Abstract
The impact of caffeine on the behavioral effects of ethanol, including ethanol consumption and abuse, has become a topic of great interest due to the rise in popularity of the so-called energy drinks. Energy drinks high in caffeine are frequently taken in combination with ethanol under the popular belief that caffeine can offset some of the intoxicating effects of ethanol. However, scientific research has not universally supported the idea that caffeine can reduce the effects of ethanol in humans or in rodents, and the mechanisms mediating the caffeine–ethanol interactions are not well understood. Caffeine and ethanol have a common biological substrate; both act on neurochemical processes related to the neuromodulator adenosine. Caffeine acts as a nonselective adenosine A1 and A2A receptor antagonist, while ethanol has been demonstrated to increase the basal adenosinergic tone via multiple mechanisms. Since adenosine transmission modulates multiple behavioral processes, the interaction of both drugs can regulate a wide range of effects related to alcohol consumption and the development of ethanol addiction. In the present review, we discuss the relatively small number of animal studies that have assessed the interactions between caffeine and ethanol, as well as the interactions between ethanol and subtype-selective adenosine receptor antagonists, to understand the basic findings and determine the possible mechanisms of action underlying the caffeine–ethanol interactions.
Caffeine as a Modulator of Ethanol Abuse
Caffeine and ethanol are widely consumed recreational drugs.1,2 Alcohol abuse is a worldwide health problem, with serious medical, economic, and social consequences.3,4 On the other hand, caffeine intake, even in excess, appears to be relatively well accepted, because methylxanthines have activating and attention-preserving properties that can help productivity and enhance the performance. Interest in caffeine has grown ever since the introduction to the market of the so-called energy drinks, which contain caffeine and related substances in quite high concentrations. These drinks are being increasingly consumed, often in combination with substances that have abuse potential.5 In addition, research with animals has demonstrated the ability of methylxanthines, and in particular caffeine, to modulate the psychopharmacological effects of drugs of abuse such as methamphetamine,6 amphetamine,7 nicotine,8,9 cocaine,10 and ethanol.11 The reasons for combining caffeine with ethanol may stem from the popular belief that caffeine can antagonize the intoxicating effects of alcohol.12 Some studies have supported this hypothesis, demonstrating that caffeine attenuates ethanol-induced changes in psychological parameters in humans such as information processing, memory, psychomotor performance, and others (for a review13).
Caffeine has been shown to indirectly modulate the activity of many neurotransmitters and neuromodulators, including dopamine, acetylcholine, or glutamate14–17 in various brain areas. However, in terms of direct actions, caffeine is most widely described as an adenosine receptor antagonist that is nonselective for the A1 and A2A subtypes of adenosine receptors in the central nervous system.1,17–19 Several articles have demonstrated that there are interactions between adenosine and ethanol. Ethanol can increase extracellular adenosine levels by increasing adenosine release,20,21 and by decreasing adenosine uptake22 that takes place via a facilitative nucleoside transporter.23,24 Inhibition of this transporter in the presence of ethanol would lead to an increase in the extracellular adenosine and could thereby modulate some of the effects of ethanol.21 Secondarily, ethanol increases the adenosine levels because acetate generated by ethanol metabolism promotes adenosine synthesis25 (see Fig. 1).
FIG. 1.
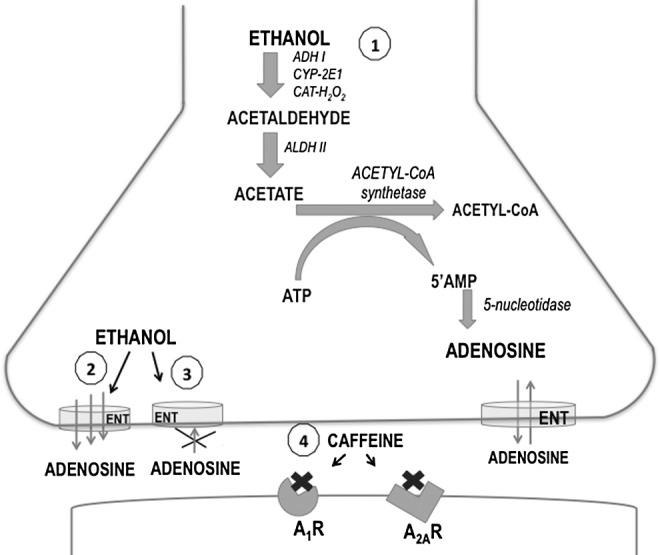
Schematic showing ethanol regulation of adenosine production (1), release (2), and uptake (3), as well as caffeine blockade of adenosine receptors (4) in the central nervous system. A1R and A2AR, adenosine A1 and A2A receptors; ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; ATP, adenosine triphosphate; AMP, adenosine monophosphate; CAT-H2O2, catalase; CYP-2E1, cytochrome P4502E1; ENT, equilibrative nucleoside transporters.
In contrast to the studies showing that caffeine can blunt the effects of ethanol, there also is evidence that fails to support the idea of an antagonistic behavioral interaction between caffeine and ethanol, either in humans26,27 (for review13) or in rodents.28–30 A considerable number of studies employing experimental animal models have been performed to elucidate the impact of caffeine on the effects of ethanol and on ethanol consumption. In the present review, we have emphasized those studies addressing behaviors that can be relevant for the development of alcohol consumption, abuse, and addiction as a compulsive habit, as well as studies that evaluate signs of dependence after withdrawal, such as physical abstinence and craving, which are factors that can lead to relapse.
Drug addictions, including alcoholism, can be conceptualized as disorders of motivation characterized by an excessive control of the drug over behavior.31–33 This disorder involves a reorganization of the preference structure of the person, dramatic changes in the allocation of behavioral resources toward the addictive substance,34,35 and alterations in the elasticity of demand for the drug.36 Typically, there is a heightened tendency to engage in drug-reinforced instrumental behavior and drug consumption, often at the expense of other behavioral activities. Addicts will go to great lengths to obtain the drug, overcoming numerous obstacles and constraints. In addition, the development of addiction is attributed to a profound sensitization in the neural processes that mediate the drug-seeking behavior, which can facilitate the incentive properties of drugs and drug-related stimuli as the addiction process proceeds.37,38 Thus, as addiction progresses, the drug itself, as well as drug-associated stimuli, trigger an automatic seeking response that ultimately resolves in the consumption of the drug. This automatism has compulsive characteristics that are devoid of instrumental feedback, leading to the formation of drug-related habits.39,40 Thus, addiction is a very complex set of behavioral and physiological processes that range all the way from drug consumption, to tolerance for some effects, sensitization of motor activity, establishment of implicit and explicit learning, initial sensitivity to reward and punishment, attention shifts, responsivity to Pavlovian cues, and other processes.
In the present review, studies addressing the impact of caffeine on some of those behaviors modulated by ethanol will be summarized. Because of the opposing actions of ethanol and caffeine on the adenosine system, studies focusing on the effects of selective adenosine receptor agonists and antagonists and their interaction with ethanol will be also presented in an attempt to shed light upon the potential receptor mechanisms involved.
Caffeine–Ethanol Interactions: Effects on Locomotion
The behavioral stimulant or suppressant actions of drugs are frequently evaluated by analyzing the locomotor activity of animals.41,42 Although ethanol is generally classed as a sedative hypnotic and caffeine is considered to be a minor stimulant, both drugs are able to stimulate the locomotor activity in rodents at some dose,43–48 typically with bell-shaped (or inverted-U) dose–response functions. Rodents (more in mice than rats) show a time- and dose-dependent locomotor response to acute ethanol administration, with low doses stimulating and high doses reducing locomotion.46,49–52 Methylxanthines such as caffeine also can affect locomotor activity in a biphasic way.53–56 However, few studies have evaluated the caffeine–ethanol interactions using locomotion as a measure.51,53,57,58 Waldeck51 evaluated the effect of ethanol (1, 3, or 4 g/kg, intraperitoneal [IP]) and caffeine (25, 50, or 100 mg/kg, IP) on locomotor activity in female mice, and observed that a moderate dose of caffeine (25 mg/kg) that stimulated locomotion also potentiated the stimulation induced by ethanol administered at the lowest dose (1 g/kg), although it abolished the stimulant effect of a higher dose of ethanol (3 g/kg). On the other hand, a motor-suppressant dose of caffeine (100 mg/kg) totally blocked the stimulant effect of ethanol (1 g/kg). Moreover, the motor-suppressant effect of the higher dose of ethanol (4 g/kg) was potentiated by all doses of caffeine employed.51 These results with female mice are in close agreement with the observations obtained from cats reported by Pilcher57. This author concluded that “when small doses of caffeine and alcohol are combined, the result is generally a qualitative algebraic summation of both actions, that is, each drug produces, qualitatively, its ordinary effects. However, when large doses of the two drugs are combined, the effects of the stimulant drug tend to be reversed, resulting in a greater suppression than the suppressant drug alone.”57
Oral administration of both drugs in mice could be a useful tool for studying the effects of the ethanol–caffeine interactions, since both drugs are consumed orally in humans. Indeed, as mentioned above, energy drinks contain high concentrations of caffeine, and their consumption in combination with alcoholic beverages is a common practice among young people. The popular belief suggests that, in humans, energy drinks could reduce the intensity of the motor-suppressant effects of ethanol.26 However, only one study has explored the effects of ethanol on the stimulant effects of energy drinks in animal models.59 In this study done in mice, oral administration of energy drinks did not significantly alter the effects of moderate oral doses of ethanol (0.5, 1.0, or 1.5 g/kg), but was able to reduce the suppressant effects of a higher dose of ethanol (2.5 g/kg). It is possible that in this study, some effects could be attributed to other stimulant components of the energy drinks, such as taurine, which has been shown to interact with ethanol on locomotion.60,61 However, acute oral coadministration of caffeine at a low dose (10 mg/kg) combined with ethanol (1.6, 2.4, and 3.2 g/kg) was demonstrated to increase locomotor activity compared with the effect observed after separate administration of each individual drug.53
It is also relevant to consider the effects of acute administration of caffeine or ethanol on the chronic actions of these substances.58,62–64 Chronic caffeine intake reduces spontaneous locomotion in mice62 and rats.58 However, chronic caffeine consumption (0.1% during 30 days) increased sensitivity (relative to water consumption) to the activating effects of an acute dose of ethanol (1.5 g/kg, IP) in rats.58 In contrast, in mice exposed to chronic caffeine (1 g/L during 7 days), acute doses of ethanol (1.5 and 2.5 g/kg, IP) significantly induced locomotion, but never to the level of animals in the water control group.62–64 Further, acute caffeine administration (10–35 mg/kg) increased locomotion to a similar extent in mice chronically consuming ethanol (5%, v/v) and those in the water control group (in this case, ethanol did not affect spontaneous locomotion). Thus, chronic consumption of ethanol did not change the acute stimulant effects of caffeine.62 The same pattern of results was found after acute administration of 5′-N-ethylcarboxamidoadenosine (NECA), an adenosine agonist with high affinity for both the A1 and A2A adenosine receptors. In this case, NECA suppressed locomotion in a similar manner in mice chronically consuming either water or ethanol.62
Adolescence is a vulnerable time for organisms exposed to drugs of abuse such as ethanol.65 It is widely acknowledged that the human adolescent brain is not fully mature,66,67 and there is evidence from animal studies that exposure to alcohol during adolescence can affect subsequent brain/behavior development.68,69 Voluntary consumption of ethanol (at a concentration of 8.5 g/L that led to a dose of 1.0–1.5 g/kg), caffeine (at a concentration of 170 mg/L that led to a dose of 20–30 mg/kg), or an ethanol–caffeine combination during late adolescence in male and female rats had effects on the subsequent adult behavior that were dependent on the sex of the rats.70 Males showed more ambulation after exposure to the alcohol–caffeine mixture, while females exposed to the mixture showed the opposite effects, that is, suppressed ambulation.70 This pattern of results could be related to sex differences in the sensitivity to the neurotoxic effects of caffeine.71 In hippocampal cultures pre-exposed to 5 mM ethanol for 10 days, caffeine (5 or 20 μM) produced greater neurotoxicity in cultures from female tissues than from male ones, specifically in the dentate gyrus and the CA1 region.71 These results demonstrate the importance of including both sexes in investigations of this sort.
In summary, the interacting effects of caffeine and ethanol on locomotor activity are quite complex. It seems that at low doses, acute caffeine administration can increase the stimulant effects of acute doses of ethanol. However, when caffeine or ethanol doses are higher, a potentiation of the suppressant effects of both substances is most evident. On the other hand, chronic administration of either substance does not appear to change the acute doses at which locomotion can be stimulated.
Caffeine–Ethanol Interactions: Effects on Motor Coordination
At medium-to-high doses, a typical action of ethanol is to impair motor coordination.72–76 This effect generally shows tolerance with repeated ethanol exposure.77,78 The development of tolerance appears to be relevant for the emergence of ethanol abuse and dependence, because it can attenuate the performance impairing the effect of the drug, which promotes the use of escalating doses.79 Several studies have investigated the ability of caffeine to modulate ethanol-induced motor incoordination and have explored the possible involvement of adenosine receptors.28,29,76,80–82
A single injection of a broad range of doses of caffeine (5–75 μg) administered in the brain ventricles intracerebroventricular (ICV) or peripherally (2.5–62.5 mg/kg, IP) did not alter motor coordination in mice evaluated in the rotarod test.80,81 However, pretreatment with low doses of caffeine (2.5–25.0 μg ICV, or 2.5–5.0 mg/kg IP) was effective in decreasing the degree and duration of motor incoordination produced by a single dose of ethanol (2 g/kg, IP). The antagonism by caffeine of ethanol-induced motor incoordination was dose related, since higher doses of caffeine (75 μg ICV, or 62.5 mg/kg IP) enhanced ethanol-induced motor incoordination.80,81 The methylxanthine (and caffeine metabolite) theophylline was less potent, but dose-dependently attenuated (100–150 μg, ICV, 50 mg/kg IP) the motor incoordinating effect of acute ethanol (1.5–2 g/kg, IP).73,74 On the other hand, potentiation of ethanol-induced ataxia was also observed after pretreatment with another methylxanthine, 3-isobutyl-1-methylxanthine (IBMX).81
Chronic oral administration of caffeine for 10 days (45 and 90 mg/kg/day) and IBMX (30 and 60 mg/kg/day) potentiates acute ethanol-induced motor incoordination (1.5 g/kg, IP), an effect that was associated with increased adenosine A1 receptor binding compared to tap water controls.28 However, no interaction with ethanol-induced motor incoordination (1.5 g/kg, IP) was observed after chronic theophylline (75 and 150 mg/kg/day) consumption.28 This lack of effect of chronic theophylline on motor incoordination induced by ethanol was paralleled with the lack of changes in the A1 receptor density.28
More recently, it has been demonstrated that acute oral coadministration of caffeine (20 mg/kg) and ethanol (2.5 g/kg) attenuated the ethanol-induced motor impairment in rats evaluated in the accelerating rotarod.29 This effect was also observed after acute IP administration of an A1-selective receptor antagonist (8-cyclopentyl-1,3-dipropylxanthine; DPCPX) injected after oral ethanol administration, but not with an A2A selective receptor antagonist 2-(2-Furanyl)-7-(2-phenylethyl)-7H- pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-a mine (SCH 58261), suggesting again that A1 adenosine receptors are involved in motor incoordination induced by ethanol.29 However, microinfusions of both the A1 receptor-selective agonist cyclohexyladenosine (CHA) and the A2A-selective agonist 5′-N-ethylcarboxamido-2-[2-(4-phenyl-(3-propanoic acid)] (CGS21680) into the rat motor cortex significantly accentuated motor incoordination induced by ethanol (1.5 g/kg IP) in a dose-related manner.76 CHA was more potent than CGS21680 in producing this effect. However, the potentiation induced by the A1 and A2A agonists was attenuated by the A1-selective antagonist DPCPX, but not by the A2A receptor-selective antagonist 8-(3-chlorostyryl)caffeine, further emphasizing the involvement of the adenosine Al receptor subtype in these effects.76
The involvement of different adenosine receptors in the development of rapid tolerance to ethanol-induced motor incoordination in mice has also been evaluated.82 A single administration of caffeine (3, 10, or 30 mg/kg, IP) or selective antagonists of the A1 or A2A receptors did not change the performance of animals treated with ethanol (2.5 g/kg) on the first day of testing. However, caffeine administered on the first day was able to block the development of tolerance to ethanol that was manifested on the second day. Moreover, caffeine's blockade of the rapid tolerance to ethanol-induced incoordination appears to be mediated by the A1 rather than A2A receptors, because DPCPX, but not 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385), also blocked rapid tolerance. These data are in agreement with previous studies,29,76 and it is reasonable to suggest that this effect may be due to the high number of A1 receptors in the areas controlling motor coordination, such as the cortex and cerebellum.83
To summarize, acute low doses of caffeine can reduce the incoordination effects of ethanol, but high doses of caffeine can potentiated them. Moreover, the adenosine A1 receptors appear to be more important for these effects than the A2A receptors. The ability of caffeine to attenuate the rapid tolerance to ethanol-induced incoordination effects also has been attributed more to the A1 than A2A receptors.
Caffeine–Ethanol Interactions: Sedation and Narcosis
Ethanol intoxication produces sedative and, at high doses, even hypnotic effects.72,84–86 In contrast, caffeine enhances wakefulness and alertness, effects that are associated with its ability to block adenosine receptors.87–91 Although the effects of ethanol or caffeine on sedation and alertness have been widely described, their interaction is much less well characterized, and only a few studies have explored the impact of caffeine on the narcosis or loss of the righting reflex (LORR) induced by ethanol in rodents.28,92–95
For example, it has been demonstrated in mice that when coffee (15 mg/mL) or caffeine (0.5 mg/mL) were orally administered before ethanol (75% v/v), the latency to the LORR increased.92 However, this effect was not observed when caffeine was administered after ethanol. Moreover, this effect was not due to pharmacokinetic interference, since no decrease in plasma ethanol levels was detected in mice pretreated with coffee or caffeine.92 In another study in mice, an intermediate dose of caffeine (25 mg/kg, IP) administered before an IP injection of narcotic doses of ethanol also blunted the effect of ethanol, in this case by reducing the duration of the LORR.93 This effect was not seen with higher doses of caffeine (40–100 mg/kg).81,93 Theophylline (50 mg/kg, IP) produced the same pattern of effects, prolonging the onset and shortening the duration of ethanol-induced LORR81,73; however, IBMX (12.5 mg/kg IP) did not alter the LORR induced by ethanol.81
Caffeine and theophylline have also been compared in long-sleep (LS) and short-sleep (SS) mice, which are selectively bred for differences in sensitivity to the LORR induced by ethanol, but also have differential sensitivity to purinergic agonists and antagonists.94 LS and SS mice showed differences in sensitivity to the nonselective adenosine antagonists, theophylline and caffeine.95 These drugs also produced a distinct pattern of effects in the two strains of mice; while theophylline reduced the duration of LORR induced by ethanol in both strains of animals (at a broader range of doses in LS mice), caffeine only did so in LS mice. Moreover, caffeine at doses of 10 and 20 mg/kg increased the LORR in SS mice. Theophylline did not change the blood or brain ethanol elimination rate, but the effects of caffeine on blood ethanol levels were affected.95 The A1 receptor-selective agonists CHA and l-phenyl isopropyl adenosine (PIA), as well as the nonselective A1-A2A agonists, 2-chloroadenosine and N-ethylcarboxamidoadenosine, increased the LORR in both LS and SS mice.95 In general, LS mice were more affected than SS mice by purinergic drugs, suggesting that there may be differences in the adenosine systems of these lines of mice; this observation may aid in understanding how they differ in ethanol sensitivity as well.
As discussed above, adenosine is involved in mediating many of ethanol's intoxicating effects, such as ataxia74,96,97 and sedation (for review98,99). However, in rodents, adenosine analogs seem to increase LORR only during interactions with hypnotic drugs, rather than causing a direct deep hypnotic effect or unconsciousness.100 Thus, dipyrimadole (30–40 mg/kg IP), an inhibitor of adenosine uptake, increased the duration of LORR in mice only after the administration of hypnotic doses of ethanol (3.5–4.0 g/kg, IP).73,93 In regard to the specific adenosine receptors implicated in the modulation of the hypnotic effects of alcohol, more recent studies using novel selective A2A antagonists suggest that A2A rather than A1 receptors seem to mediate this effect. The A2A antagonist SCH58261, but not the A1 antagonist DPCPX, blocked LORR induced by ethanol.93 In addition, female and male mice lacking the adenosine A2A receptor (i.e., A2A KO mice) showed a reduced duration of LORR compared to their wild-type (WT) siblings after ethanol administration.93,101
In summary, adenosine agonists seem to potentiate the duration of LORR, while adenosine antagonists reduce the LORR induced by high doses of ethanol. In general, nonselective adenosine receptor antagonists, as well as selective A2A antagonism or genetic deletion, reduce ethanol induced LORR.
Caffeine–Ethanol Interactions: Effects on Learning and Memory
High doses of ethanol can also cause learning impairments, amnesia, or impaired retrieval of information, effects that can persist long after the drug wears off.102–104 Complete or partial memory impairment occurs commonly from the episodes of binge drinking in both alcoholics and nonalcoholics.105 This memory impairment may reflect a disruption of encoding, storage, consolidation, and/or retrieval capability.106,107 Other studies have shown that moderate doses of ethanol delivered after learning generally enhance or have little effect on memory examined the next day,108,109 and caffeine at moderate doses has been shown to facilitate memory acquisition and retention in animals assessed on various learning tasks.110–113
A few articles have focused on the interaction between caffeine and ethanol on the memory in rodents.114,115 Ethanol and caffeine coadministration has demonstrated to be neuroprotective in different models of ischemia.114,116,117 Thus, an acute administration of caffeinol (combination of 10 mg/kg caffeine plus 0.65 g/kg alcohol, IP) 15 minutes after traumatic brain injury in rats, produced an improvement in working memory tasks in the Morris water maze, compared to the vehicle-treated animals.114 This protection was not due to effects on motor performance.
Retrograde amnesic effects of ethanol, caffeine, or a combination of both agents have been evaluated in rats with an olfactory memory test that uses social odors.115 A high dose of ethanol (3.0 g/kg, IP) administered after exposure to a novel odor produced memory recall or retrograde memory impairments the following day, and caffeine (5 mg/kg, IP), either 20 minutes before or 1 hour after exposure to the novel odor, prevented this ethanol disruption in recognition memory.115
In humans, ethanol and caffeine can also produce state-dependent memory effects.118,119 State-dependent learning or memory is the term applied to the condition in which a behavior that is learned in a drug state is most readily recalled when the organism is in the same drug state.120 In rodents, administration of ethanol before training can impair the retrieval of tasks learned in a state-dependent manner, which is reversible by readministering ethanol before the retrieval test.121,122 This type of study also reflects the ability of ethanol to serve as an interoceptive cue that can aid learning and performance of a specific operant response.123 Defined in this way, acute ethanol administration can exert state-dependent effects on conditioned avoidance responding.124,125 However, caffeine (100 mg/kg, IP) does not change the performance of rats already trained to discriminate the interoceptive cue produced by ethanol administration (1.5 g/kg, IP) in an active avoidance task performed in a typical three-chamber apparatus.126
The interaction between caffeine and ethanol also has been evaluated using the acquisition of an avoidance task performed in a plus-maze discrimination apparatus.127 This apparatus uses an elevated plus-maze consisting of two opposing open arms and two opposing enclosed arms. During training, animals are free to explore all four arms, but are conditioned to avoid one of the enclosed arms (the aversive arm) by the presentation of both light and white noise stimuli when they enter that arm. During the testing session (24 hours after the training session), animals are free to explore all four arms again, but no cues are presented. Time in the aversive arm was used as an index of memory. Ethanol alone (1.0 and 1.4 g/kg, IP) or in combination with caffeine (20 and 40 mg/kg, IP) administered before the training session produced a learning deficit manifested during the test session. Only the highest dose of caffeine alone (40 mg/kg) produced that effect. However, that was not due to a state-dependent effect, since the administration of this dose of caffeine before the test did not reverse the learning deficit.127
Caffeine also does not change the conditioned avoidance of a sweet solution produced by ethanol. This conditioned taste avoidance (CTA) is produced by administering an acute dose of ethanol after voluntary consumption of saccharine, and is observed as a reduction in saccharine consumption the following day.30 Caffeine (2.5–10 mg/kg, IP) did not block the association between taste and ethanol effects (1.0–1.5 g/kg, IP); thus, saccharine consumption was not restored. However, caffeine by itself was able to produce CTA at a moderate dose (20 mg/kg, IP).30
Taken together, these studies indicate that caffeine appears to prevent explicit memory deficits induced by high doses of ethanol, but does not affect the perception of the interoceptive cue generated by ethanol, and it does not prevent the disruptive effects of ethanol on avoidance learning in discriminative procedures, suggesting a lack of effect of caffeine on implicit learning processes regulated by ethanol.
Caffeine–Ethanol Interactions: Effects on Anxiety and Stress
Considerable evidence indicates that ethanol is capable of reducing anxiety levels in humans and other animals,128–130 and adenosine has been proposed as a mediator of this anxiolytic effect.131–133 In this regard, adenosine itself, as well as adenosine receptor agonists, has anxiolytic effects as assessed by a number of ethological tests in rodent models.134,135 On the other hand, methylxantines such as caffeine and theophylline have been demonstrated to increase anxiety in humans136–139 and in rodents in different anxiety paradigms.127,140–143
Caffeine modulation of the effects of ethanol on anxiety has been explored in a handful of studies,70,127,131 which also assessed the role of adenosine receptor subtypes in this interaction. Thus, caffeine, across a broad range of doses that extended into the anxiogenic range (10–40 mg/kg), was shown to reduce the anxiolytic-like effect of ethanol (1.0–1.4 g/kg, IP) in the elevated plus-maze in mice.127,131 The effects of caffeine on acutely administered ethanol appeared to be mediated by the A1 adenosine receptors, since the selective adenosine A1 receptor antagonist DPCPX, but not the A2A receptor antagonist ZM241385, significantly reduced the anxiolytic-like effect of ethanol (1.2 g/kg).131 Moreover, an anxiolytic response was observed after coadministration of nonanxiolytic doses of the A1 adenosine agonist 2-chloro-N6-cyclopentyladenosine (CCPA) and ethanol.131
A different pattern emerges when these substances are administered chronically. The anxiety-related effects of chronic oral consumption of alcohol (1.0–1.5 g/kg) combined with oral consumption of caffeine (20–30 mg/kg) during adolescence were evaluated in male and female rats when they reached mid-adulthood.70 Males that had previously consumed alcohol plus caffeine showed anxiolysis in the light and dark box and in the open field. However, females exposed to the drug mixture showed an anxiogenic-like effect.70 Thus, as described above, the results in females and males seem to be opposite.
Caffeine and ethanol not only regulate anxiety-like behavior but also regulate the stress responses involving activation of the hypothalamo–pituitary–adrenal (HPA) axis.143–150 HPA axis activation ultimately leads to increases in the biosynthesis and systemic secretion of adrenocorticosteroids. The effects of alcohol and other drugs of abuse on this axis are relevant, because a link between the stress response and drug abuse and addiction has been observed. Stress is one of the main factors stimulating drug consumption and the relapse to drug taking in abstinent addicts.151,152 Further, chronic drug exposure affects the brain stress response systems. Thus, drug abuse is often accompanied by enhanced brain stress responses, which in turn may contribute to the addiction process.152
In regard to ethanol and caffeine, moderate acute doses of ethanol144–147 or caffeine143,148–150 have been shown to increase the plasma corticosterone levels in rodents and cortisol in humans. However, only one study so far has explored the interaction of caffeine and ethanol on corticosterone release.153 In this study, a low dose of caffeine (5 mg/kg IP) delivered before a low dose of ethanol (0.8 g/kg IP) elevated plasma corticosterone levels. This increase was not observed after ethanol or caffeine was administered alone.153
In summary, more studies need to evaluate this complex interaction, but so far, the evidence suggests that caffeine and ethanol can counteract each other's effects on acute anxiety levels in rodents, and some of this evidence points to A1 adenosine receptors as being responsible for the anxiolytic effects of ethanol as well as of the reversal of this effect by caffeine. It would be very important to have a clearer view of the interaction between these substances after chronic consumption, because tension reduction theories suggest that the anxiolytic effects of alcohol facilitate alcohol use by anxious individuals.154,155 Moreover, a growing body of evidence shows that corticosterone may directly modulate alcohol drinking.156–159
Effect of Caffeine on Alcohol Self-Administration
Epidemiology studies have shown that a positive correlation may exist between the consumption of caffeine and that of ethanol.160,161 Moreover, it has been demonstrated that people who use energy drinks consume alcohol more frequently than people who do not (for review13). Studies in rodents have shown a complex relationship between caffeine and ethanol intake.11,162–164 Caffeine administered in the diet of malnourished female rats has been shown to facilitate voluntary ethanol drinking in a free access two-bottle paradigm,162,163 and removal of caffeine from the diet restored alcohol consumption to baseline levels. This effect was not taste-related, because quinine did not produce the same pattern as caffeine.163 However, slow-release caffeine pellets (200 mg/day during 21 days) failed to alter ethanol intake in an unlimited free-choice paradigm in female rats.165 This lack of effect was specific to caffeine, since slow-release pellets containing other stimulants did increase ethanol consumption.165 Caffeine administered acutely did not produce a consistent pattern of effects; a low dose of caffeine (5 mg/kg, IP) promoted ethanol drinking in male rats using a limited-access two-bottle choice paradigm.11 However, a high acute dose of caffeine (50 mg/kg, IP) decreased ethanol as well as food intake in deprived male and female rats.166 The lack of caffeine effects on ethanol intake has been also demonstrated in a recent study.167 The presence of caffeine (1 g/L) in alcoholic solutions (10% v/v) did not increase the ethanol consumption of male rats exposed to a free-choice procedure during 50 days. Interestingly, it did prevent the alcohol deprivation effect (ADE), blocking an increase of ethanol intake after an abstinent period of 7 days.167 Because ADE has been suggested as an animal model of human alcohol craving and relapse,168 the effect of caffeine on such effect is a very relevant finding.
Research on the role of adenosine receptor subtypes in ethanol intake has mainly focused on the A2A receptors. Ethanol intake and preference were increased in male and female KOA2A mice compared to their WT counterparts in a free-choice task.101 Results in the same direction have been observed in studies employing pharmacological manipulation of adenosine transmission. Both acute and subchronic (7 days) IP administration of the A2A receptor antagonist 8-ethoxy-9-ethyl-9H-purin-6-amine (ANR94) increased the levels of ethanol intake in alcohol-preferring rats assessed in a free choice task.169 Conversely, a reduction of ethanol intake was observed after acute IP administration of the A2A receptor agonists CGS21680 and 5′-N-ethylcarboxamido-2-(2-phenethylthio) (VT7).169
The involvement of adenosine A2A receptors in ethanol seeking and intake also has been evaluated in operant chambers in which animals have to exert various levels of effort to have access to ethanol (e.g., lever pressing on fixed ratio [FR] schedules ranging from FR1 to FR3).169–172 In this case, the pattern of effects produced by different A2A receptor antagonists was more complex. While SCH58261 reduced the number of ethanol-reinforced responses and ethanol consumption,172 ANR94 increased responding.169 Moreover, 3,7-dimethyl-1-propargylxanthine had a multiphasic effect on the number of lever presses and amount of ethanol consumed during operant self-administration.170,171 The A2A agonists CGS21680 and VT7 decreased lever pressing and alcohol consumption in alcohol-preferring rats tested on an FR1 schedule.169 Using the same behavioral procedure, no effect was observed with an adenosine A1 antagonist DPCPX.170,172
Taken together, it appears that the results so far are not conclusive (see summary in Table 1). The specific effects of adenosine antagonism on ethanol self-administration may depend on factors such as food restriction, sex, ethanol-intake or reinforcement paradigms, or other factors. For instance, it has been suggested that the suppressive effects of caffeine on ethanol intake seen in some studies could be due to the use of high toxic doses of caffeine.165,166 However, the fact that chronic caffeine blocked the ADE effect167 suggests that caffeine could be promising as a treatment for protective abstinence, although more studies should assess this point.
Table 1.
Summary of the Effects of Pharmacological and Genetic Manipulations of Adenosine Receptors on Free Ethanol Intake and Operant Self-Administration
|
Free intake | |||||
|---|---|---|---|---|---|
| Drug | Mechanism of action | Sex/species | Ethanol concentration | Ethanol Intake | Refs. |
| Caffeine | Non selective antagonist A1/A2A | Male and female rats | 10% (v/v) | Increase | 11, 163, 164 |
| Male and female rats | 5% (w/v) 10% (v/v) |
Decrease | 166, 167 | ||
| Male rats | 10% (v/v) | No effect | 168 | ||
| ANR94 | A2A antagonist | Male alcohol-preferring rats | 10% (v/v) | Increase | 170 |
| A2A genetic deletion | Male and female mice | 3%–20% (v/v) | Increase | 101 | |
| CGS 21680 | A2A agonist | Male alcohol-preferring rats | 10% (v/v) | Decrease | 170 |
| VT7 | A2A agonist | Male alcohol-preferring rats | 10% (v/v) | Decrease | 170 |
|
Operant self-administration | |||||
|---|---|---|---|---|---|
| Drug | Mechanism of action | Sex/species | Ethanol concentration/schedule | Ethanol intake | Refs. |
| ANR94 | A2A antagonist | Male alcohol-preferring rats | 10% (v/v), FR1 | Increase | 170 |
| SCH58261 | A2A antagonist | Male alcohol-preferring rats | 10% (v/v), FR3 | Decrease | 173 |
| DMPX | A2A antagonist | Male rats | 10% (w/v) FR1 | Decrease | 172 |
| Male rats | 10% (v/v), FR3 | Bimodal effect | 171 | ||
| DPCPX | A1 antagonist | Male alcohol-preferring rats | 10% (v/v), FR3 | No effect | 171, 173 |
| CGS21680 | A2A agonist | Male alcohol-preferring rats | 10% (v/v), FR1 | Decrease | 170 |
| VT7 | A2A agonist | Male alcohol-preferring rats | 10% (v/v), FR1 | Decrease | 161 |
DMPX, 3,7-dimethyl-1-propargylxanthine.
Effect of Caffeine on Ethanol Withdrawal
Withdrawal is a defining characteristic of drug dependence and is often characterized by an impaired physiological function and enhanced negative effect, symptoms strongly associated with relapse.173 Symptoms of ethanol withdrawal appear between 12 and 24 hours after the time when ethanol levels in blood are no longer detectable. For instance, acute withdrawal appears several hours after a high dose of ethanol has been administered, and produces a mild set of symptoms (i.e., hangover) that, among other effects, can include increased anxiety.132 Moreover, the withdrawal syndrome after chronic administration or chronic consumption of significant amounts of ethanol is also characterized by an increased anxiety response (for review174). Other common symptoms of this syndrome in rodents are marked hyperalgesia,175 tremors, piloerection,176,177 changes in cardiovascular178 and gastrointestinal functions,176 seizures, or convulsions,179,180 which correspond to the withdrawal symptoms observed in humans (for review see174,176).
Although there are no animal studies focusing on the impact of caffeine on anxiety induced by ethanol withdrawal, other adenosine receptor modulators have been shown to regulate the signs of ethanol withdrawal. The administration of adenosine 18 hours after an acute ethanol injection in mice, which is at the onset of the peak of withdrawal as characterized by high levels of anxiety, reduced increases in anxiety observed in an elevated plus-maze.132 This reversal effect was also observed after the administration of a selective adenosine A1 receptor agonist CCPA, but not after a selective adenosine A2A receptor agonist N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine.132 Moreover, the anxiolytic effect of CCPA on ethanol withdrawal-induced anxiety was reversed by the selective adenosine A1 antagonist DPCPX.132 The results from studies involving chronic ethanol administration appear to be different from those observed after acute ethanol administration. In this case, the A1 receptor antagonist 8-cyclopentyltheophylline reduced the anxiogenic effect produced by ethanol withdrawal in the elevated plus-maze and in the dark/light test in rats.175
Removal of a liquid diet containing ethanol (6.7%, v/v) after chronic exposure led to handling-induced hyperexcitability, a less-frequently used behavioral measure of withdrawal.181 Administration of an adenosine A1 receptor agonist R-PIA and the adenosine A2A receptor agonist CGS21680 significantly reduced this withdrawal sign, suggesting the involvement of both the A1 and A2A receptors.181 In this study, there were no changes in the adenosine A1 and A2A receptors or in adenosine transporter-binding sites in the frontal cortex and the cerebellum. However, a reduction in adenosine transporter-binding sites was observed in the striatum of ethanol-withdrawn mice.181
The administration of adenosine, adenosine analogs, or dipyridamole (an inhibitor of adenosine reuptake) has been shown to reduce the number of rats in which audiogenic convulsions appeared during ethanol withdrawal.179 The adenosine A1 receptor agonist CCPA also produced a dose-dependent reduction of the convulsions induced by an intense audiogenic stimulus, as well as tremors, which were apparent 24 hours after repeated high doses of oral ethanol administration (12–18 g/kg per day) in rats.182 Moreover, administration of the adenosine A1 antagonist DPCPX completely abolished the antagonistic effects of the adenosine A1 agonist CCPA on both tremors and audiogenic seizures during ethanol withdrawal.182 The A2A adenosine receptor also has been implicated in withdrawal-induced convulsions.183,184 In fact, these receptors are expressed in areas of the brain involved in epileptogenesis, including the striatum, neocortex, and hippocampus.185 A2AR KO mice are less susceptible to seizures caused by ethanol withdrawal that was induced by the cessation after 10 consecutive days of ethanol intake (up to 6.3% v/v). This effect has also been observed when the A2A adenosine receptor antagonist ZM 241385 was administered during the last 5 of 10 days of ethanol intake.180 Similarly, subchronic coadministration of theophylline (1 g/kg, IP; twice daily) during chronic ethanol intake (6.5% w/v) was demonstrated to decrease hyperalgesia and withdrawal scores in rats during ethanol withdrawal.186 However, the protective effect of A2A receptor antagonism or repeated theophylline administration was not observed after the acute administration of caffeine or theophylline (5–25 mg/kg, IP); in this case, there was no effect on the audiogenic seizures observed during ethanol withdrawal in rats.179 However, caffeine and theophylline did antagonize the suppressive effects of adenosine analogs on these withdrawal symptoms.179
In summary, adenosine seems to play an important role in the regulation of ethanol withdrawal. Agonism of the adenosinergic system, especially via stimulation of A1 adenosine receptors, reduces some of the withdrawal symptoms that occur after acute or chronic ethanol administration. More importantly, pharmacological antagonism or genetic deletion of the adenosine A1 and/or A2A receptors could have a role in prevention of withdrawal during ethanol intake.180,186 Nevertheless, most of these studies have employed manipulations affecting specific adenosine receptor subtypes rather than caffeine itself, and therefore have not directly assessed the popular belief that a cup of strong coffee can antagonize some of the symptoms of ethanol withdrawal, especially after an acute episode of alcohol consumption in nonalcoholic individuals.
Future Directions
After reviewing the literature on the caffeine–ethanol interactions, one can see that a significant body of work has been performed. However, a clear pattern of results does not easily emerge. Further experiments are needed to establish the specific range of doses, patterns of administration, sex differences, and other factors that could clarify some of the apparent contradictions in the results observed in many of the studies presented above.
More importantly, there is a dearth of studies about the interactions of both agents on processes that are particularly relevant for addiction, such as Pavlovian conditioning, habit formation, or motor sensitization, which seem to contribute to the acquisition and intensification of a compulsive drug-seeking behavior.38,40 Although sensitization of locomotor activity by caffeine as well as cross-sensitization with other drugs such as amphetamine187 and nicotine188 has been observed, so far there are no studies of possible cross-sensitization between ethanol and caffeine. In fact, preliminary studies from our laboratory show that caffeine reduces locomotion in animals repeatedly exposed to a sensitizing dose of ethanol.189 Further, the effects of the caffeine–ethanol interactions on learning processes are not well understood, in part due to the complexity of learning processes per se. Caffeine has been demonstrated to induce a conditioned place preference,190–192 and also to modulate a conditioned place preference induced by methamphetamine or cocaine.6 It also would be important to study the effects of caffeine on the acquisition of Pavlovian cues associated with ethanol in this paradigm.
In summary, despite the fact that this area of inquiry has grown increasingly important due to the potential dangers of combining high-caffeine energy drinks with ethanol, animal researchers have only scratched the surface of this complex and multifaceted field. Additional investigations will be required to identify how caffeine and ethanol interact to modulate the behavioral processes related to ethanol consumption, dependence, abuse, and addiction.
Acknowledgments
This research was supported by a grant from Plan Nacional de Drogas (2010/024) and from Ministerio de Educación, FPU (AP2010-3793), Spain.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fredholm BB. Bätting K. Holmén J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 2.DeWit H. Pierri J. Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmachology. 1988;1:113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- 3.Rehm JT. Bondy SJ. Sempos CT. Vuong CV. Alcohol consumption and coronary heart disease morbidity and mortality. Am J Epidemiol. 1997;146:495–501. doi: 10.1093/oxfordjournals.aje.a009303. [DOI] [PubMed] [Google Scholar]
- 4.Hingson RA. Howland J. Alcohol and non traffic unintended injuries. Addiction. 1993;88:877–883. doi: 10.1111/j.1360-0443.1993.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 5.Morelli M. Simola N. Methylxanthines and drug dependence: a focus on interactions with substances of abuse. In: Fredholm B.B., editor. Methylxanthines. Heidelberg, Germany: Springer; 2011. pp. 484–501. [DOI] [PubMed] [Google Scholar]
- 6.Tuazon DB. Suzuki T. Misawa M, et al. Methylxanthines (caffeine and theophylline) blocked methamphetamine-induced conditioned place preference in mice but enhaned that induced by cocaine. Ann N Y Acad Sci. 1992;654:531–533. doi: 10.1111/j.1749-6632.1992.tb26022.x. [DOI] [PubMed] [Google Scholar]
- 7.Simola N. Tronci E. Pinna A. Morelli M. Subchronic-intermittent caffeine amplifies the motor effects of amphetamine in rats. Amino Acids. 2006;31:359–363. doi: 10.1007/s00726-006-0373-3. [DOI] [PubMed] [Google Scholar]
- 8.Shoaib M. Swanner LS. Yasar S. Golberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology (Berl) 1999;142:327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- 9.Gasior M. Jaszyna M. Munzar P. Witkin JM. Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology. 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- 10.Green TA. Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 11.Kunin D. Gaskin S. Rogan F. Smith BR. Amit Z. Caffeine promotes ethanol drinking in rats. Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–277. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- 12.Hasenfratz M. Bunge A. Dal Prá G. Bättig K. Antagonistic effects of caffeine and alcohol on mental performance parameters. Pharmacol Biochem Behav. 1993;46:463–465. doi: 10.1016/0091-3057(93)90380-c. [DOI] [PubMed] [Google Scholar]
- 13.Ferré S. O'Brien MC. Alcohol and caffeine: the perfect storm. J Caffeine Res. 2011;1:153–162. doi: 10.1089/jcr.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferré S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;20(Suppl 1):S35–S49. doi: 10.3233/JAD-2010-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acquas E. Tanda G. Di Chiara G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology. 2002;27:182–193. doi: 10.1016/S0893-133X(02)00290-7. [DOI] [PubMed] [Google Scholar]
- 16.Acquas E. Vinci S. Ibba F. Spiga S. De Luca MA. Di Chiara G. Role of dopamine D(1) receptors in caffeine-mediated ERK phosphorylation in the rat brain. Synapse. 2010;64:341–349. doi: 10.1002/syn.20732. [DOI] [PubMed] [Google Scholar]
- 17.Cauli O. Morelli M. Caffeine and the dopaminergic system. Behav Pharmacol. 2005;16:63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Fredholm BB. Ijzerman AP. Jacobson KA. Klotz KN. Linden J. International Union of Pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 19.Ferré S. Ciruela F. Borycz J, et al. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 20.Clark M. Dar MS. Effect of acute ethanol on release of endogenous adenosine from rat cerebellar synaptosomes. J Neurochem. 1989;52:1859–1865. doi: 10.1111/j.1471-4159.1989.tb07268.x. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm BB. Wallman-Johansson A. Effects of ethanol and acetate on adenosine production in rat hippocampal slices. Pharmacol Toxicol. 1996;79:120–123. doi: 10.1111/j.1600-0773.1996.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 22.Diamond I. Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Nagy LE. Diamond I. Casso DJ. Franklin C. Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 24.Nagy LE. Ethanol metabolism and inhibition of nucleoside uptake lead to increased extracellular adenosine in hepatocytes. Am J Physiol. 1992;262:1175–1180. doi: 10.1152/ajpcell.1992.262.5.C1175. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael FJ. Israel Y. Crawford M, et al. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Therapeut. 1991;259:403–408. [PubMed] [Google Scholar]
- 26.Ferreira SE. de Mello MT. Pompéia S. de Souza-Formigoni ML. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30:598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- 27.Liguori A. Robinson JH. Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend. 2001;63:123–129. doi: 10.1016/s0376-8716(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 28.Dar MS. Wooles WR. Effect of chronically administered methylxanthines on ethanol-induced motor incoordination. Life Sci. 1986;39:1429–1437. doi: 10.1016/0024-3205(86)90547-3. [DOI] [PubMed] [Google Scholar]
- 29.Connole L. Harkin A. Maginn M. Adenosine A1 receptor blockade mimics caffeine's attenuation of ethanol-induced motor incoordination. Basic Clin Pharmacol Toxicol. 2004;95:299–304. doi: 10.1111/j.1742-7843.2004.pto950509.x. [DOI] [PubMed] [Google Scholar]
- 30.Kunin D. Bloch RT. Terada Y. Rogan F. Smith BR. Amit Z. Caffeine promotes an ethanol-induced conditioned taste aversion: a dose-dependent interaction. Exp Clin Psychopharmacol. 2001;9:326–333. doi: 10.1037//1064-1297.9.3.326. [DOI] [PubMed] [Google Scholar]
- 31.Di Chiara G. A motivational learning hypothesis of the role of dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien CP. Childress AR. McLellan AT. Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE. Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 34.Heyman GM. Cambridge, MA: Harvard University Press; 2009. Addiction: A Disorder of Choice. [Google Scholar]
- 35.Vezina P. Lorrain DS. Arnold GM. Austin JD. Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heyman GM. An economic approach to animal models of alcoholism. Alcohol Res Health. 2000;24:132–139. [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart J. De Wit H. Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 38.Robinson TE. Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 39.Everitt BJ. Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1981–1989. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 40.Belin D. Jonkman S. Dickinson A. Robbins TW. Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Dunne F. O'Halloran A. Kelly JP. Development of a home cage locomotor tracking system capable of detecting the stimulant and sedative properties of drugs in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1456–1463. doi: 10.1016/j.pnpbp.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Himmel HM. Safety pharmacology assessment of central nervous system function in juvenile and adult rats: effects of pharmacological reference compounds. J Pharmacol Toxicol Methods. 2008;58:129–146. doi: 10.1016/j.vascn.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Read GW. Cutting W. Furst A. Comparison of excited phases after sedatives and tranquilizers. Psyhopharmacologia (Berl) 1960;1:346–350. doi: 10.1007/BF00404231. [DOI] [PubMed] [Google Scholar]
- 44.Carlsson A. Engel J. Svensson TH. Inhibition of ethanol-induced excitation in mice and rats by a-methyl-p-tyrosine. Psychopharmacology (Berl) 1972;26:307–312. doi: 10.1007/BF00422706. [DOI] [PubMed] [Google Scholar]
- 45.Correa M. Miquel M. Sanchis-Segura C. Aragon CMG. Acute lead acetate administration potentiates ethanol-induced locomotor activity in mice: the role of brain catalase. Alcohol Clin Exp Res. 1999;23:799–805. [PubMed] [Google Scholar]
- 46.Correa M. Sanchis-Segura C. Pastor R. Aragon CMG. Ethanol intake and motor sensitization: the role of brain catalase activity in mice with different genotypes. Physiol Behav. 2004;82:231–240. doi: 10.1016/j.physbeh.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Correa M. Viaggi C. Escrig MA. Pascual M. Guerri C. Vaglini F. Aragon CMG. Corsini GU. Ethanol intake and ethanol-induced locomotion and locomotor sensitization in Cyp2e1 knockout mice. Pharmacogenet Genomics. 2009;19:217–225. doi: 10.1097/FPC.0b013e328324e726. [DOI] [PubMed] [Google Scholar]
- 48.Sanchis-Segura C. Correa M. Aragon CM. Lesion on the hypothalamic arcuate nucleus by estradiol valerate results in a blockade of ethanol-induced locomotion. Behav Brain Res. 2000;114:57–63. doi: 10.1016/s0166-4328(00)00183-2. [DOI] [PubMed] [Google Scholar]
- 49.Phillips TJ. Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. In: Bradley R.J., editor; Harris R.A., editor; Jenner P., editor. International Review of Neurobiology. San Diego, CA: Academic Press; 1996. pp. 243–282. [DOI] [PubMed] [Google Scholar]
- 50.Pohorecky LA. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–240. [Google Scholar]
- 51.Waldeck B. Ethanol and caffeine: a complex interaction with respect to locomotor activity and central catecholamines. Psychopharmacologia (Berl) 1974;36:209–220. doi: 10.1007/BF00421803. [DOI] [PubMed] [Google Scholar]
- 52.Correa M. Arizzi MN. Betz A. Mingote S. Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003:197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Kuribara H. Asahi T. Tadokoro S. Ethanol enhances, but diazepam and pentobarbital reduce the ambulation-increasing effect of caffeine in mice. Arukoru Kenkyuto Yakubutsu Ison. 1992;27:528–539. [PubMed] [Google Scholar]
- 54.Haghgoo S. Hasegawa T. Nadai M, et al. Brain distribution characteristics of xanthine derivatives and relation to their locomotor activity in mice. J Pharm Pharmacol. 1995;47:412–419. doi: 10.1111/j.2042-7158.1995.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 55.El Yacoubi M. Ledent C. Menard JF, et al. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q. Yu YP. Ye YL. Zhang JT. Zhang WP. Wei WQ. Spatiotemporal properties of locomotor activity after administration of central nervous stimulants and sedatives in mice. Pharmacol Biochem Behav. 2011;97:577–585. doi: 10.1016/j.pbb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Pilcher JD. Alcohol and caffeine: a study of antagonism and synergism. J Pharmacol exp Ther. 1911;8:267–298. [Google Scholar]
- 58.Sudakov SK. Rusakova IV. Medvedeva OF. Effect of chronic caffeine consumption on changes in locomotor activity of WAG/G and Fischer-344 rats induced by nicotine, ethanol, and morphine. Bull Exp Biol Med. 2003;136:563–565. doi: 10.1023/b:bebm.0000020204.54037.be. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira SE. Hartmann Quadros IM. Trindade AA. Takahashi S. Koyama RG. Souza-Formigoni ML. Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiol Behav. 2004;82:841–847. doi: 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Aragon CM. Trudeau LE. Amit Z. Effect of taurine on ethanol induced changes in open-field locomotor activity. Psychopharmacology (Berl) 1992;107:337–340. doi: 10.1007/BF02245158. [DOI] [PubMed] [Google Scholar]
- 61.Miquel M. Correa M. Sanchis-Segura C. Aragon CM. The ethanol-induced open-field activity in rodents treated with isethionic acid, a central metabolite of taurine. Life Sci. 1999;64:1613–1621. doi: 10.1016/s0024-3205(99)00098-3. [DOI] [PubMed] [Google Scholar]
- 62.Daly JW. Shi D. Wong V. Nikodijevic O. Chronic effects of ethanol on central adenosine function of mice. Brain Res. 1994;650:153–156. doi: 10.1016/0006-8993(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 63.Nikodijević O. Jacobson KA. Daly JW. Effects of combinations of methylxanthines and adenosine analogs on locomotor activity in control and chronic caffeine-treated mice. Drug Dev Res. 1993;30:104–110. doi: 10.1002/ddr.430300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikodijević O. Jacobson KA. Daly JW. Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav. 1993;44:199–216. doi: 10.1016/0091-3057(93)90299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- 66.Casey BJ. Getz S. Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- 68.Gilpin NW. Karanikas CA. Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C. Titus JA. Bell RL. Kapros T. Chen J. Huang R. A mouse model for adolescent alcohol abuse: stunted growth and effects in brain. Alcohol Clin Exp Res. 2012;36:1728–1737. doi: 10.1111/j.1530-0277.2012.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Butler TR. Smith KJ. Berry JN. Sharrett-Field LJ. Prendergast MA. Sex differences in caffeine neurotoxicity following chronic ethanol exposure and withdrawal. Alcohol Alcohol. 2009;44:567–574. doi: 10.1093/alcalc/agp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chuck TL. McLaughlin PJ. Arizzi-LaFrance MN. Salamone JD. Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 73.Dar MS. Mustafa SJ. Wooles WR. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983;33:1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- 74.Dar MS. Central adenosinergic system involvement in ethanol-induced motor incoordination in mice. J Pharmacol Exp Ther. 1990;255:1202–1209. [PubMed] [Google Scholar]
- 75.Dar MS. Mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination: possible involvement of cAMP. Brain Res. 1997;792:263–274. doi: 10.1016/s0006-8993(96)01263-2. [DOI] [PubMed] [Google Scholar]
- 76.Barwick VS. Dar MS. Adenosinergic modulation of ethanol-induced motor incoordination in the rat motor cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:587–607. doi: 10.1016/s0278-5846(98)00025-6. [DOI] [PubMed] [Google Scholar]
- 77.Crabbe JC. Rigter H. Uijlen J. Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–133. [PubMed] [Google Scholar]
- 78.Khanna JM. Morato GS. Kalant H. Effect of NMDA antagonists, an NMDA agonist, and serotonin depletion on acute tolerance to ethanol. Pharmacol Biochem Behav. 2002;72:291–298. doi: 10.1016/s0091-3057(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 79.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: DSM IV; 1994. [Google Scholar]
- 80.Dar MS. The biphasic effects of centrally and peripherally administered caffeine on acute ethanol-induced MI in mice. J Pharm Pharmacol. 1988;40:482–487. doi: 10.1111/j.2042-7158.1988.tb05282.x. [DOI] [PubMed] [Google Scholar]
- 81.Dar MS. Jones M. Close G. Mustafa SJ. Wooles WR. Behavioral interactions of ethanol and methylxanthines. Psychopharmacology. 1987;91:1–4. doi: 10.1007/BF00690916. [DOI] [PubMed] [Google Scholar]
- 82.Batista LC. Prediger RD. Morato GS. Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology (Berl) 2005;181:714–721. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- 83.Ribeiro JA. Sebastiao AM. de Mendoça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 84.Correa M. Sanchis-Segura C. Aragon CMG. Influence of brain catalase on ethanol-induced loss of righting reflex in mice. Drug Alcohol Depend. 2001;65:9–15. doi: 10.1016/s0376-8716(01)00142-9. [DOI] [PubMed] [Google Scholar]
- 85.Correa M. Pascual M. Sanchis-Segura C. Guerri C. Aragon CM. Lead-induced catalase activity diferentially modulates behaviors induced by short-chain alcohols. Pharmacol Biochem Behav. 2005;82:443–452. doi: 10.1016/j.pbb.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Miquel M. Correa M. Aragon CMG. Methionine enhances alcohol-induced narcosis in mice. Pharmacol Biochem Behav. 1999;64:89–93. doi: 10.1016/s0091-3057(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 87.Fredholm BB. Are methylxanthine's effects due to antagonism of endogenous adenosine? Trends Pharmacol Sci. 1980;1:129–132. [Google Scholar]
- 88.Snyder SH. Katims JJ. Annau Z. Bruns RF. Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daly JW. Butts-Lamb P. Padjett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Moll Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yanik G. Glaum S. Radulovacki M. The dose–response effects of caffeine on sleep in rats. Brain Res. 1987;403:177–180. doi: 10.1016/0006-8993(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 91.Ledent C. Vaugeois JM. Schiffmann SN, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 92.Koo MW. Effects of ginseng on ethanol induced sedation in mice. Life Sci. 1999;64:153–160. doi: 10.1016/s0024-3205(98)00545-1. [DOI] [PubMed] [Google Scholar]
- 93.El Yacoubi M. Ledent C. Parmentier M. Costentin J. Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;45:977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 94.Proctor WR. Dunwiddie TV. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984;224:519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- 95.Smolen TN. Smolen A. Purinergic modulation of ethanol-induced sleep time in long-sleep and short-sleep mice. Alcohol. 1991;8:123–130. doi: 10.1016/0741-8329(91)91320-2. [DOI] [PubMed] [Google Scholar]
- 96.Dar MS. Involvement of kappa-opioids in the mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination. Alcohol Clin Exp Res. 1998;22:444–454. [PubMed] [Google Scholar]
- 97.Dar MS. Mustafa SJ. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 2002;957:53–60. doi: 10.1016/s0006-8993(02)03599-0. [DOI] [PubMed] [Google Scholar]
- 98.Ruby CL. Adams CA. Knight EJ. Nam HW. Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev. 2010;3:163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asatryan L. Nam HW. Lee MR. Thakkar MM, et al. Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 101.Naassila M. Ledent C. Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodwin DW. Alcohol amnesia. Addiction. 1995;90:315–317. doi: 10.1111/j.1360-0443.1995.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 103.Hartzler B. Fromme K. Fragmentary and en bloc blackouts: similarity and distinction among episodes of alcohol-induced memory loss. J Stud Alcohol. 2003;64:547–550. doi: 10.15288/jsa.2003.64.547. [DOI] [PubMed] [Google Scholar]
- 104.Wixted JT. A theory about why we forget what we once knew. Curr Dir Psychol Sci. 2005;14:6–9. [Google Scholar]
- 105.White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- 106.Gold PE. The many faces of amnesia. Learn Memory. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- 107.Weitemier AZ. Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- 108.Alkana RL. Parker ES. Memory facilitation by post-training injection of ethanol. Psychopharmacology. 1979;66:117–119. doi: 10.1007/BF00427617. [DOI] [PubMed] [Google Scholar]
- 109.Manrique HM. Miquel M. Aragon CM. Brain catalase mediates potentiation of social recognition memory produced by ethanol in mice. Drug Alcohol Depend. 2005;79:343–350. doi: 10.1016/j.drugalcdep.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 110.Angelucci ME. Cesário C. Hiroi RH. Rosalen PL. Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Braz J Med Biol Res. 2002;35:1201–1208. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- 111.Izquierdo JA. Costas SM. Justel EA. Rabiller G. Effect of caffeine on the memory of the mouse. Psychopharmacology. 1979;61:29–30. doi: 10.1007/BF00426806. [DOI] [PubMed] [Google Scholar]
- 112.Furusawa K. Drug effects on cognitive function in mice determined by the non-matching to sample task using a 4-arm maze. Jpn J Pharmacol. 1991;56:483–493. doi: 10.1254/jjp.56.483. [DOI] [PubMed] [Google Scholar]
- 113.Molinengo L. Scordo I. Pastorello B. Action of caffeine, L-PIA and their combination on memory retention in the rat. Life Sci. 1994;54:1247–1250. doi: 10.1016/0024-3205(94)00851-5. [DOI] [PubMed] [Google Scholar]
- 114.Dash PK. Moore AN. Moody MR. Treadwell R. Felix JL. Clifton GL. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma. 2004;21:1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- 115.Spinetta MJ. Woodlee MT. Feinberg LM, et al. Alcohol-induced retrograde memory impairment in rats: prevention by caffeine. Psychopharmacology. 2008;201:361–371. doi: 10.1007/s00213-008-1294-5. [DOI] [PubMed] [Google Scholar]
- 116.Strong R. Grotta JC. Aronowski J. Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology. 2000;39:515–522. doi: 10.1016/s0028-3908(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 117.Piriyawat P. Labiche LA. Burgin WS. Aronowski JA. Grotta JC. Pilot dose-escalation study of caffeine plus ethanol (caffeinol) in acute ischemic stroke. Stroke. 2003;34:1242–1245. doi: 10.1161/01.STR.0000067706.23777.04. [DOI] [PubMed] [Google Scholar]
- 118.Kelemen WL. Creely CE. State-dependent memory effects using caffeine and placebo do not extend to metamemory. J Gen Psychol. 2003;130:70–86. doi: 10.1080/00221300309601276. [DOI] [PubMed] [Google Scholar]
- 119.Duka T. Weissenborn R. Dienes Z. State-dependent effects of alcohol on recollective experience, familiarity and awareness of memories. Psychopharmacology. 2011;153:295–306. doi: 10.1007/s002130000564. [DOI] [PubMed] [Google Scholar]
- 120.Overton DA. State-dependent learning produced by alcohol and its relevance to alcoholism. In: Kissen B., editor; Begleiter H., editor. The Biology of Alcoholism, Vol. II: Physiology and Behavior. New York: Plenum Press; 1974. pp. 368–373. [Google Scholar]
- 121.Nakagawa Y. Iwasaki T. EtOH-induced state-dependent learning is mediated by 5-hydroxytryptamine3 receptors but not by N-methyl-D-aspartate receptor complex. Brain Res. 1996;706:227–232. doi: 10.1016/0006-8993(95)01040-8. [DOI] [PubMed] [Google Scholar]
- 122.Rezayof A. Motevasseli T. Rassouli Y. Zarrindast MR. Dorsal hippocampal dopamine receptors are involved in mediating ethanol state-dependent memory. Life Sci. 2007;80:285–292. doi: 10.1016/j.lfs.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Solinas M. Panlilio LV. Justinova Z. Yasar S. Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- 124.Crow, Lowell T. Effects of alcohol on conditioned avoidance responding. Physiol Behav. 1966;1:89–91. [Google Scholar]
- 125.Overton D. State-dependent learning produced by depressant and atropine-like drugs. Psychopharmacology (Berl) 1966;10:6–31. doi: 10.1007/BF00401896. [DOI] [PubMed] [Google Scholar]
- 126.Schechter MD. Effect of propranolol, d-amphetamine and caffeine on ethanol as a discriminative cue. Eur J Pharmacol. 1974;29:52–57. doi: 10.1016/0014-2999(74)90169-1. [DOI] [PubMed] [Google Scholar]
- 127.Gulick D. Gould TJ. Effects of ethanol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009;123:1271–1278. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Correa M. Manrique HM. Font L. Escrig MA. Aragon CM. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacology. 2008;200:455–464. doi: 10.1007/s00213-008-1219-3. [DOI] [PubMed] [Google Scholar]
- 129.Kushner M. Sher K. Beitman B. The relation between alcohol problems and the anxiety disorders. Am J Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- 130.Cappell H. Greeley J. Alcohol and tension reduction: an update on research and theory. In: Blaine H., editor; Leonard K., editor. Psychological Theories of Drinking. New York: Guilford; 1987. pp. 15–51. [Google Scholar]
- 131.Prediger RD. Batista LC. Takahashi RN. Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol. 2004;499:147–154. doi: 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- 132.Prediger RD. Da Silva GE. Batista LC. Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- 133.Correa M. Font L. Is there a major role for adenosine A2A receptors in anxiety? Front Biosci. 2008;1:4058–4070. doi: 10.2741/2994. [DOI] [PubMed] [Google Scholar]
- 134.Okuyama E. Ebihara H. Takeuchi H. Yamazaki M. Adenosine, the anxiolytic-like principle of the Arillus of Euphoria longana. Plant Med. 1992;65:115–119. doi: 10.1055/s-1999-14055. [DOI] [PubMed] [Google Scholar]
- 135.Florio C. Prezioso A. Papaioannou A. Vertua R. Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacology. 1998;136:311–319. doi: 10.1007/s002130050572. [DOI] [PubMed] [Google Scholar]
- 136.Uhde TW. Boulenger JP. Jimerson DC. Post RM. Caffeine: relationship to human anxiety, plasma MHPG and cortisol. Psychopharmacol Bull. 1984;20:426–430. [PubMed] [Google Scholar]
- 137.Bruce MS. The anxiogenic effects of caffeine. Postgrad Med J. 1990;66(Suppl 2):18–24. [PubMed] [Google Scholar]
- 138.Boulenger JP. Uhde TW. Wolf EA., 3rd Post RM. Increased sensitivity to caffeine in patients with panic disorders: preliminary evidence. Arch Gen Psychiatry. 1984;41:1067–1071. doi: 10.1001/archpsyc.1983.01790220057009. [DOI] [PubMed] [Google Scholar]
- 139.Gea J. de Pablo J. Marti J. Picado C. Pujol A. Agustí-Vidal A. Anxiety crisis (panic attack) secondary to the administration of theophylline in an oral sustained-release preparation. Rev Clin Esp. 1988;183:280. [PubMed] [Google Scholar]
- 140.El Yacoubi M. Ledent C. Parmentier M. Costentin J. Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- 141.Bhattacharya SK. Satyan KS. Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- 142.Correa M. López-Cruz L. Pardo M. Valverde O. Ledent C. Salamone JD. Effects of the nonselective adenosine antagonists caffeine and theophylline on measures of motor activity and anxiety in mice: studies of acute and chronic administration and comparison with adenosine A2A receptor KO mice. Soc Neurosci. 2011 (Online. 103.09/XX6). [Google Scholar]
- 143.López-Cruz Pardo M. Dosda A. Salamone JD. Correa M. Comparison between high doses of caffeine and theophylline on motor and anxiogenic effects in CD1 mice: studies of acute and chronic administration. Behav Pharmacol. 2011;eS22:71–72. [Google Scholar]
- 144.Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- 145.Kakihana R. Butte JC. Noble EP. Corticosterone response to ethanol in inbred strains of mice. Nature. 1968;218:360–336. doi: 10.1038/218360a0. [DOI] [PubMed] [Google Scholar]
- 146.Roberts AJ. Crabbe JC. Keith LD. Genetic differences in hypothalamic–pituitary–adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;579:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- 147.Escrig MA. Pardo M. Aragon CM. Correa AM. Anxiogenic and stress-inducing effects of peripherally administered acetaldehyde in mice: similarities with the disulfiram-ethanol reaction. Pharmacol Biochem Behav. 2012;100:404–412. doi: 10.1016/j.pbb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 148.Nicholson SA. Stimulatory effect of caffeine on plasma corticosterone and pituitary-adrenocortical axis in the rat. J Endocrinol. 1989;122:535–543. doi: 10.1677/joe.0.1220535. [DOI] [PubMed] [Google Scholar]
- 149.Patz MD. Day HE. Burow A. Campeau S. Modulation of the hypothalamo-pituitary adrenocortical axis by caffeine. Psychoneuroendocrinology. 2005;31:493–500. doi: 10.1016/j.psyneuen.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin AS. Uhde TW. Slate SO. McCann UD. Effects of intravenous caffeine administered to healthy males during sleep. Depress Anxiety. 1997;5:21–28. [PubMed] [Google Scholar]
- 151.Delaney WP. Grube JW. Greiner B. Fisher JM. Ragland DR. Job stress, unwinding and drinking in transit operators. J Stud Alcohol. 2002;63:420–429. doi: 10.15288/jsa.2002.63.420. [DOI] [PubMed] [Google Scholar]
- 152.Koob GF. Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kunin D. Gaskin S. Rogan F. Smith BR. Amit Z. Augmentation of corticosterone release by means of a caffeine-ethanol interaction in rats. Alcohol. 2000;22:53–56. doi: 10.1016/s0741-8329(00)00113-0. [DOI] [PubMed] [Google Scholar]
- 154.Kalodner CR. Delucia JL. Ursprung AW. An examination of the tension reduction hypothesis: the relationship between anxiety and alcohol in college students. Addict Behav. 1989;14:649–654. doi: 10.1016/0306-4603(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 155.Spencer RL. Hutchison KE. Alcohol, aging and the stress response. Alcohol Res Health. 1999;23:272–283. [PMC free article] [PubMed] [Google Scholar]
- 156.Fahlke C. Hard E. Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology (Berl) 1996;127:133–139. doi: 10.1007/BF02805986. [DOI] [PubMed] [Google Scholar]
- 157.Lamblin F. De Witte P. Adrenalectomy prevents the development of alcohol preference in male rats. Alcohol. 1996;13:233–238. doi: 10.1016/0741-8329(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 158.Pastor R. Reed C. Burkhart-Kasch S, et al. Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berl) 2011;218:169–177. doi: 10.1007/s00213-011-2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vendruscolo LF. Barbier E. Schlosburg JE. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Itsvan J. Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- 161.Kozlowski LT. Henningfield JE. Keenan RM, et al. Patterns of alcohol, cigarette, and caffeine and other durg use in tow drug abusing populations. J Subs Abuse Treat. 1993;10:171–179. doi: 10.1016/0740-5472(93)90042-z. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert RM. Dietary caffeine and EtOH consumption by rats. J Stud Alcohol. 1976;37:11–18. doi: 10.15288/jsa.1976.37.11. [DOI] [PubMed] [Google Scholar]
- 163.Gilbert RM. Augmentation of EtOH-consumption by caffeine in malnourished rats. J Stud Alcohol. 1979;40:19–27. doi: 10.15288/jsa.1979.40.19. [DOI] [PubMed] [Google Scholar]
- 164.Register UD. Marhs SR. Thurston CT, et al. Influence of nutrients on the intake of alcohol. J Am Diet Assoc. 1972;61:159–162. [PubMed] [Google Scholar]
- 165.Potthoff AD. Ellison G. Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- 166.Dietze MA. Kulkosky PJ. Effects of caffeine and bombesin on ethanol and food intake. Life Sci. 1991;48:1837–1844. doi: 10.1016/0024-3205(91)90239-8. [DOI] [PubMed] [Google Scholar]
- 167.Carvalho CR. Cruz JS. Takahashi RN. Prolonged exposure to caffeinated alcoholic solutions prevents the alcohol deprivation effect in rats. J Caffeine Res. 2012;2:83–89. [Google Scholar]
- 168.Heyser CJ. Schulteis G. Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- 169.Micioni Di Bonaventura MV. Cifani C. Lambertucci C, et al. Effects of A2A adenosine receptor blockade or stimulation on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 2012;219:945–957. doi: 10.1007/s00213-011-2430-1. [DOI] [PubMed] [Google Scholar]
- 170.Arolfo MP. Yao L. Gordon AS. Diamond I. Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- 171.Thorsell A. Johnson J. Heilig M. Effect of the adenosine. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 172.Adams CL. Cowen MS. Short JL. Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- 173.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 174.Finn DA. Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol Health Res World. 1997;21:149–156. [PMC free article] [PubMed] [Google Scholar]
- 175.Gatch MB. Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333. [PubMed] [Google Scholar]
- 176.Friedman HJ. Assessment of physical dependence on and withdrawal from ethanol in animals. In: Rigter H., editor; Crabbe J.C., editor. Alcohol Tolerance and Dependence. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 93–121. [Google Scholar]
- 177.Frye GD. McCown TJ. Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Therapeut. 1983;226:720–725. [PubMed] [Google Scholar]
- 178.Crandall DL. Ferraro GD. Lozito RJ. Cervoni P. Clark LT. Cardiovascular effects of intermittent drinking: assessment of a novel animal model of human alcoholism. J Hypertens. 1989;7:683–687. doi: 10.1097/00004872-198908000-00013. [DOI] [PubMed] [Google Scholar]
- 179.Malec D. Michalska E. Pikulicka J. Influence of adenosinergic drugs on ethanol withdrawal syndrome in rats. Pol J Pharmacol. 1996;48:583–588. [PubMed] [Google Scholar]
- 180.El Yacoubi M. Ledent C. Parmentier M. Daoust M. Costentin J. Vaugeois J. Absence of the adenosine A(2A) receptor or its chronic blockade decrease ethanol withdrawal-induced seizures in mice. Neuropharmacology. 2001;40:424–432. doi: 10.1016/s0028-3908(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 181.Kaplan GB. Bharmal NH. Leite-Morris KA. Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–162. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 182.Concas A. Mascia MP. Cuccheddu T, et al. Chronic ethanol intoxication enhances [3H]CCPA binding and does not reduce A1 adenosine receptor function in rat cerebellum. Pharmacol Biochem Behav. 1996;53:249–255. doi: 10.1016/0091-3057(95)00208-1. [DOI] [PubMed] [Google Scholar]
- 183.Morgan PF. Durcan MJ. Caffeine-induced seizures: apparent proconvulsant action of N-ethyl carboxamidoadenosine (NECA) Life Sci. 1990;47:1–8. doi: 10.1016/0024-3205(90)90559-a. [DOI] [PubMed] [Google Scholar]
- 184.De Sarro G. De Sarro A. Di Paola ED. Bertorelli R. Effects of adenosine receptor agonists and antagonists on audiogenic seizure-sensible DBA/2 mice. Eur J Pharmacol. 1999;371:137–145. doi: 10.1016/s0014-2999(99)00132-6. [DOI] [PubMed] [Google Scholar]
- 185.Johansson B. Georgiev V. Parkinson FE. Fredholm BB. The binding of the adenosine A2A receptor selective agonist [3H]CGS 21680 to rat cortex differs from its binding to rat striatum. Eur J Pharmacol. 1999;247:103–110. doi: 10.1016/0922-4106(93)90066-i. [DOI] [PubMed] [Google Scholar]
- 186.Gatch MB. Selvig M. Theophylline blocks ethanol withdrawal-induced hyperalgesia. Alcohol Alcohol. 2002;37:313–317. doi: 10.1093/alcalc/37.4.313. [DOI] [PubMed] [Google Scholar]
- 187.Simola N. Cauli O. Morelli M. Sensitization to caffeine and cross-sensitization to amphetamine: influence of individual response to caffeine. Behav Brain Res. 2006;172:72–79. doi: 10.1016/j.bbr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 188.Celik E. Uzbay IT. Karakas S. Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:50–55. doi: 10.1016/j.pnpbp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 189.López-Cruz L. Pardo M. San Miguel N. Salamone JD. Correa M. Impact of high doses of caffeine on acute and sensitized motor activity induced by ethanol in mice. Presented at: Federation of European Neuroscience Societies; Barcelona. 2012. [Google Scholar]
- 190.Hsu CW. Chen CY. Wang CS. Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology (Berl) 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- 191.Bedingfield JB. King DA. Holloway FA. Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol Biochem Behav. 1998;61:291–296. doi: 10.1016/s0091-3057(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 192.Patkina NA. Zvartau EE. Caffeine place conditioning in rats: comparison with cocaine and ethanol. Eur Neuropsychopharmacol. 1998;8:287–291. doi: 10.1016/s0924-977x(97)00086-2. [DOI] [PubMed] [Google Scholar]


