Abstract
Aim:
The aim was to compare patients' morbidity and response of bacillus Calmette–Guérin (BCG) prophylaxis after the intravesical instillation of low-dose Tokyo 172 strain and regular dose Connaught strain in patients with nonmuscle invasive bladder cancer (NMIBC).
Patients and Methods:
This was a randomized, active-controlled, open-label, monocenter study. Thirty-eight, NMIBC patients were treated sequentially, in a random order, with low-dose Tokyo 172 strain and regular dose Connaught strain, receiving each therapy for 6 weeks. A total of 18 and 20 patients were randomly assigned to a Tokyo 172 strain arm and a Connaught strain arm, respectively. Complication, morbidity, and recurrence-free survival (RFS) after each treatment were compared.
Results:
There was no significant difference in the 1-year RFS rate in patients treated with Tokyo 172 strain and Connaught strain (72.2% vs. 83.5%, respectively; P = 0.698). There were no significant differences in adverse events between the arms. Severe adverse events (>Grade 3) were seen in 15% of the Connaught strain group while no severe adverse events were observed as a result of Tokyo 172 strain.
Conclusion:
Our results indicated that low-dose Tokyo 172 strain decreased adverse events although it was not significant, and the RFS difference was not statistically significant between the two arms. Further investigation is warranted.
Keywords: Bacillus Calmette–Guérin, bladder cancer, intravesical instillation, Tokyo 172 strain
INTRODUCTION
Approximately half of nonmuscle invasive bladder cancer (NMIBC) patients are at risk for disease recurrence and progression. Therefore, they require adjuvant intravesical treatment after tumor resection. Currently, the gold standard treatment for NMIBC is bacillus Calmette–Guérin (BCG) instillation into the bladder. Despite the initial treatment success, many patients with NMIBC eventually have recurrence after intravesical BCG treatments. No consensus has been reached about the optimal dose for BCG therapy or about how the toxicity of BCG treatment can be reduced. Mack et al. reported the results of a phase II study with low-dose BCG therapy in high-risk NMIBC and concluded that low-dose BCG therapy is effective in high-risk T1 bladder cancer, especially with maintenance therapy to prevent progression and recurrence.[1] Kamal et al. also reported the efficacy of low-dose BCG.[2]
BCG daughter strains are divided into the early strains Japan, Birkhaug, Russia and Brazil, which were brought to each country between 1924 and 1926, and the late strains Pasteur, Danish, Glaxo, and Connaught, which were obtained after 1931. Although all of these strains are descendants of the original Mycobacterium bovis isolate, subsequent passages under different conditions have resulted in a variety of strains with unique genetic alterations. Differences in the antitumor effect between the strains are unknown so far. Only a few studies report the efficacy of different strains. Dutch South East Cooperative Urological Group evaluated BCG-Tice versus BCG-RIVM in 469 patients with pTa/pT1 carcinoma and found that there were no statistical differences in the toxicity between the two strains of BCG.[3] Tokyo 172 strain is unique to contain a higher number of colony-forming units (CFU) than other strains; even at half the standard dose, it is conceivable to reduce the dose of instillation without affecting the therapeutic efficacy. Irie et al. prospectively evaluated the efficacy and adverse events of low-dose (40 mg) Tokyo 172 strain. There was no significant difference in the tumor recurrence rates between the low-dose (40 mg) group and the standard dose (80 mg) group.[4] Mugiya et al. retrospectively reviewed 43 patients who underwent low-dose (40 mg) BCG therapy for carcinoma in situ (CIS) of the bladder. Of the 36 (84%) patients who achieved complete remission (CR), 12 showed intravesical recurrence while one developed ureteral cancer. The median CR duration of all patients was 37.5 months,[5] suggesting the comparable effect of low-dose Tokyo 172 strain with the regular dose.[6] Similarly, Takashi et al. reported that the dose (40 versus 80 mg) of BCG was not a significant determinant for CR in patients with CIS of the bladder.[7] In a randomized trial, Ozono et al. evaluated the recurrence-free rate in patients with CR and adverse events between groups treated by Tokyo 172 strain instillation at a dose of 80 mg eight times or at a dose of 40 mg 10 times, and observed no differences between two groups.[8] These studies suggest that 40 mg would be an adequate dose for Tokyo 172 strain, and a comparable study is possible with a dose of 40 mg. No prospective studies have been conducted to compare low-dose Tokyo 172 strain (40 mg) with other BCG substrains. In the present study, we compared patients' morbidity and response of prophylaxis after the intravesical instillation of low-dose Tokyo 172 with regular dose Connaught strain, which is used worldwide for the therapy of NMIBC.
PATIENTS AND METHODS
The present study included patients with histologically proven, single or multiple, primary or recurrent, stage Ta, T1, grades 1-3 urothelial carcinoma of the bladder, or CIS. Exclusion criteria were a tumor size >3 cm; stage T2 or higher tumors; age <20 year; ECOG performance status 3 or 4; presence of pneumonitis or active tuberculosis or strong positive PPD skin test; intravesical treatment during the previous 1 month; presence of a malignant tumor of the upper urinary tract or the urethra as a complication; a history of intravenous chemotherapy or intraarterial chemotherapy for bladder cancer; coexisting primary cancers; >grade 2 dysuria according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE); pregnancy; or any other case that the investigator considered to be inappropriate for the study. The present study was fully reviewed and approved by IRB. Informed consent was obtained in accordance with the Declaration of Helsinki. Patients were randomized to two treatment groups: Group 1, Tokyo 172 strain, 40 mg in 40 ml of saline weekly for six consecutive weeks starting 14 days after transurethral resection, and group 2, Connaught strain, 81 mg in 40 ml of saline weekly for six consecutive weeks starting 14 days after transurethral resection. Adverse events were monitored following NCI-CTCAE v3.0. Major complications were defined as meeting or exceeding grade 3. Cystoscopy and urine cytology were repeated every 3 months during the follow-up period. All visible lesions were resected with recurrence determined by histological confirmation. The treatment groups were compared on a primary end point of first bladder recurrence (any stage or grade). Secondary end points were BCG-related adverse events, adverse events occurring more frequently, severe adverse events, as well as BCG discontinuation. Adverse events were recorded by the investigators irrespective of their requirement of medication or discontinuation of BCG.
Results were expressed as means ± standard errors (SEs) for quantitative variables, while frequencies and proportions (%) were used for categorical variables. The main statistical test was the generalized Wilcoxon test. Recurrence-free survival (RFS) was determined by the Kaplan–Meier technique and compared using the two-sided log-rank test. Missing data were not replaced. Patients without an event were censored at the date of the last follow-up. Results were considered to be significant at the 5% critical level (P < 0.05).
RESULTS
Thirty-eight patients were randomized between February 2008 and December 2009. Table 1 summarizes patient background characteristics for the patients by treatment group. A total of 18 patients on low-dose Tokyo 172 strain, and 20 on Connaught strain started treatment. The median follow-up was of 16.4 months in the Tokyo 172 strain group and 16.5 months in the Connaught strain group. The median age of the Tokyo 172 group was 73.5 years; 77.8% had primary tumors, 44.4% had solitary tumors, 44.4% had Ta tumors, and 27.8% had grade 3 tumors. The median age of the Connaught group was 72.5 years; 80.0% had primary tumors, 45.0% had solitary tumors, 30.0% had Ta tumors, and 40.0% had grade 3 tumors. Globally, in 26.3% recurrence was found, and no one progressed to muscle invasive disease or developed distant metastases, and no one died during follow-up. The number of recurrence or progression was 5 (27.9%) in the Tokyo 172 group, and 5 (25%) in the Connaught group. Patient characteristics were well balanced in the two treatment groups [Table 1]. As shown in Figure 1, the 1-year recurrence-free probability was 72.2% in the Tokyo 172 strain group and 83.5% in the Connaught strain group. There was no significant difference in the 1-year RFS between the treatment groups (P = 0.698). Because there was no beneficial effect of the low-dose Tokyo 172 strain over the regular dose Connaught strain [Figure 1], the main interest of the current study centered on the comparison of adverse events between two groups. Global adverse events were noted in 73.7% of all patients, of which most were mild and transient [Table 2]. Compared to the Connaught strain, and as summarized in Table 2, the low-dose Tokyo 172 strain did not reduce the global number of adverse events (P = 0.7718; Table 2). The most common adverse events were fever and pollakisuria during treatment sessions, followed by hematuria and micturition pain [Tables 2 and 3]. Of four common adverse events, which occurred more frequently (≥10%), number of micturition pain events stratified by the NCI-CTCAE grade was significantly lower in the Tokyo 172 strain group, compared with the Connaught strain group (Wilcoxon's signed-rank test, P = 0.0455; Table 3). Table 4 extracts the severe adverse events with grade 3 or greater and comparisons according to therapy. Although the analyses are exploratory and are based on very small numbers of cases, the results suggest that the number of severe adverse events at least was minimized, if not statistically significant, in the Tokyo 172 strain group as compared with the Connaught strain group.
Table 1.
Patients' background characteristics
Figure 1.
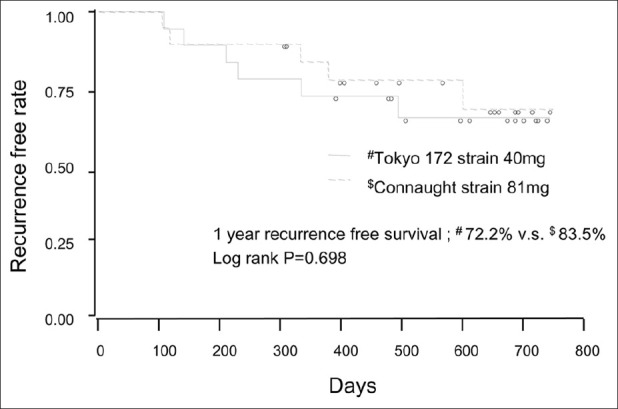
Recurrence-free survival (RFS) by the BCG strain. Solid curve indicates the Connaught strain. Broken curve indicates Tokyo 172. Vertical lines indicate censored
Table 2.
Adverse events
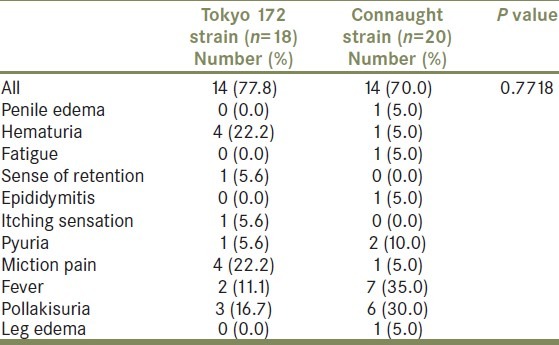
Table 3.
Adverse events occurring more frequently (≥10%)
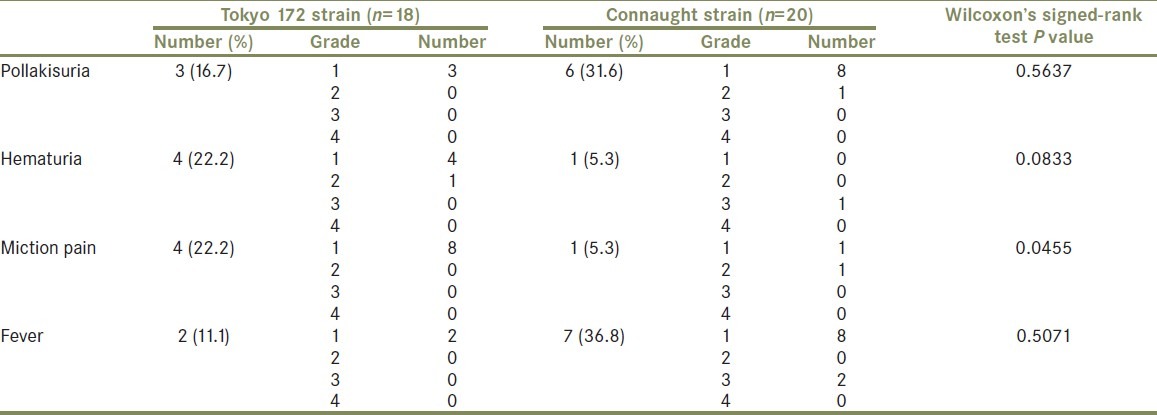
Table 4.
Adverse events of grade 3 or greater
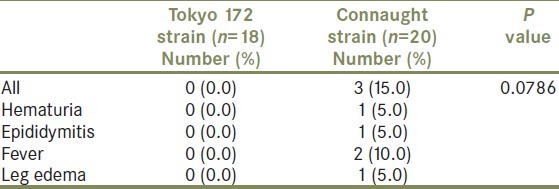
Table 5 shows the treatment completion rate by group. There was no significant difference in the treatment completion rates between the Tokyo 172 strain group and the Connaught strain group (P = 0.0523). However, 17 (94.4%) of 18 patients who underwent Tokyo 172 strain instillation completed the instillation protocol, while 14 (70.0%) of 20 in the Connaught strain group completed the therapy. Main reasons for stopping treatment were adverse events, including severe fever, hematuria, and epididymitis.
Table 5.
Completion rate of each group

DISCUSSION
Approximately 70% of patients with newly diagnosed bladder cancer present with stage Ta, CIS, or T1. Half of these patients are at risk for disease recurrence and/or progression. Currently, the mainstay in the intravesical instillation therapy treatment for NMIBC is BCG. The initial failure rate in patients treated with BCG is significant. In addition to disease recurrence, treatment fails in some patients due to adverse events, especially when given in a maintenance schedule. Lamm et al. reported that only 16% of patients on a maintenance schedule complete the planned treatment sessions, mostly due to adverse events.[9] Side-effects are commonly manifested during BCG therapy. Delay or interruption in instillation due to side effects may actually be detrimental to efficacy. So an important issue is whether a low-dose regimen can reduce toxicity while maintaining efficacy.
There are some reports available so far which verified the low-dose BCG regimen, and the overall response rate of the low-dose regimen ranges from 28% to 84%.[1,5,10–18] These results compare to the 72.2% of a 6-week course in our series, which possibly account for the short duration of follow-up in our series. In our cohort, Connaught strain-related adverse events rates were higher in most domains than were low-dose Tokyo 172-related complications. This result reinforces the value in reducing treatment-related toxicity with a lower dose. Most studies indicate lower toxicity with low-dose BCG.[13,19,7] In a phase III randomized trial comparing low-dose versus standard dose BCG (Pasteur strain, 75 mg vs. 150 mg), Pagano et al. reported a significant decrease in most of the common side effects (cystitis, fever, hematuria; P < 0.05), clarifying the relationships between dose and toxicity.[13] Mack evaluated the side effects of quarter dose BCG on Ta/T1 bladder cancer and found that there were modest local side effects including dysuria in 54% of cases and macroscopic hematuria in 39%.[20] Most studies, however, provided only short-term follow-up ranging from few months to 2 years, and data on the long-term condition of bladder patients originally treated with low-dose BCG are very rare. Losa et al. retrospectively reviewed 70 consecutive patients with primary or secondary carcinoma in situ with or without concomitant solitary or multifocal papillary tumor treated with weekly instillations of low-dose Pasteur strain for 6 weeks with a median follow-up of 74 months.[21] The mean time was 18 months (range 6-69) to treatment failure and 13 months (range 7-53) to progression.[21] They concluded that low-dose BCG is similarly effective, with a lower incidence of side effects and long-lasting positive outcome. Similarly, Kamel et al. retrospectively evaluated 74 patients with G3, T1 bladder cancer treated by a 6-week course of low-dose Pasteur strain with a median follow-up of 61 months.[2] The median time to treatment failure was 20 months. Regarding toxicity, irritative symptoms occurred in 24% of patients, fever in 9%, and microscopic hematuria in 14%, which appeared to be lower when compared with the rates reported for regular doses of BCG.[2] These data corroborate our findings and suggest that low-dose BCG therapy is effective and safe. Our study might have been underpowered to detect effectiveness associated with low-dose BCG because of the small number of patients prescribed low-dose Tokyo 172 and short-term follow-up. Larger studies are needed to better determine the effect of low-dose BCG on NMIBC recurrence. Connaught (Canada), Pasteur (France), Armand Frappier (Canada), and Tokyo 172 (Japan) were employed and studied for the efficacy in low-dose regimens.[1,5,10–18] Lamm reported that there were differences in complication rates with various BCG strains.[22] The incidence of cystitis-like symptoms, hematuria, and fever in our series was 64%, 40%, and 20%, respectively.[22]
The usage of low-dose BCG for aggressive bladder cancer is controversial. Hurle et al. assessed the effectiveness of low-dose BCG in high-risk T1/G3 bladder cancer patients who had weekly instillations of low-dose Pasteur strain for 6 weeks.[23] With a median follow-up of 33 months, 28 of 51 (54.9%) patients were disease free, and the risk of treatment failure was significantly greater for solid than papillary tumors (P = 0.0006), recurrent than primary tumors (P = 0.0052) and coexisting carcinoma in situ (P = 0.124) in multivariate analysis, suggesting that this low-dose Pasteur BCG is effective in the treatment of high-risk NMIBC, except for some tumor characteristics, such as solid appearance, coexisting carcinoma in situ, history of superficial transitional cell carcinoma, and early relapse after the initial induction course.[23] In contrast, Herr concluded that patients with highly malignant bladder cancer would not benefit from a dose reduction.[24] A large prospective cohort study is awaited to evaluate if low-dose BCG is safe for high-risk bladder cancer.
CONCLUSIONS
Given the favorable tolerability rate and the initial encouraging results of our study, it seems that the low-dose instillation of Tokyo 172 strain can be an effective option for NMIBC. The low-dose administration of Tokyo 172 strain might be suitable for repeated administration, including the maintenance schedule.
ACKNOWLEDGMENT
This study was partially supported by Japan BCG Laboratory (Tokyo, Japan).
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Mack D, Frick J. Low-dose bacille Calmette-Guérin (BCG) therapy in superficial high-risk bladder cancer: A phase II study with the BCG strain Connaught Canada. Br J Urol. 1995;75:185–7. doi: 10.1111/j.1464-410x.1995.tb07308.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamel AI, El Baz AG, Abdel Salam WT, El Din Ryad ME, Mahena AA. Low dose BCG regimen in T1 transitional cell carcinoma of the bladder: Long term results. J Egypt Natl Canc Inst. 2009;21:151–5. [PubMed] [Google Scholar]
- 3.Witjes WP, Witjes JA, Oosterhof GO, Debruyne MJ. Update on the Dutch Cooperative Trial: Mitomycin versus bacillus Calmette-Guérin-Tice versus bacillus Calmette-Guérin RIVM in the treatment of patients with pTA-pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Semin Urol Oncol. 1996;14:10–6. [PubMed] [Google Scholar]
- 4.Irie A, Uchida T, Yamashita H, Matsumoto K, Satoh T, Koh H, et al. Sufficient prophylactic efficacy with minor adverse effects by intravesical instillation of low-dose bacillus Calmette-Guérin for superficial bladder cancer recurrence. Int J Urol. 2003;10:183–9. doi: 10.1046/j.0919-8172.2003.00607.x. [DOI] [PubMed] [Google Scholar]
- 5.Mugiya S, Ozono S, Nagata M, Takayama T, Ito T, Maruyama S, et al. Long-term outcome of a low-dose intravesical bacillus Calmette-Guerin therapy for carcinoma in situ of the bladder: Results after six successive instillations of 40 mg BCG. Jpn J Clin Oncol. 2005;35:395–9. doi: 10.1093/jjco/hyi111. [DOI] [PubMed] [Google Scholar]
- 6.Akaza H, Kameyama S, Kakizoe T, Kojima H, Koiso K, Aso Y, et al. Ablative and prophylactic effects of BCG Tokyo 172 strain for intravesical treatment in patients with superficial bladder cancer and carcinoma in situ of the bladder. Bladder cancer BCG Study Group. Nihon Hinyokika Gakkai Zasshi. 1992;83:183–9. doi: 10.5980/jpnjurol1989.83.183. [DOI] [PubMed] [Google Scholar]
- 7.Takashi M, Wakai K, Ohno Y, Murase T, Miyake K. Evaluation of a low-dose intravesical bacillus Calmette-Guerin (Tokyo strain) therapy for superficial bladder cancer. Int Urol Nephrol. 1995;27:723–33. doi: 10.1007/BF02552138. [DOI] [PubMed] [Google Scholar]
- 8.Ozono S, Takahashi K, Tanaka N, Momose H, Hirao Y, Okajima E. Intravesical bacillus Calmette–Guerin (BCG) therapy for carcinoma in situ of the urinary bladder—outcomes of a randomized study. Ann Soc BCG BRM Immunother. 1996;20:95–100. [Google Scholar]
- 9.Lamm DL. Preventing progression and improving survival with BCG maintenance. Eur Urol. 2000;37:9–15. doi: 10.1159/000052376. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Chen X, Downs TM, DeWolf WC, O'Donnell MA. IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guerin immunotherapy. J Immunol. 1999;162:2399–405. [PubMed] [Google Scholar]
- 11.Yoneyama T, Ohyama C, Imai A, Ishimura H, Hagisawa S, Iwabuchi I, et al. Low-dose instillation therapy with bacille Calmette-Guerin Tokyo 172 strain after transurethral resection: Historical cohort study. Urology. 2008;71:1161–5. doi: 10.1016/j.urology.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 12.Stricker P, Pryor K, Nicholson T, Goldstein D, Golovsky D, Ferguson R, et al. Bacillus Calmette-Guerin plus intravesical interferon alpha-2b in patients with superficial bladder cancer. Urology. 1996;48:957–61. doi: 10.1016/s0090-4295(96)00375-5. discussion 961-2. [DOI] [PubMed] [Google Scholar]
- 13.Pagano F, Bassi P, Piazza N, Abatangelo G, Drago Ferrante GL, Milani C. Improving the efficacy of BCG immunotherapy by dose reduction. Eur Urol. 1995;27:19–22. doi: 10.1159/000475204. [DOI] [PubMed] [Google Scholar]
- 14.Pagano F, Bassi P, Milani C, Meneghini A, Tuccitto G, Garbeglio A, et al. Low-dose BCG-Pasteur strain in the treatment of superficial bladder cancer: Preliminary results. Prog Clin Biol Res. 1989;310:253–61. [PubMed] [Google Scholar]
- 15.Mack D, Frick J. Five-year results of a phase II study with low-dose bacille Calmette-Guerin therapy in high-risk superficial bladder cancer. Urology. 1995;45:958–61. doi: 10.1016/s0090-4295(99)80115-0. [DOI] [PubMed] [Google Scholar]
- 16.Mack D, Frick J. Low-dose BCG in superficial bladder cancer with strain Connaught Canada-as effective as strain Pasteur Paris? Eur J Cancer. 1994;30A:1728–9. doi: 10.1016/0959-8049(94)90603-3. [DOI] [PubMed] [Google Scholar]
- 17.Lebret T, Gaudez F, Hervé JM, Barré P, Lugagne PM, Botto H. Low-dose BCG instillations in the treatment of stage T1 grade 3 bladder tumours: Recurrence, progression and success. Eur Urol. 1998;34:67–72. doi: 10.1159/000019664. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher BL, Joudi FN, Maymí JL, O'Donnell MA. Impact of previous bacille Calmette-Guérin failure pattern on subsequent response to bacille Calmette-Guérin plus interferon intravesical therapy. Urology. 2008;71:297–301. doi: 10.1016/j.urology.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 19.Morales A, Nickel JC, Wilson JW. Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol. 1992;147:1256–8. doi: 10.1016/s0022-5347(17)37532-8. [DOI] [PubMed] [Google Scholar]
- 20.Mack D, Höltl W, Bassi P, Brausi M, Ferrari P, de Balincourt C, et al. The ablative effect of quarter dose bacillus Calmette-Guerin on a papillary marker lesion of the bladder. J Urol. 2001;165:401–3. doi: 10.1097/00005392-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Losa A, Hurle R, Lembo A. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: Long-term results. J Urol. 2000;163:68–71. discussion 71-2. [PubMed] [Google Scholar]
- 22.Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 23.Hurle R, Losa A, Ranieri A, Graziotti P, Lembo A. Low dose Pasteur bacillus Calmette-Guerin regimen in stage T1, grade 3 bladder cancer therapy. J Urol. 1996;156:1602–5. [PubMed] [Google Scholar]
- 24.Herr HW, Schwalb DM, Zhang ZF, Sogani PC, Fair WR, Whitmore WF, Jr, et al. Intravesical bacillus Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer: Ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404–8. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]


