156.Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- 157.Wallace DC. Bunn CL. Eisenstadt JM. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975;67:174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 159.Wirdefeldt K. Gatz M. Schalling M. Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- 160.Wiseman A. Attardi G. Reversible tenfod reduction in mitochondria DNA content of human cells treated with ethidium bromide. Mol Gen Genet. 1978;167:51–63. doi: 10.1007/BF00270321. [DOI] [PubMed] [Google Scholar]
- 161.Wooten GF. Currie LJ. Bennett JP. Harrison MB. Trugman JM. Parker WD., Jr. Maternal inheritance in Parkinson's disease. Ann Neurol. 1997;41:265–268. doi: 10.1002/ana.410410218. [DOI] [PubMed] [Google Scholar]
- 162.Yoshino H. Nakagawa-Hattori Y. Kondo T. Mizuno Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1992;4:27–34. doi: 10.1007/BF02257619. [DOI] [PubMed] [Google Scholar]
- 163.Zameitat E. Gojkovic Z. Knecht W. Piskur J. Loffler M. Biochemical characterization of recombinant dihydroorotate dehydrogenase from the opportunistic pathogenic yeast Candida albicans. FEBS J. 2006;273:3183–3191. doi: 10.1111/j.1742-4658.2006.05327.x. [DOI] [PubMed] [Google Scholar]