Abstract
Cancer, one of the leading causes of death worldwide is estimated to increase to approximately 13.1 million by 2030. This has amplified the research in oncology towards the exploration of novel targets. Recently there has been lots of interest regarding the hedgehog (Hh) pathway, which plays a significant role in the development of organs and tissues during embryonic and postnatal periods. In a normal person, the Hh signaling pathway is under inhibition and gets activated upon the binding of Hh ligand to a transmembrane receptor called Patched (PTCH1) thus allowing the transmembrane protein, smoothened (SMO) to transfer signals through various proteins. One of the newer drugs namely vismodegib involves the inhibition of Hh pathway and has shown promising results in the treatment of advanced basal-cell carcinoma as well as medulloblastoma. It has been granted approval by US Food and Drug Administration's (US FDA) priority review program on January 30, 2012 for the treatment of advanced basal-cell carcinoma. The drug is also being evaluated in malignancies like medulloblastoma, pancreatic cancer, multiple myeloma, chondrosarcoma and prostate cancer. Moreover various Hh inhibitors namely LDE 225, saridegib, BMS 833923, LEQ 506, PF- 04449913 and TAK-441 are also undergoing phase I and II trials for different neoplasms. Hence this review will describe briefly the Hh pathway and the novel drug vismodegib.
Keywords: Advanced basal-cell carcinoma, hedgehog pathway, medulloblastoma
INTRODUCTION
Cancer, one of the leading causes of death in both developed and developing countries, account for nearly 13% of all deaths. In 2008, the global cancer-related mortality was found to be 7.6 million and was estimated to increase to approximately 13.1 million in 2030.[1] Hence there is a need for newer treatment strategies with novel drugs acting at different pathways for treatment of malignancies and there has been a tremendous pressure on the healthcare researchers and pharmaceutical industries to explore newer approaches for the treatment of cancer. The newer targets being investigated in cancer therapy include mammalian target of rapamycin (mTOR) signaling, epidermal growth factor receptor (EGFR) pathway, transforming growth factor–β (TGF-β) signaling, nuclear factor κB (NFκB), Ras etc.[2] In the last few years, there has been a growing attention towards a distinct newer target namely Hedgehog (Hh) pathway. The aberrant signaling in Hh pathway has been found to be associated with the occurrence of basal-cell carcinoma (BCC), medulloblastoma, multiple myeloma, breast cancer, pancreatic cancer, glioma and lymphoma.[3–7] Currently a newer drug, vismodegib acting through inhibition of the Hh pathway has been approved by the US Food and Drug Administration's (US FDA) priority review program on January 30, 2012, for the treatment of advanced form of basal-cell carcinoma.[8,9] This has further accelerated the focus towards the novel targets for cancer therapy and this review will discuss briefly on the newer Hh pathway inhibitor vismodegib.
The hedgehog pathway was first recognized in mutant fruit flies by Christiane Nusslein and Eric Wieschaus in 1980. It was named after the larvae of mutant fruit fly, which resembled hedgehogs and the discovery was awarded Nobel Prize for physiology or medicine in 1995.[10] The Hh pathway plays a significant role in the development of organs and tissues during embryonic and postnatal periods. It is involved in determining the site of growth of fingers in the limb buds and the formation of motor neurons in the developing spinal cord.[11] Under physiological conditions, the Hh signaling pathway is under inhibition and gets activated upon the binding of Hh ligand to a transmembrane receptor called Patched (PTCH1) as shown in Figure 1. The activation of Hh pathway allows the transmembrane protein, smoothened (SMO) to transfer signals through various proteins.[4] The abnormal Hh signaling resulting in new tumors or acceleration of existing tumors could be due to mutation in the Hh pathway, paracrine or autocrine activation by Hh ligands, involvement of stroma or activation of cancer stem cells (CSCs).[6,12] It has been hypothesized that the activation of cancer stem cells (CSCs) through abnormal Hh signaling could be a reason for uncontrolled self-renewal of tumor cells and the occurrence of metastasis. Among the various cancers, studies have shown the significance of mutations in PTCH leading to the pathogenesis of sporadic BCCs and medulloblastoma.[13,14]
Figure 1.
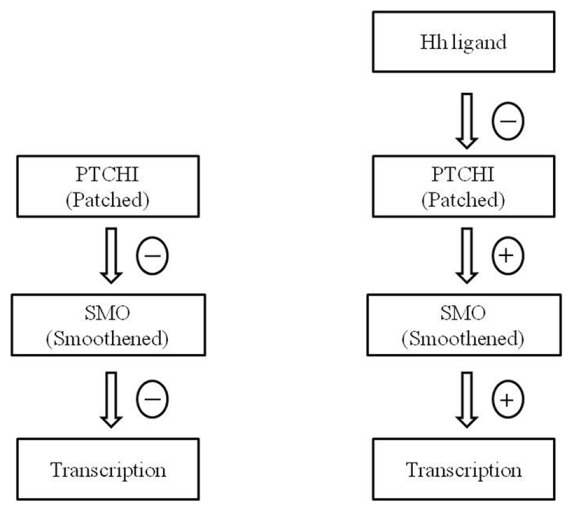
Schematic diagram explaining the mechanism of action of Hedgehog (Hh) pathway. PTCH1 - Patched 1, transmembrane receptor; SMO - smoothened, transmembrane protein; Hh - Hedgehog. (a) shows that during normal conditions, PTCH1 keeps SMO under repression. (b) shows that Hh ligand binds to PTCH1 receptor and inhibits its inhibitory effect on SMO, thus promoting the signal transduction via Hh pathway
The discovery of cyclopamine, a natural product known to bind SMO protein, resulting in the inhibition of Hh signaling, opened a new pathway in the designing and discovery of Hh pathway inhibitors.[15,16] Presently vismodegib, a novel small molecule Hh inhibitor, which acts by inhibiting SMO protein involved in Hh signal transduction has shown promising anti-tumor responses in advanced BCC and has been approved by the US FDA for the same.[7,17]
Efficacy
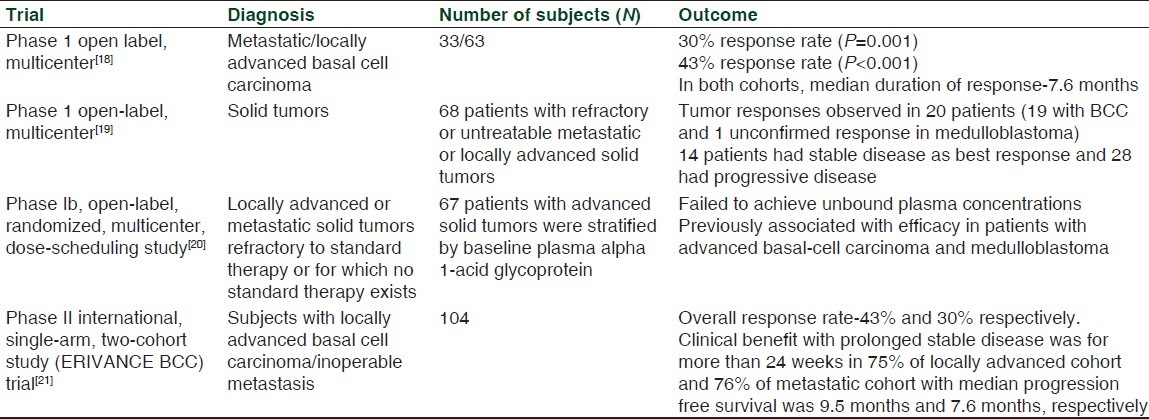
Some of the clinical trials done in advanced cancer patients with vismodegib are shown in Table 1.[18–21] In an open label, multicenter, two-stage phase I trial, vismodegib was found to have acceptable safety profile and anti-tumor activity in 68 patients with advanced BCC and medulloblastoma. Similarly, another multicenter clinical trial among 96 patients with locally advanced or metastatic BCC showed 30% partial response in patients with metastatic disease and 43% complete or partial response in patients with locally advanced disease, thus demonstrating the safety and efficacy of vismodegib. In a phase 2 study involving 41 patients with basal-cell carcinoma, vismodegib group was found to have only four new tumors over a period of one year compared to 24 new tumors in the placebo group. The same study also demonstrated shrinkage of existing skin lesions in patients receiving vismodegib, while the placebo group had modest growth. A phase II international, single-arm, two-cohort study (ERIVANCE BCC) trial with vismodegib on subjects with inoperable metastatic or locally advanced basal-cell carcinoma achieved the primary endpoint of overall response rate as assessed by an independent review. This interim analysis revealed that at a dose of 150 mg given orally once daily till disease progression or toxicity resulted in an overall response rate of 43% and 30% in locally advanced and metastatic cohorts, respectively. The analysis also showed prolonged stable disease for more than 24 weeks in 75% of the locally advanced cohort and 76% of the metastatic cohort. The study subjects in advanced and metastatic cohorts were found to have 9.5 months and 7.6 months of median progression free survival, respectively.[20] Based on the trials, vismodegib was approved on January 30, 2012, by US FDA's priority review program and is the first FDA-approved drug to be used in advanced form of basal-cell carcinoma.[22]
Table 1.
Pharmacokinetics
Vismodegib is absorbed orally and its absorption is not interfered by food. It has high affinity to human serum albumin (HSA) and alpha-1-acid glycoprotein (AAG). The drug has solubility limited absorption with slow elimination and is excreted as an unchanged product. Vismodegib gets metabolized to several minor metabolites by multiple cytochrome P450 (CYP) enzymes and is a substrate for CYP2C9, CYP3A4 and P-glycoprotein (P-gp). The drug has not shown any alteration with CYP inhibitors but following co-administration with P-gp inhibitors like clarithromycin, erythromycin, azithromycin etc., there is a higher chance of adverse drug reactions with vismodegib. The drug may have reduced bioavailability with proton pump inhibitors, H2-receptor blockers and antacids, owing to less solubility at a higher pH.[23]
Indications
Vismodegib (ERIVEDGE™), a Hh pathway inhibitor, has been approved by US FDA for the treatment of adults with metastatic basal-cell carcinoma or with locally advanced basal-cell carcinoma that has recurred following surgery or in those patients who are not candidates for surgery or radiation. The recommended dose of vismodegib is 150 mg given once daily through oral route. The drug has to be prescribed in the above-mentioned disease states until the disease progresses or unacceptable toxicity occurs.
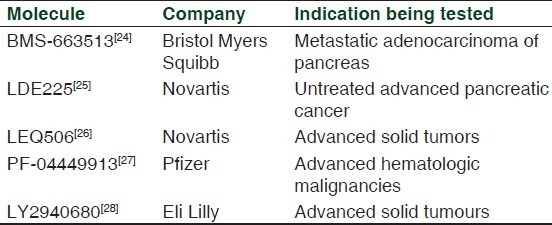
Vismodegib has not been approved by other regulatory agencies so far. It is being evaluated in phase II trials for operable basal-cell carcinoma. Disappointing results seen with vismodegib in ovarian and colorectal cancers have not limited its manufacturer Genentech to expand its clinical development in other areas. The drug is being tested in malignancies such as medulloblastoma, pancreatic cancer, multiple myeloma, chondrosarcoma and prostate cancer. Currently, various Hh inhibitors namely LDE 225, saridegib, BMS 833923, LEQ 506, PF-04449913 and TAK-441 are being evaluated in phase I and II trials for different neoplasms as shown in Table 2.[24–29] However, saridegib, another Hh inhibitor failed to improve overall response rate in patients with pancreatic carcinoma. The fact that Hh inhibitors have shown varied response in different malignancies makes it clear that the Hh pathway does not have the same role in all cancers.[30]
Table 2.
Adverse effects
The most common adverse events reported from clinical trials with vismodegib include muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea and decreased appetite. All these adverse events were seen in 20 to 40% of patients. CTCAE (Common Terminology Criteria for Adverse Events) Grade 4 events experienced by the trial patients included hyponatremia, pyelonephritis and presyncope. Hyponatremia and fatigue were reversible on temporary discontinuation of the drug.[16] As with all chemotherapeutic agents, acquired mutations in SMO receptor can result in resistance to vismodegib.[31]
Special situations
Vismodegib is considered as a category D drug and carries a boxed warning stating that the drug can cause fetal death or severe birth defects during pregnancy. Hence pregnancy needs to be ruled out at least 7 days before starting treatment, and both male and female patients need to be informed regarding the risk. Advice needs to be given about the need for contraception in female patients and the potential risk for vismodegib exposure through semen in male patients.
In lactating mothers, the decision to continue the drug during lactation should be based on the disease status of lactating mother and the risk benefit ratio. It should not be prescribed in children as the safety and effectiveness of the drug has not been established in this age group. In addition, patients treated with vismodegib should be advised not to donate blood or blood products while receiving the drug and for at least 7 months after the last dose.[32]
CONCLUSION
Targeting the Hh pathway appears to be a promising strategy in cancer chemotherapy with the approval of vismodegib. Hh pathway inhibitors could become a boon in the future for the treatment of various chemoresistant tumors. Although vismodegib has shown encouraging results in early clinical studies that has led to its accelerated approval for advanced BCC, the long-term safety and efficacy in larger populations need to be proven using well-designed robust phase 3 clinical trials, if it has to remain a formidable weapon against aggressive tumors in the long run. Besides vismodegib, other new molecules that inhibit Hh pathway at several distinct sites are being tested in number of solid tumors and hematologic malignancies, most of which are in early phases of clinical development. Since Hh signaling is also essential for the viability of various normal stem cells like bone marrow and skin, the concerns of major toxicity are not unfounded and warrant intensive monitoring.[33] Hence regulatory authorities need to carefully review trials with such group of drugs before taking any decision regarding the approval of Hh pathway inhibitors. Nevertheless, Hh pathway inhibitors may offer a ray of hope to those battling with advanced malignancies with a dismal prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cancer. [Last accessed on 2012 July 25]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009;1:6. doi: 10.1186/1758-3284-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy S, Ingham PW. Hedgehogs tryst with the cell cycle. J Cell Sci. 2002;115:4393–7. doi: 10.1242/jcs.00158. [DOI] [PubMed] [Google Scholar]
- 4.Kudchadkar R, Lewis K, Gonzalez R. Advances in the treatment of Basal cell carcinoma: Hedgehog inhibitors. Semin Oncol. 2012;39:139–44. doi: 10.1053/j.seminoncol.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Ailles L, Siu LL. Targeting the Hedgehog pathway in cancer: Can the spines be smoothened? Clin Cancer Res. 2011;17:2071–3. doi: 10.1158/1078-0432.CCR-11-0211. [DOI] [PubMed] [Google Scholar]
- 6.Merchant AA, Matsui W. Targeting Hedgehog-a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vismodegib approved by US FDA for treatment of basal cell carcinoma. [Last accessed on 2012 April 29]. Available from: http://www.medwirenews.md/46/97315/Oncology/Vismodegib_approved_by_US_FDA_for_treatment_of_basal_cell_carcinoma.html .
- 9.FDA approval for vismodegib. [Last accessed on 2012 April 29]. Available from: http://www.cancer.gov/cancertopics/druginfo/fda-vismodegib .
- 10.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12(20 Pt 1):5924–8. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 12.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–4. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 13.Reifenberger J, Wolter M, Knobbe CB, Köhler B, Schönicke A, Scharwächter C, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MD, Liu L, Raffel C, Hui C, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 15.Warzecha J, Dinges D, Kaszap B, Henrich D, Marzi I, Seebach C. Effect of the Hedgehog-inhibitor cyclopamine on mice with osteosarcoma pulmonary metastases. Int J Mol Med. 2012;29:423–7. doi: 10.3892/ijmm.2011.851. [DOI] [PubMed] [Google Scholar]
- 16.Gould A, Missailidis S. Targeting the hedgehog pathway: The development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev Med Chem. 2011;11:200–13. doi: 10.2174/138955711795049871. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 18.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorusso PM, Jimeno A, Dy G, Adjei A, Berlin J, Leichman L, et al. Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:5774–82. doi: 10.1158/1078-0432.CCR-11-0972. [DOI] [PubMed] [Google Scholar]
- 21.Interim results of phase II trial of vismodegib. [Last accessed on 2012 April 29]. Available from: http://www.centerwatch.com/clinical-trials/results/new-therapies/nmt details.aspx?CatID=37 .
- 22.Guha M. Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat Rev Drug Discov. 2012;11:257–8. doi: 10.1038/nrd3714. [DOI] [PubMed] [Google Scholar]
- 23.Graham RA, Lum BL, Cheeti S, Jin JY, Jorga K, Von Hoff DD, et al. Pharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: The role of alpha-1-acid glycoprotein binding. Clin Cancer Res. 2011;17:2512–20. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A Follow-up Evaluation for Safety in Subjects Who Participated in a Phase 1 Study With BMS-986094 (INX-08189) [Last accessed on 2012 July 26]. Available from: http://www.clinicaltrials.gov/ct2/show?term=BMS+-833923 and rank=12 .
- 25.LDE225 with Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan for Untreated Advanced Pancreatic Cancer. [Last accessed on 2012 July 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01485744?term=Hh+pathway+inhibitor+in+cancer and rank=3 .
- 26.A Dose Finding and Safety Study of Oral LEQ506 in Patients With Advanced Solid Tumors. [Last accessed on 2012 July 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01106508?term=leq506 and rank=1 .
- 27.A Study of PF-04449913 In Select Hematologic Malignancies. [Last accessed on 2012 July 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00953758?term=PF-04449913 and rank=3 .
- 28.A Study in Advanced. [Last accessed on 2012 July 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01226485?term=LY2940680 and rank=1 .
- 29.Allison M. Hedgehog hopes lifted by approval.and stung by failure. Nat Biotechnol. 2012;30:203. doi: 10.1038/nbt0312-203. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Su X, Xie J. Activation of Hedgehog pathway in gastrointestinal cancers. Vitam Horm. 2012;88:461–72. doi: 10.1016/B978-0-12-394622-5.00020-1. [DOI] [PubMed] [Google Scholar]
- 31.Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erivedge [Package insert] South Sanfranciso, CA: Genentech Inc; 2012. [Google Scholar]
- 33.Garber K. Hedgehog drugs begin to show results. J Natl Cancer Inst. 2008;100:692–7. doi: 10.1093/jnci/djn169. [DOI] [PubMed] [Google Scholar]


