Abstract
Dietary restriction (DR) extends lifespan across multiple species including mouse. Antioxidant plant extracts rich in polyphenols have also been shown to increase lifespan. We hypothesized polyphenols might potentiate DR-induced lifespan extension. Twenty week old C57BL/6 mice were placed on one of three diets: continuous feeding (control), alternate day chow (Intermittent fed, IF), or IF supplemented with polyphenol antioxidants (PAO) from blueberry, pomegranate, and green tea extracts (IF+PAO). Both IF and IF+PAO groups outlived the control group and the IF+ PAO group outlived the IF group (all p < 0.001). In the brain, IF induced the expression of inflammatory genes and p38 MAPK phosphorylation, while the addition of PAO reduced brain inflammatory gene expression and p38 MAPK phosphorylation. Our data indicate that while IF overall promotes longevity, some aspects of IF-induced stress may paradoxically lessen this effect. Polyphenol compounds, in turn, may potentiate IF-induced longevity by minimizing specific components of IF-induced cell stress.
Keywords: Longevity, dietary restriction, polyphenol, tea, blueberry, pomegranate
1. Introduction
Dietary restriction (DR) extends lifespan in multiple species including flies, yeast, worms, mice, and rats [1]. Different DR regimens, including alternate day or intermittent fasting (IF), have been extensively studied in rodents [2] and shown to modify expression of genes involved in oxidative stress, tumorigenesis, cell bioenergetics, inflammation, and mRNA splicing [3]. DR influences the energy sensing AMP kinase system [4] and the sirtuin family of NAD-dependent deacetylases [5]. It affects mitochondrial maintenance [4], and alters chromatin architecture [6]. These changes are demonstrable in a variety of tissues and can be tissue-specific. It has been hypothesized that DR slows the basic aging process through effects that involve a number of interactive gene networks. While DR has not yet been shown to increase human longevity, it does appear to mitigate age-associated increases in insulin resistance, cholesterol, and blood pressure [7].
Several plant polyphenols have also been shown to increase longevity. In particular, crude blueberry extracts and green tea polyphenols prolong life spans of worms and mice, respectively [8, 9]. These effects have been attributed both to antioxidant effects and to the direct activation of SIRT1 by compounds such as resveratrol that are present in the polyphenol mixtures. While pomegranate has not been reported to extend lifespan, polyphenol-rich pomegranate fruit extract inhibited oxidative stress in human keratinocytes [10]. Although it has been proposed that polyphenols mediate life prolongation primarily via their anti-oxidant properties, some studies suggest some antioxidant chemicals not only fail to provide a life prolonging effect, but also unexpectedly reduce the life-extension capacity of DR. In mice, an antioxidant mixture containing 2-mercaptoethylamine plus ethoxyquin countered DR-induced lifespan extension [11]. N-acetylcysteine, vitamin C, and the vitamin E derivative trolox abolished a DR mimetic-induced lifespan increase in C. elegans [12]. Similarly, in humans, vitamins C and E reduced the benefits of endurance training [13]. In this study, we examined the impact of polyphenol-plant derived antioxidants (PAO) on DR’s longevity-promoting effects.
2. Material and methods
2.1. Survival Experiment
Survival studies were performed using 148 (12 control, 68 IF and 68 IF+PAO) male C57BL/6 mice that were obtained from Jackson Laboratories at 4–6 weeks of age. Individual mice were housed in separate clear plastic cages on a 12:12 hour light:dark schedule. All mice were maintained on an AL diet until 20 weeks of age. At 20 weeks of age the mice were randomized to 3 different dietary groups. AL-fed mice (the control group) had uninterrupted food access. IF mice had 24 hours of no food access followed by 24 hours of food access (fasted 3 days per week and fed high fat chow ad libitum 4 days per week). IF+PAO mice had 24 hours of no food access followed by 24 hours of food access (fasted 3 days per week and fed high fat chow supplemented with blueberry, green tea, and pomegranate extracts ad libitum 4 days per week). The control and IF groups consumed TD.94045 AIN-93G chow (Harlan-Teklad). The IF+PAO mice consumed TD.07226 (Harlan Teklad), which is TD.94045 modified to contain 2% blueberry extract, 115 ppm Sunphenon (epigallocatechin gallate; EGCG), and 0.3% pomegranate powder at the expense of corn starch. All mice had uninterrupted access to drinking water.
2.2. Microarray Experiments
In addition to the mice used in the survival experiment, another 8 male mice and 16 female mice were maintained on the above specified diets in order to generate biospecimens for microarray experiments. Brain tissue was collected from these mice at 2 years of age, flash frozen in liquid nitrogen, and stored at −80C until use. For male mice, brains were collected at the end of a feeding day for comparisons of the control (n=2), IF (n=3) and IF+PAO (n=3) groups. For female mice, brains were collected at the end of a feeding day for comparisons of IF (n=4) and female IF+PAO mice (n=4), as well as at the end of a fasting day (IF group n=4; IF+PAO group n=4). For each specimen total RNA was isolated from 50–100 mg of freshly ground tissue using TRIzol reagent with phase lock gel, and subjected to quality assessment with Agilent RNA kits before reverse transcription. Total cDNAs were amplified once and hybridized to Affymetrix Genechip Array Mouse 430 2.0. Data were analyzed by Affymetrix Expression Console RMA-PARTEK (2 data points) and by GeneSpring for gene annotation. To evaluate the expression levels of gene sets, Gene Set Enrichment Analysis (GSEA, http://www.broadinstitute.org/gsea/index.jsp) software was used. The enrichment score (ES) and normalized enrichment score (NES) were calculated to reflect the over-represented degree [14]. GSEA gene ontology collection v3.0 was used (http://www.broadinstitute.org/gsea/msigdb/index.jsp) for pathway expression analysis. False Discovery Rate (FDR) q values and nominal p values were calculated by GSEA for each gene set.
2.3. Immunoblotting
Another 15 male C57BL/6 mice were placed onto the different diets and used to prepare brain protein lysates. For this part of the analysis, the control, IF, and IF+PAO groups each contained five mice. After one month on their respective diets these mice were sacrificed by decapitation. The control group had access to food up to the time of sacrifice, while the IF and IF+PAO groups had no food access for 24 hours prior to sacrifice. Brains were rapidly removed and placed in liquid nitrogen within one minute of decapitation. The frozen brains were later thawed, on ice, to the point that homogenates could be generated and protein lysates were subsequently prepared using a NE-PER kit (Pierce) according to the manufacturer’s instructions. A primary antibody to the phospho-p38 (Cell Signaling Technology) mitogen-activated protein kinase (MAPK) was used to measure p38 activation in the cytosolic lysate fraction. Primary antibody binding was revealed using horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) and Supersignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Densitometry was performed using a ChemiDoc XRS with Quantity One software. To ensure equivalent protein loading, the blot was subsequently stained with a primary antibody to actin (Santa Cruz Biotechnology). The phospho-p38 band densities were divided by their corresponding actin densities to yield a relative density value. Relative density values were summarized by mean and standard error. Means were compared by one-way ANOVA followed by a Least Squares Deviation test, with p values less than 0.05 considered significant.
3. Results
To examine the effects of PAO on DR lifespan extension, we placed four month old C57BL/6 mice on three different diets. The control diet consisted of a high-fat chow, which was selected over standard chow in order to better reflect diet patterns typical of Western countries. We established two treatment groups: an IF group that underwent repeated fasting and refeeding (in 24 hour cycles) with the high fat chow, and an IF group whose high fat chow was supplemented with blueberry, pomegranate, and green tea extracts (the IF+PAO group).
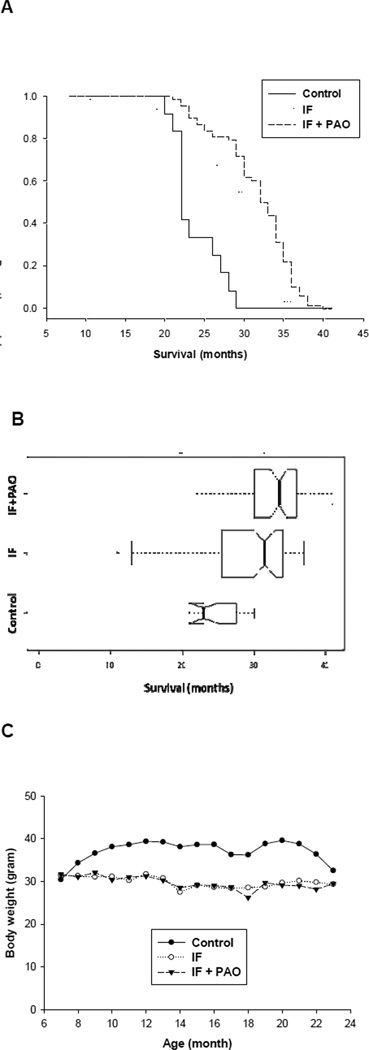
As shown in Figure 1a, compared to the control group the IF and IF+PAO groups had significant life span prolongation (p < 0.001 and p < 0.001, respectively) (see also Table 1). IF+PAO mice also significantly outlived IF mice with a median survival of 33.5 months compared to 31.5 months for the IF group (p < .001) (Figure 1a and 1b, Table 1). Major postmortem pathologic findings did not differ between IF and IF+PAO mice (Table 2). Relative to the control group, body weight was significantly reduced in both the IF and IF+PAO groups but IF and IF+PAO body weights were themselves comparable (Fig. 1c).
Figure 1. Comparison of Survival (A,B) and Body Weights (C) for male C57BL/6 mice.
Mice were fed a high fat chow ad libitum (control, n=12), high fat chow on alternate days four days a week (intermittent fed group, IF, n = 68), or alternate day high fat chow supplemented with blueberry, pomegranate, and green tea extracts (IF + PAO, n = 68). (A) Kaplan-Meier survival curves are used to represent the distribution of survival time in months. (B) Boxplots represent the distribution of survival time in months for the three groups. The median survival time, first and third quartile, and outlying times are represented by the center line, left and right bounds of the box, and plotted points, respectively. Boxes are drawn with widths proportional to the number of observations per group. Whiskers extend to the most extreme observation within a distance of 1.5 times the interquartile range (IQR = Q2 − Q1). The notches in the side of each box extend to and represent an asymptotic but robust 95% confidence interval estimate for median survival time. Strong evidence of a difference in the median survival time between two groups is indicated by non-overlapping notches of the plots of those groups. (C) Mean body weights across the duration of the study. Control mice stabilized at 37 grams. IF and IF+PAO groups stabilized at 29 grams.
Table 1.
Survival analysis and Kruskal-Wallis rank sum test (χ22 = 24.47, p = < 0.001) results.
| A. Kaplan-Meier estimates of median survival | ||
|---|---|---|
| Group | N | Median Survival (months) |
| Control | 12 | 23.0 |
| IF | 68 | 31.5 |
| IF+PAO | 68 | 33.5 |
| B. Kruskal-Wallis rank sum tests of significance | ||
|---|---|---|
| χ22, p | Control | IF |
| Control | — | — |
| IF | 17.9 (<0.001) | — |
| IF+PAO | 43.9 (<0.001) | 12 (<0.001) |
Table 2.
Deceased mice necropsy results.
| IF | IF+PAO | |
|---|---|---|
| died naturally & underwent necropsy | 32 | 30 |
| severely enlarged liver, likely liver tumor | 12 (34%) | 12 (32%) |
| visibly enlarged liver, possibly due to liver tumor | 6 (17%) | 7 (18%) |
| short or no top teeth | 14 (40%) | 11 (30%) |
GSEA was used to identify gene ontology pathways that were differently expressed in IF+PAO versus IF mouse brain tissue. These studies were performed using both male and female aged C57BL/6 mice. For 2 year-old male and female mice sacrificed at the end of a feeding day, no pathways were significantly altered. Although we did not analyze gene expression in aged male mice at the end of a fasting day, in female mice sacrificed at the end of a fasting day GSEA identified 20 gene sets that were down-regulated by addition of PAO to IF (FDR q < 0.0001, NOM p < 0.001). Table 3 lists these 20 sets by biological function. Of the 20, 11 were related to immune response or inflammation, 4 to tumorigenesis/metastasis, 3 to cell differentiation, 1 to adipogenesis, and 1 to oxidative stress.
Table 3.
20 gene sets were significantly down-regulated by addition of PAO to IF (FDR q < 0.0001, NOM p < 0.001).
| Pathway | Description | ES | NES | NOM p-val |
FDR q-val |
|---|---|---|---|---|---|
| WIELAND_HEPATITIS_B_INDUCED | immune response | −0.69 | −2.63 | 0.00 | 0.00 |
| ICHIBA_GVHD | immune response | −0.52 | −2.44 | 0.00 | 0.00 |
| SANA_IFNG_ENDOTHELIAL_UP | immune response | −0.63 | −2.26 | 0.00 | 0.00 |
| FLECHNER_KIDNEY_TRANSPLANT_REJECTION_UP | immune response | −0.56 | −2.17 | 0.00 | 0.00 |
| NEMETH_TNF_UP | immune response | −0.55 | −2.16 | 0.00 | 0.00 |
| DER_IFNG_UP | immune response | −0.59 | −2.15 | 0.00 | 0.00 |
| SANA_TNFA_ENDOTHELIAL_UP | immune response | −0.56 | −2.13 | 0.00 | 0.00 |
| IGLESIAS_E2FMINUS_UP | immune response | −0.52 | −2.20 | 0.00 | 0.00 |
| LAL_KO_6MO_UP | inflammation | −0.61 | −2.27 | 0.00 | 0.00 |
| LAL_KO_3MO_UP | inflammation | −0.61 | −2.15 | 0.00 | 0.00 |
| CARIES_PULP_UP | inflammation, DNA repair, cell differentiation, | −0.53 | −2.35 | 0.00 | 0.00 |
| LIAN_MYELOID_DIFF_GRANULE | cell differentiation | −0.80 | −2.54 | 0.00 | 0.00 |
| TGFBETA_EARLY_UP | cell differentiation | −0.66 | −2.29 | 0.00 | 0.00 |
| TAKEDA_NUP98_HOXA9_8D_DN | cell differentiation | −0.49 | −2.13 | 0.00 | 0.00 |
| YU_CMYC_DN | tumorigenesis | −0.58 | −2.15 | 0.00 | 0.00 |
| LEE_MYC_E2F1_UP | tumorigenesis | −0.61 | −2.14 | 0.00 | 0.00 |
| EMT_UP | metastasis/tumorigenesis | −0.59 | −2.15 | 0.00 | 0.00 |
| GILDEA_BLADDER_UP | metastasis/tumorigen | −0.70 | −2.15 | 0.00 | 0.00 |
| NADLER_OBESITY_UP | adipogenesis | −0.63 | −2.30 | 0.00 | 0.00 |
| AGEING_BRAIN_UP | antioxidant and DNA repair | −0.55 | −2.43 | 0.00 | 0.00 |
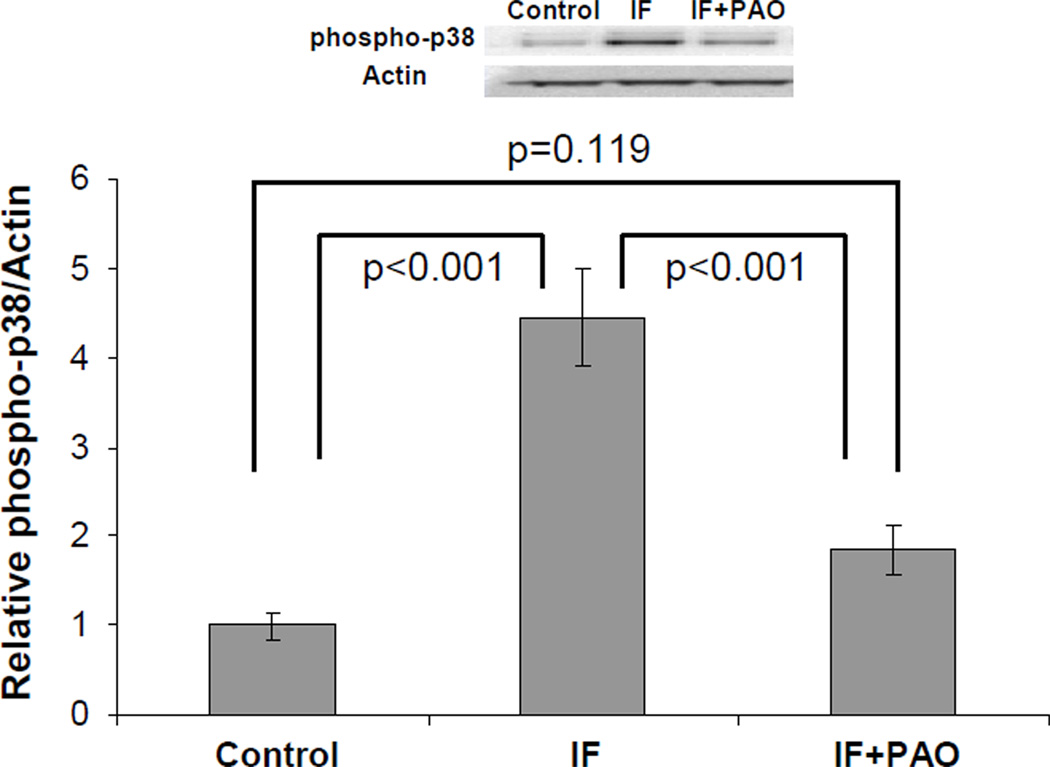
These gene array findings suggest that addition of PAO to the IF protocol prevents the activation of pro-inflammatory pathways. In support of this, we analyzed the activation status of brain p38 MAPK protein. The p38 MAPK acts as a pro-inflammatory signaling pathway intermediate that is activated by oxidative stress and oxidative stress-related conditions. We found that relative to the control diet the unsupplemented IF diet actually induced p38 phosphorylation, while the addition of PAO to the IF diet blocked this effect (Figure 2).
Figure 2. p38 phosphorylation in brain protein lysates from control, IF, and IF+PAO mice.
A Western blot approach was used to determine phospho-p38 and actin band densities. The density value for each phospho-p38 band was divided by its corresponding actin band density value to correct for differences in protein loading. Each group contained brain lysates from five different mice. Data are presented as mean values ± SEM. The groups were analyzed by oneway ANOVA. Phospho-p38 in the IF group was higher than it was in the control group, which suggests 24 hours of fasting activated p38 MAPK signaling. PAO supplementation prevented the apparent fast-induced increase in p38 phosphorylation. The groups were analyzed by oneway ANOVA.
4. Discussion
This study investigated the impact of PAO on DR-induced lifespan extension. While our data show both IF and IF+PAO significantly prolong lifespan, adding PAO to IF further extends lifespan significantly beyond IF alone.
We previously reported that the addition of this PAO combination to this IF DR protocol did not alter insulin sensitivity or glucose tolerance in male C57BL/6 mice [19]. We now report addition of PAO to the IF protocol did not affect the weight of the mice. Together, results from our previous and current study suggest changes in weight, insulin sensitivity, and glucose tolerance cannot explain the beneficial effect of PAO supplementation on mouse longevity. Rather, our data suggest this benefit may be explained by changes in immune response, inflammation, cell differentiation, oxidative stress, DNA repair, and adipogenesis-related gene expression. In particular, eleven of twenty PAO-induced GSEA changes involved inflammation/immunity gene sets. Our data, therefore, further suggest that although IF extends longevity in caged mice, it also simultaneously induces stress that may paradoxically counteract its overall beneficial effects.
Epidemiological data suggest that in humans DR may prolong lifespan and reduce mortality from cancer and coronary heart disease [20]. Uncontrolled data from people practicing selfimposed DR suggests it protects against diabetes, left ventricular diastolic dysfunction, and inflammation [21]. Examining whether polyphenols potentiate DR benefits in humans will likely require logistically complex randomized clinical studies. Our data on polyphenol -DR combinations can potentially inform the design of such studies.
We selected a high-fat chow for these experiments because we wanted to mimic the effects of a Western diet. Our use of a high-fat diet does, however, raise several interpretive considerations. One caveat arises from the possibility that even though the mice were on an alternate day feeding protocol, our high-fat baseline diet may have rendered the mice obese. To assess this, we reviewed prior CR studies of male C57BL/6 mice [15–18]. Body weights in both our control and IF groups were comparable to those from other studies (Table 4).
Table 4.
Comparison of approximate body weights and median life spans for C57BL/6 mice from different DR studies.
| A. Comparison of approximate body weights among various studies (in gram) | ||
|---|---|---|
| Control | DR | |
| Non-obese | ||
| Turturro | 41 | 23 |
| Aires | 37 | 29 |
| Pearson | 34 | 30 |
| Pugh | 33 | 25 |
| Weindruch | 32 | 25 |
| Obese | ||
| Pearson Obese mice | 47 | |
| B. Comparison of approximate median life span among various studies (in months) | ||
|---|---|---|
| Control | DR | |
| Non-obese | ||
| Turturro | 26.8 | 32.1 |
| Aires | 23 | 31.5 |
| Pearson | 29.5 | 31.3 |
| Pugh | 29.8 | 34.6 |
| Weindruch | 30 | 31.2 |
| Obese | ||
| Pearson Obese mice | 27 | |
Control group longevity in our study was reduced relative to what was reported in these other studies (Table 4), which could be due to the high fat chow these ad libitum-fed mice were maintained on. However, compared to the other studies the high fat chow did not appear to negatively impact the life expectancy of our IF group. Since our study did not focus on differences between IF and control, but rather on differences between IF and IF+PAO, the fact that our IF group lived as long as non-high-fat IF groups from other studies indicates IF-induced lifespan extension in this study was not simply an artifact of reduced life expectancy in our control group.
Our study has several procedural limitations. The fasting GSEA data were obtained only in female mice and the survival data were obtained only from male mice. This occurred because the male mice were sacrificed before the need to sacrifice mice on a fasting day was appreciated, and thus no gene array data were obtained from the post-fasted male mice. The fact that these pathway changes were only observed in mice on the fasting day raises the possibility that PAO benefits require the presence of DR, and that PAO without DR might not prolong longevity. Finally, the p38 MAPK biochemical analysis was performed on relatively young male mice, as opposed to aged mice. Repeating these measurements in appropriately aged mice would be required to determine whether or not the ability of PAO to reduce IF-induced oxidative stress is age-dependent or age-independent.
5. Conclusion
Our study is the first to demonstrate that polyphenols, when used in conjunction with an intermittent feeding DR protocol, can enhance the longevity-promoting effects of that DR protocol (at least in mice). The underlying mechanism appears to involve reduction of cell stress and inflammation-related activity, some of which seems to be a byproduct of DR itself. In the absence of definitive clinical trials that address the health benefits of DR-polyphenol combinations, our data may have practical relevance for humans seeking to engage in healthpromoting behaviors.
Acknowledgements
This work was partly funded through a generous donation from Mr. and Mrs. Thomas Docking, and partly by NIH grants NIDDK R01DK067355 and NIA P30AG035982 (which supports the University of Kansas Alzheimer’s Disease Center).
References
- 1.Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- 2.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Swindell WR. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics. 2009;10:585. doi: 10.1186/1471-2164-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 6.Mostoslavsky R, Esteller M, Vaquero A. At the crossroad of lifespan, calorie restriction, chromatin and disease: meeting on sirtuins. Cell Cycle. 2010;9:1907–1912. doi: 10.4161/cc.9.10.11481. [DOI] [PubMed] [Google Scholar]
- 7.Rae M. It's never too late: calorie restriction is effective in older mammals. Rejuvenation Res. 2004;7:3–8. doi: 10.1089/154916804323105026. [DOI] [PubMed] [Google Scholar]
- 8.Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaid MA, Afaq F, Syed DN, Dreher M, Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem Photobiol. 2007;83:882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 11.Harris SB, Weindruch R, Smith GS, Mickey MR, Walford RL. Dietary restriction alone and in combination with oral ethoxyquin/2-mercaptoethylamine in mice. J Gerontol. 1990;45:B141–B147. doi: 10.1093/geronj/45.5.b141. [DOI] [PubMed] [Google Scholar]
- 12.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res. 1999;59:1642–1648. [PubMed] [Google Scholar]
- 17.Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:B379–B389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- 18.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, L E, Wang W, Frontera J, Zhu H, Wang WT, Lee P, Choi IY, Brooks WM, Burns JM, Aires D, Swerdlow RH. Alternate day fasting impacts the brain insulin-signaling pathway of young adult male C57BL/6 mice. J Neurochem. 2011;117:154–163. doi: 10.1111/j.1471-4159.2011.07184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- 21.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]