Abstract
Objective:
To evaluate the clinical efficacy and safety of Tinospora cordifolia lotion including its cure rate and clearance time compared with permethrin lotion.
Materials and Methods:
A single blind, randomized, controlled, pilot clinical study was performed in three government institutions to investigate clinical efficacy of T. cordifolia lotion in sixty-six clinically-diagnosed scabies-infected patients. The patients were treated with T. cordifolia or permethrin lotions for three consecutive days for two weeks and clinical assessment of each patient was performed for five weeks.
Results:
T. cordifolia lotion and permethrin significantly reduced the mean global evaluation score after four weeks of treatment. The two lotions showed comparable effects as anti-scabies agent. Moreover, the clearance time (days) and cure rate using the two lotions did not differ. Clinical improvement, mean clearance time and cure rate of T. cordifolia lotion are comparable with permethrin.
Conclusions:
Tinospora cordifolia lotion exhibits anti-scabies activity comparable with permethrin. Its incorporation as therapeutic reagent in Sarcoptes scabiei infections is highly recommended.
Keywords: Anti-scabies, Sarcoptes scabiei, scabies, Tinospora cordifolia
INTRODUCTION
Scabies is a contagious, parasitic skin infestation caused by Sarcoptes scabiei mite. It is characterized by intractable, nocturnal pruritus with mild cutaneous lesions.[1] Around 300 million scabies cases worldwide are reported each year.[2,3] In the absence of a comprehensive national registry, the exact figures for the incidence of scabies in the Philippines are not known but a sizable number of patients have been reported in several Metro Manila hospitals.
Tinospora cordifolia Boerl is synonymous with T. rumphii and T.crispa, commonly known as makabuhay, a medicinal plant used for the treatment of tropical wound and ulcers.[4,5] Studies have shown its efficacy as hypolipidemic,[6] antiamoebic,[7] antioxidant,[8] hepatoprotective,[9] and immunomodulatory agents.[9,10] So far, a diverse group of pharmacologically active substances like clerodane furanoditerpene glycosides,[11] aporphine,[12] isoquinoline[13] and quaternary alkaloids,[14] octacosanol,[15] arabinogalactan polysaccharide[16] and G1-4 A[17] have been isolated and identified in T. cordifolia.
It has been previously shown that the T. cordifolia aqueous stem extract exhibited anti-scabies activity with complete cure of the lesions in eight out of 26 patients after 3-5 days of treatment.[18] The anti-scabies activity of T. cordifolia is similar with Crotamiton.[19] In this study, we aimed to investigate the efficacy and safety of the formulated T. cordifolia lotion compared with permethrin including its cure rate and clearance time.
MATERIALS AND METHODS
Preparation of the plant material
Fresh, mature stems of Tinospora cordifolia collected last February 2011 from Angat, Bulacan, Philippines were authenticated by a botanist of the Bureau of Plant Industry. Stems were air dried for about seven days prior to grinding using Wiley Mill. One kilogram of air-dried powdered T. cordifolia stems was percolated with 3L of 80% ethanol until exhaustion. The percolate was concentrated by means of a rotary evaporator.
Preparation of the Tinospora cordifolia lotion
The T. cordifolia extract was mixed with 0.15g methyl paraben and 39.70g water to form the water phase. 7.0g stearic acid, 2.5g tween 80, 0.5g lanolin, 0.15g propyl paraben were triturated to form a creamy paste. The oil and water phases were stirred until a complete dispersion was formed. The lotion is yellowish brown, non-staining with agreeable odor and slightly acidic pH (5.26). The lotion was stored in a refrigerated area until use.
Permethrin lotion, as the positive control is a white, viscous, pourable, odorless lotion. T. cordifolia and permethrin lotions were transferred and supplied in 60 ml white opaque plastic bottles. All study subjects were given identical dosage instructions.
Experimental subjects
Subjects, 2-22 years of age, were recruited after clinical diagnosis. Consent and clinical research forms were provided. A licensed pediatrician with three medical doctors facilitated and assessed the patients using a clinical research form for the weekly assessment of the symptoms and appearance of local adverse reactions. The clinical testing was approved by the University Santo Tomas College of Rehabilitation Sciences Ethics Committee with clinical protocol no.041 and assigned no.041/11-12.
Written informed consent was obtained from parents/guardians and assent was provided by the children before the culmination of the trial. A signed written and dated informed consent was obtained from each patient or his nearest of kin or legal guardian if the patient was unable to give consent. All pertinent aspects of the study were explained to the subject, his/her guardian before he or she signed the consent form or was enrolled in the study. All patients enrolled in the study agreed with the clinical trial/procedures/intervention in which they were enrolled. The patient was informed that he/she was free to voluntarily discontinue his/her participation in the study without prejudice.
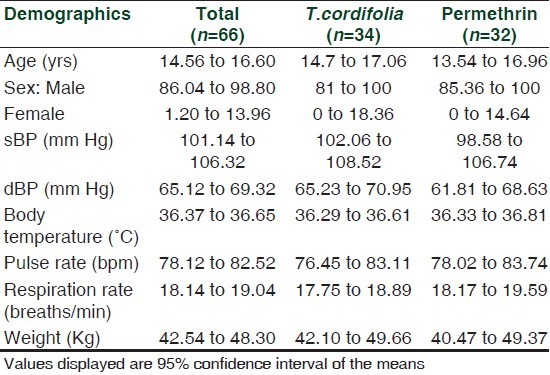
Subjects were assessed as having scabies based on the following clinical features: Nocturnal pruritus, occurrence of burrows, vesicles and papules and the distribution of lesions on the following 15 sites of predilection: Face, head, palms, interdigits, sides of fingers, upper and lower extremities, wrists, axilla, nipple, umbilical area and/or lower abdomen, genitalia, inguinal, buttocks and back area.[1] Scabies patients were characterized as either having a mild infestation (<6 sites of predilection); moderate infestation (>6 sites) or severe infestation (>10 sites).[18] Test medications were given to each subject randomly. Sixty-six children were assessed including their vital signs and dormmates history of similar lesions within the center were noted [Table 1].
Table 1.
Demographic profile of the patients included in the study
Clinical trial
A single blind, randomized, controlled, parallel clinical trial comparing the effectiveness of the T. cordifolia lotion against 5% permethrin lotion in treating 66 pediatric patients diagnosed with scabies at the Manila Youth Reception Center, Reception Action Center and Tanglao Detention Center in Malolos, Bulacan, Philippines was done from July-October 2011 [Table 1]. To detect a decrease of δ=2.24 with a standard deviation σ =4.65 in the severity scores of the erythema after one month of T. cordifolia lotion treatment, at 80% power of the statistical test, observing 0.05 level of significance, a sample size of at least 30 in each group was required for the study. Sample size was calculated using GPower ver 3.1.2.[20,21]
Patients were randomly assigned to two groups using Microsoft Excel in which thirty-four subjects were blindly given Lotion A (T. cordifolia) and thirty-two subjects were applied with Lotion B (permethrin).
Exclusion criteria included subjects who used topical or oral scabicidal treatment and corticosteroid four weeks before the trial, subjects with concomitant secondary bacterial infections with systemic manifestation like fever, malaise, chills, and subjects who were enrolled in other clinical study.[22]
The patients were instructed to apply the assigned lotion from neck down to the feet especially on the sites of predilection after a night bath using a mild soap.[1,20,22–24] This was done daily for three consecutive days per week for two weeks until clear response was achieved which is the clearing of lesions seen as post-inflammatory hyper/hypopigmentation and disappearance of pruritus, burrow or vesicles during the observation period. The treatment period was two weeks for both T. cordifolia and permethrin lotions.[20,22,24] Assessments of the patient conditions were made every week using the global evaluation score of signs and symptoms. Standard instructions were provided to all subjects for the control of mite transmission. They were informed not to apply any medications during the trial.[1,20,24,25]
Treatment was discontinued in patients who exhibited exacerbation of clinical condition, with significant adverse reactions and those who received concomitant scabicidal drugs. Patients with lesions that worsened or failed to clear after 28 days were given permethrin as rescue medication. This was reassessed one week post therapy.[20]
Efficacy assessment
The severity of skin lesions was assessed using the global evaluation scoring system. The pruritus, erythema, presence of primary lesions like papules, vesicles and pustules as well as the secondary lesions like excoriations, erosions and ulcerations were assessed. This was graded as 0 if there were no symptoms, 1 as mild, 2 if moderate and 3 if with severe signs and symptoms. The global evaluation score was computed based on the cumulative scores of the above parameters.[20,26]
Efficacy endpoints
Improvement of skin lesions was graded based on the Physical Clinical Global Evaluation of Clinical Response. Clear response was graded as 1 following clearing of all lesions described as post-inflammatory hyper/hypopigmentation and disappearance of pruritus, burrow and vesicles. Excellent graded as 2 if there's greater than 75% but less than 100% reduction of skin lesions and pruritus. Marked improvement was graded as 3 if there is 50 to 75% reduction of skin lesions and pruritus. Moderate improvement was graded as 4 if there is 25 to 50% reduction of skin lesions and pruritus. Slight improvement was graded as 5 if there's less than 25% improvement of the skin lesions and pruritus. Failure was graded as 6 if at the end of three weeks, there's no improvement in pruritus and skin lesions while Worsen was graded as 7 if there was appearance of new lesions during the course of treatment.[20,26] Post-evaluation assessment was graded as Clinical Cure when complete disappearance of signs and symptoms of the disease was noted. Clinical improvement was given if clinical findings subsided significantly at the completion of the medication period but with incomplete resolution of symptoms. Reinfestation, if there was reappearance of signs and symptoms three weeks after therapy and failure, if therapy was discontinued due to an adverse reaction associated with the study drug.
Statistical analysis
Descriptive statistics was used to summarize the data collected and the adverse effects in all visits. Mann Whitney U Test was used to compare the weekly effect of T. cordifolia lotion with permethrin in terms of severity of infestation, global evaluation scores and various parameters of scabies. Friedman tests were used for intra-group comparison. Fisher's exact test was used to compare the number of patients cleared of scabies after 28 days and McNemar's test was used to compare the adverse effects in both lotions. P values of less than 0.05 were considered statistically significant.
RESULTS
Eighty six patients were diagnosed with scabies and were eligible for the study. However, 20 patients were excluded from the study due to secondary bacterial infections, sulfur treatment, presence of edema while others declined to participate in the study. A total of 66 subjects were enrolled in the study using clinical diagnosis. Six patients (9.09%) did not return after the baseline treatment. These patients were seen during the initial visit and considered as drop-outs from the study.
A total of 62 subjects (93.94%) completed the treatment period up to day 14. Sixty patients were followed up one week post therapy. All the subjects were diagnosed with scabies based on the criteria of having lesions on the classic sites, nocturnal pruritus and presence of the same lesions within the household members [Flow chart 1].
Flow chart 1.
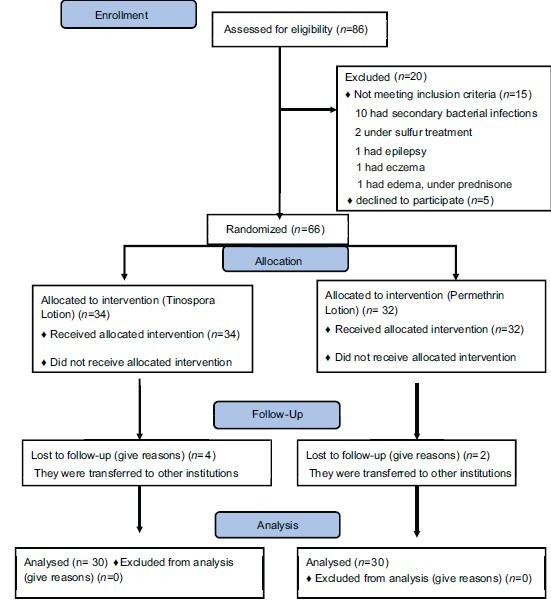
Recruitment, allocation and follow-up of participants
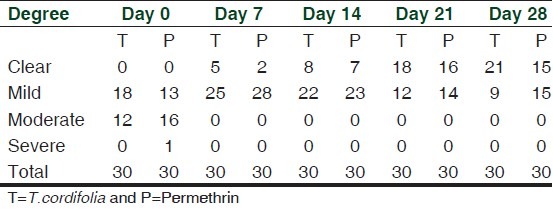
Degree of infestation
The degree of infestation showed that during the pre-treatment period (n = 60), majority of the patients (51.62%) had mild infestation, 46.66% had moderate infestation and 1.66% with generalized infestation. At baseline, 60% vs 43.33% had minimal infestation while 40 vs 53.33% of patients had moderate infestation for T. cordifolia and permethrin-treated patients. On day 28, 70 vs 50% exhibited complete cure while 30 vs 50% of the T. cordifolia and permethrin-treated patients had mild infestation [Table 2].
Table 2.
Number of patients according to degree of infestation on the 28-day treatment
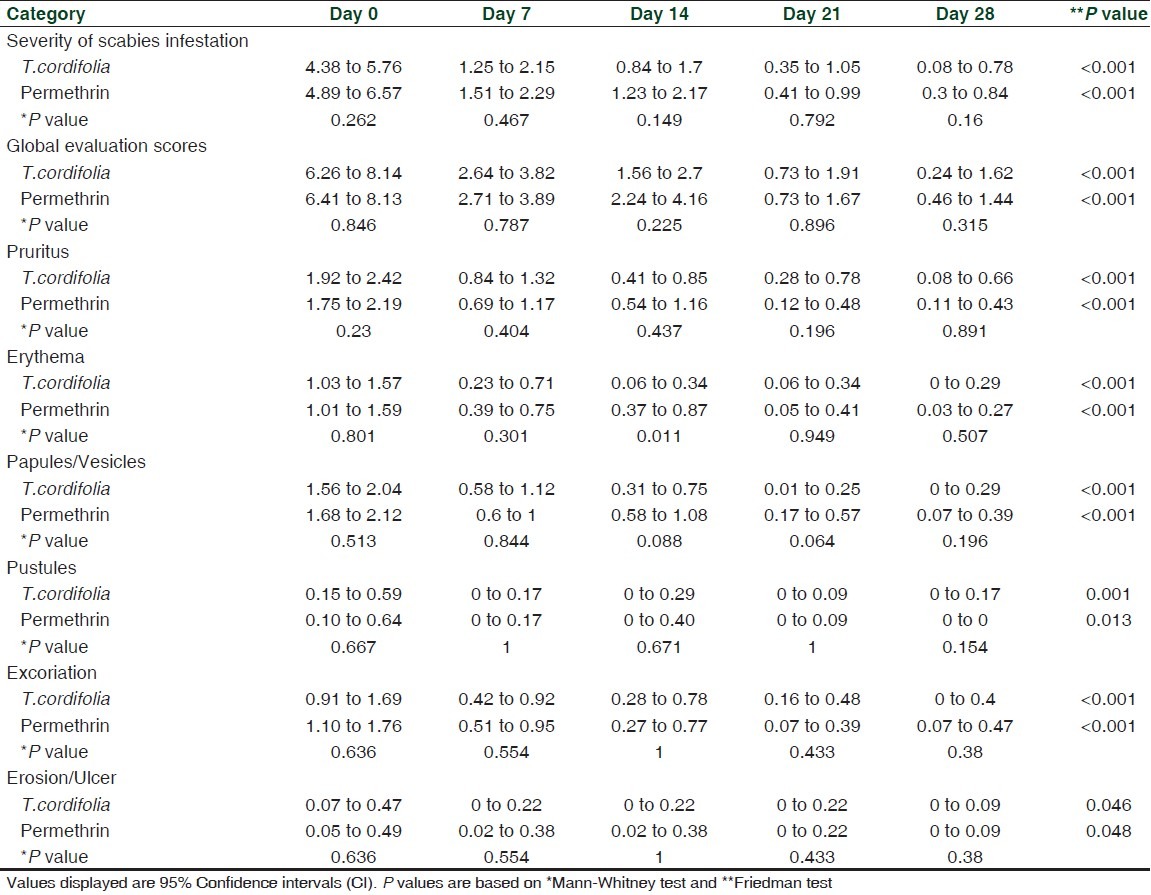
The reduction in the severity of disease and improvement of the patients' condition were observed through time. A significant decrease in the degree of infestation was observed using T. cordifolia [Baseline (Mean, 95% CI): 5.07, 4.38 to 5.76; After: 0.43, 0.08 to 0.78; P < 0.001] and permethrin [Baseline: 5.73, 4.89 to 6.57; After: 0.57, 0.30 to 0.84), P < 0.001] by both lotions after 28 days. Using Mann Whitney u-test, the reduction in the degree of infestation after 28 days using T. cordifolia and permethrin (P = 0.160) were found to be comparable [Table 3].
Table 3.
Comparison of the severity of infestation, global evaluation scores and various parameters/symptoms of scabies
Global evaluation scores
Selecting only the patients who completed the follow-up (n = 60), there was a significant decrease in the mean global evaluation score among the patients. For the T. cordifolia-treated group, the mean global evaluation score at baseline is 7.20 (95% CI: 6.26 to 8.14) while at 28th day the mean global evaluation score is 0.93 (95% CI: 0.24 to 1.62). Reduction of mean global evaluation score was also seen in patients treated with permethrin [Table 3].
Based on the mean global evaluation score, T. cordifolia and permethrin lotions exhibited comparable anti-scabies activity (P = 0.315) after 28th days. There was a significant reduction (P < 0.001) in the mean score of patients treated by both lotions from baseline to 28th day. The mean reduction per week was statistically the same for both lotions [Table 3].
Although at 14th day, the erythema was more expressed in permethrin group than T. cordifolia [T. cordifolia: 0.2, 0.06 to 0.34; Permethrin: 0.62, 0.03 to 0.27), P = 0.011], both lotions exhibited comparable mean scores of pruritus (P = 0.891), erythema (P = 0.507), papules (P = 0.196), pustules (P = 0.154), excoriations (P = 0.380), erosion/ulcer (P = 1.000) after 28th days. Moreover, significant reduction in the mean scores of all parameters were noted from baseline to 28th days (P < 0.05) for both T. cordifolia and permethrin lotions [Table 3].
Disease control
From the T. cordifolia-treated patients who completed the follow-up, on seventh day of treatment (n = 30), 3.33% had mild improvement, 30% had moderate, while 56.66% and 6.66% had marked and excellent improvement and 3.33% were cleared of lesions. On day 28, majority of the patients (70%) had clearance of lesions while 3.33% each for failure treatment and worsened condition [Figure 1].
Figure 1.
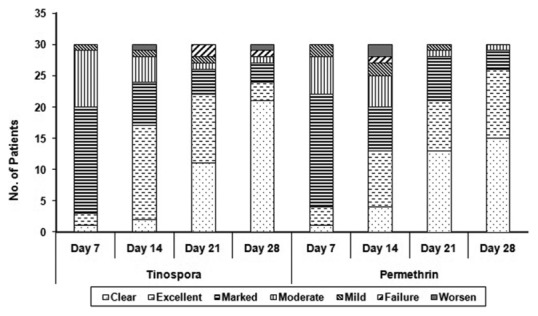
The frequency distribution of clinical improvement among patients
With regard to permethrin-treated group, on seventh day of treatment (n = 30), 6.66% had mild improvement, 20.0% had moderate and 60.0% had marked improvement while 10% had excellent improvement and 3.33% of the patients were cleared of lesions. On 28th day, majority of the patients had clearance of lesions (50%) without failure treatment nor worsened condition [Figure 1].
T. cordifolia lotion exhibited significant clinical improvement of the patients which was comparable to permethrin (P <0.315). However, a significant disease control from seventh to 28th day was seen in patients treated by both lotions [Figure 2]. Significant increase in the percent reduction of lesions was seen in both T. cordifolia (P < 0.001) and permethrin group (P < 0.001).
Figure 2.
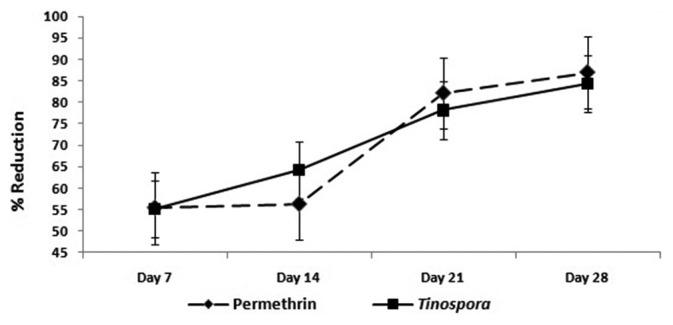
Mean Percent Reduction in Scabeitic Lesions among Patients from Day 7 to Day 28
Clearance
On 28th day, 21 T. cordifolia-treated and 15 permethrin-treated patients showed clearance of scabietic lesions. T. cordifolia and permethrin lotions exhibited comparable number of patients who had disappearance of lesions and were completely cured of the disease after treatment (P = 0.187) [Figures 4 and 5]. Nine and fifteen patients who were unclear at 28th day were noted in both T. cordifolia and permethrin lotions [Figure 1].
Figure 4.
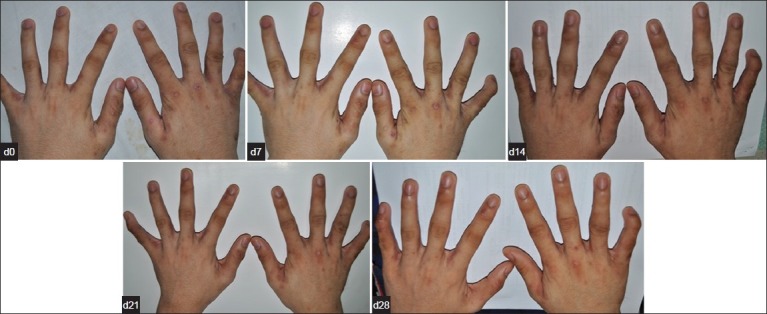
Clinical Improvement seen in a patient from Day 0 to Day 28 after treatment with Permethrin
Figure 5.
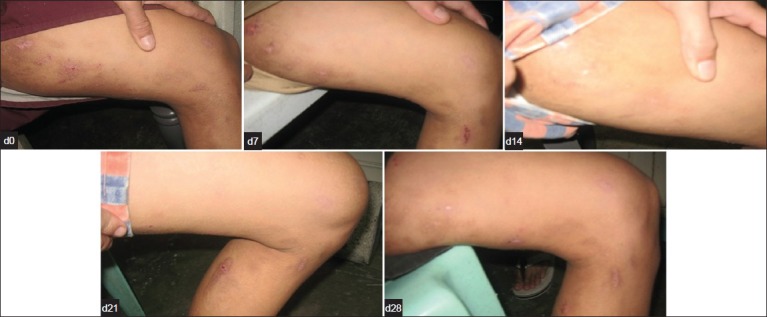
Clinical Improvement seen in a patient from Day 0 to Day 28 after treatment with Tinospora lotion showing significant decrease in scabeitic lesion
Clearance of the scabies disease in T. cordifolia and permethrin –treated patients was noted at 23 (95% CI: 20.47 to 25.53) and 21st days (95% CI: 17.39 to 23.67) for T. cordifolia and permethrin lotions. The clearance time of T. cordifolia lotion is comparable with that of permethrin (P = 0.226).
Adverse effects
Fourteen patients assigned to T. cordifolia lotion experienced pruritus, while five patients had erythema and six had burning sensation during application of the lotion. In terms of permethrin, fourteen patients had pruritus, five had erythema and another five had burning sensation during application. Most of the patients experienced mild severity of pruritus, rarely disturbing their sleep or any activity of the patient accompanied by occasional itching, with faint, barely discernible erythema and mild burning sensation [Figure 3]. Improvement in the condition was observed over time. There was reduction in the severity of the side effects experienced by the patients from the first day of application up to the last day of observation. Both T. cordifolia and permethrin lotions exhibited mild adverse effects in the same proportion of patients who had pruritus (P = 0.796), erythema (P = 0.553) and who had experienced burning sensation (P = 0.692) during treatment.
Figure 3.
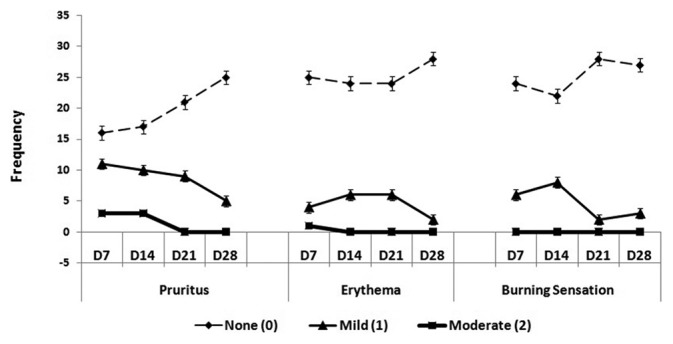
Frequency Distribution of Severity of Pruritus, Erythema and Burning Sensation among T. cordifolia-treated Patients
DISCUSSION
Scabies is a highly contagious parasitic infestation which causes intractable nocturnal pruritus since the female mites burrow deep in the stratum corneum of the epidermis at night to lay eggs.[22,23] Scabies has a long incubation period in which symptoms may develop in about four weeks following initial infestation.[2,23] Toxins, saliva and other secretions secreted by mites are probably responsible for clinical manifestations of the initial sign and symptoms like vesiculopapular lesions with pruritus. Pruritus is the result of a hypersensitivity response to the antigenic molecules of the saliva, eggs and faecal material of the mites, normally experienced at night.[22,23,25] Initial predilection sites occur in warm, moist areas where there is a thin stratum corneum like the finger webs, wrists and anterior axillary folds.[22] Secondary bacterial infections and lesions like erosions and excoriations usually develop from scratching with dirty fingernails.[3,25]
It is worth noting that majority of the scabies-infected patients belonged to the low-socioeconomic class in which overpopulation is predominant along with limited health education and inadequate sanitation. Moreover, the treatment is expensive because it requires the entire household to completely eradicate the mites. Hence, alternative, economical, and safe medications from local plants are being studied to provide cure for the disease. One of these promising plants is T. cordifolia which contains quaternary alkaloids like berberine, palmatine, jatrorrhizine and magnoflorine.[13] Berberine and its salts have antibacterial, antifungal and antipyretic properties. Berberine has cytotoxic and neoplasm inhibitory effects.[13] It also exerts spasmolytic action on muscles which may be responsible for the scabicidal activity of T. cordifolia stem extracts.
Berberine isolated from Berberis sp. exhibits anti-inflammatory activity by dose dependent inhibition of prostaglandin synthesis, reduction of exudate production in Wistar rats carrageenan- induced air pouch, inhibition of vascular permeability[27,28] and significant anti-inflammatory activity on serotonin-induced hind paw edema.[29] It can reduce intestinal mucosal inflammation by downregulation of cyclooxygenase- 2 expression.[30] These activities of berberine may lead to the reduction of inflammation and exudation in scabietic lesions and to the general improvement of the disease.
In 1983, Rivera et al., reported that Crotamiton and T. cordifolia aqueous extract are equally effective in the treatment of scabies. In a study done by Salazar et al., the aqueous stem extract stem has acaricidal property exhibiting a 31 % total cure and 69% partial cure when applied daily as a lotion for five days.
T. cordifolia lotion is a non-irritating and safe topical medication as shown by phase 1 clinical trial.[26] In a comparative analysis of T. cordifolia lotion with Crotamiton lotion and placebo, results showed same effectiveness with Crotamiton since there is no significant difference between the two while both lotions are significantly different to the placebo.[20]
The age of the subjects ranged from 2 to 22 years with a mean of 15.58 (95% CI: 14.56 to 16.60). There was no significant difference in the age of the patients assigned to both lotions (P = 0.547). At P value of 0.999, the gender of the patients assigned to both lotions were statistically the same. Neither the age nor gender served as a variable in the study [Table 1].
There was a gradual decline in the frequency of patients having moderate and mild scabies infestation after treatment with T. cordifolia lotion. Improvement in the mean global evaluation score from baseline to day 28 was observed in both lotions. Significant decrease in the signs and symptoms of scabies infestation like pruritus, papules, pustules, vesicles, erythema, excoriation and erosion was observed. The clinical improvement seen in the patients were comparable to the results of the clinical trials done by Llamasares and Reyes et al.
Sixty patients who completed the five week clinical study were included in the evaluation post-therapy. These results were comparable to the initial clinical trial done in 27 patients who completed the course with 74.07% (20 patients) clearance of lesions and 11.1% (3 patients) treatment failure at 28th day.[26]
The subjects were followed-up until 28th day, three weeks after the treatment period of two weeks. There were still significant reductions based on the location of the lesions, degree of infestation, global evaluation score and disease control.
Presence of erythema and few papules over the sites of predilection among six out of the 30 T. cordifolia–treated patients may be due “post-scabeitic hypersensitivity” which persists for 2-4 weeks and represents the body's response to dead mites or toxins produced after treatment.[1,25] Two subjects had treatment failure on 21st day. These patients had new pustules, erosion and pruritus at wrist and dorsum of the hand. The patients failed to iron their clothes and clean their respective beddings which may cause the treatment failure. Another patient was considered reinfested because marked improvement (50%) was seen on seventh day but only a mild improvement at 14th day (17%) and 21st day (17%) was observed while on 28th day, no improvement was noted and there was reappearance of new papules in the wrist, legs and interdigits and persistent papules on genitals from 14-28th days were noted.
Overall, the 50% T. cordifolia lotion showed a significant decrease in all the parameters. It showed significant decrease in the degree of infestation, sites of predilection and global evaluation score while it demonstrated significant increase in the clinical improvement of the patients during clinical assessment.
The 50% T. cordifolia lotion is a non-staining, yellowish brown product with an agreeable odor. Although, a bitter sensation was noted when the lotion is applied topically, patients were asked to apply the lotion after dinner and to washed hands after application. Aside from the mild side effects like erythema, pruritus and burning sensation, no reportable cases were noted that it is unsafe to use topically.
Results presented herein showed that, T. cordifolia lotion exhibited a comparable anti-scabies activity with permethrin having the same cure rate [T. cordifolia: 70%, 53.60 to 86.94%; Permethrin: 50%, 32.11 to 67.89%; P = 0.187] and clearance time of 23rd days, 20.47 to 25.53 days. Since the T. cordifolia lotion is inexpensive compared to the commercially available drugs, it can be used as an alternative treatment to scabies infestation.
ACKNOWLEDGMENT
The study was supported by research grants from the Science Education Institute, Department of Science and Technology, Philippines, the Commission on Higher Education, Philippines, the Fund for Assistance to Private Education, Philippines and the University of Santo Tomas Grants Office, Philippines.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fitzpatrick T, Johnson R, Wolff K. 4th ed. New York: McGraw-Hill Co; 2001. Color Atlas and Synopsis of Clinical Dermatology. Common and Serious Diseases; pp. 834–7. [Google Scholar]
- 2.Vorou R, Remoudaki HD, Maltezou HC. Nosocomial scabies- Review. J Hosp Infect. 2007;65:9–14. doi: 10.1016/j.jhin.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Orion E, Marcos B, Davidovici B, Wolf R. Itch and scratch: Scabies and pediculosis. Clin Dermatol. 2006;24:168–75. doi: 10.1016/j.clindermatol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.De Padua LS, Lugod GC, Pancho JV. Los Banos: Laguna, Phil: Documentation and Information Section, Office of the Director of Research, University of the Philippines; 1977. Handbook on Philippine Medicinal Plants; pp. 89–94. [Google Scholar]
- 5.Department of Health, Bureau of Food and Drugs. Manila, Philippines: 2004. Philippine Pharmacopeia; pp. 107–8. [Google Scholar]
- 6.Stanely Mainzen Prince P, Menon VP, Gunasekaran G. Hypolipidemic action of Tinospora cordifolia roots in alloxan diabetic rats. J Ethnopharmacol. 1999;64:53–7. doi: 10.1016/s0378-8741(98)00106-8. [DOI] [PubMed] [Google Scholar]
- 7.Sohni YR, Kaimal P, Bhatt RM. The antiamoebic effect of a crude drug formulation of herbal extracts against Entamoeba histolytica in vitro and in vivo. J Ethnopharmacol. 1995;45:43–52. doi: 10.1016/0378-8741(94)01194-5. [DOI] [PubMed] [Google Scholar]
- 8.Goel HC, Prasad J, Singh S, Sagar R, Agrawala PK, Bala M, et al. Radioprotective Potential of a Herbal Extract of Tinospora cordifolia. J Radiat Res. 2004;45:61–8. doi: 10.1269/jrr.45.61. [DOI] [PubMed] [Google Scholar]
- 9.Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–46. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- 10.Diwanay S, Chitre D, Patwardhan B. Immunoprotection by botanical drugs in cancer chemotherapy. J Ethnopharmacol. 2004;90:49–55. doi: 10.1016/j.jep.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Maurya R, Manhas LR, Gupta P, Mishra PK, Singh G, Yadav PP. Amritosides A, B, C and D: Clerodane furano diterpene glucosides from Tinospora cordifolia. Phytochemistry. 2004;65:2051–5. doi: 10.1016/j.phytochem.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Phan VK, Chau VM, Nguyen TD, La VK, Dan TH, Nguyen HN, et al. Aporphine Alkaloids, clerodane diterpenes, and other constituents from Tinospora cordifolia. Fitoterapia. 2010;81:485–9. doi: 10.1016/j.fitote.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sarma DN, Khosa RL, Sahai M. Isolation of Jatrorrhizine from Tinospora cordifolia Roots. Planta Med. 1995;61:98–9. doi: 10.1055/s-2006-958022. [DOI] [PubMed] [Google Scholar]
- 14.Bisset NG, Nwaiwu J. Quaternary Alkaloids of Tinospora Species. Planta Med. 1983;48:275–9. doi: 10.1055/s-2007-969933. [DOI] [PubMed] [Google Scholar]
- 15.Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF < kappa > B and its DNA binding activity. Eur J Pharmacol. 2008;588:141–50. doi: 10.1016/j.ejphar.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Chintalwar G, Jain A, Sipahimalani A, Banerji A, Sumariwalla P, Ramakrishnan R, et al. An immunologically active arabinogalactan from Tinospora cordifolia. Phytochemistry. 1999;52:1089–93. doi: 10.1016/s0031-9422(99)00386-6. [DOI] [PubMed] [Google Scholar]
- 17.Desai VR, Ramkrishnan R, Chintalwar GJ, Sainis KB. G1-4 A, an Immunomodulatory polysaccharide from Tinospora cordifolia, modulates macrophage responses and protects mice against lipopolysaccharide induced endotoxic shock. Int Immunopharmacol. 2007;7:1375–86. doi: 10.1016/j.intimp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Salazar N, Sabordo N, Romero R, Montalban C, Acacio M, Tarrayo M, et al. Tinospora rumphii Boerl (Makabuhay) in the Treatment of scabies. Philippine J Microbiol Infect Dis. 1987;16:25–9. [Google Scholar]
- 19.Rivera EF, Sison EM, Aligui G. A Comparativestudy of the efficacy of crotamiton and tinospora rumphii in the treatment of scabies in children. J Philippine Med Assoc. 1983;58:265–67. [Google Scholar]
- 20.Llamasares A. Quality Control and Product Formulation of the Crude Ethanolic Extract of Tinospora rumphii Boerl (Menispermaceae) Stems. Manila, Philippines: MS Pharmacy Thesis. UST Graduate School. 2006:60–75. 201–10. [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Du Vivier A. 3rd ed. London: Churchill Livingstone; 2002. Atlas of Clinical Dermatology; pp. 331–6. [Google Scholar]
- 23.Meinking T, Burkhart CN, Burkhart CG. Mosby: London; 2003. Dermatology; pp. 1321–4. [Google Scholar]
- 24.Chouela EN, Abeldano AM, Pellerano G, La Forgia M, Papale RM, Garsd A, et al. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the treatment of human scabies. Arch Dermatol. 1999;135:651–5. doi: 10.1001/archderm.135.6.651. [DOI] [PubMed] [Google Scholar]
- 25.Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767–74. doi: 10.1016/S0140-6736(06)68772-2. [DOI] [PubMed] [Google Scholar]
- 26.Reyes JM, King-Ismael D, Llamasares A, Ochoa MT. Manila, Philippines: UST Department of Dermatology; 2008. Phase I And Phase II Clinical Trial On The Efficacy And Safety Of Tinospora Rumphii Boerl. (Makabuhay) Lotion For The Treatment Of Scabies; pp. 20–50. [Google Scholar]
- 27.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in-vitro and in vivo. Cancer Lett. 2004;203:127–37. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Ivanovska N, Philipov S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int J Immunopharmacol. 1996;18:553–61. doi: 10.1016/s0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 29.Kupeli E, Kosar M, Yesilada E, Baser K. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72:645–57. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 30.Feng AW, Yu C, Mao Q, Li N, Li QR, Li JS. Berberine hydrochloride attenuates cyclooxygenase-2 expression in rat small intestinal mucosa during acute endotoxemia. Fitoterapia. 2011;82:976–82. doi: 10.1016/j.fitote.2011.05.013. [DOI] [PubMed] [Google Scholar]


