Sir,
Drug-induced liver injury is a major health problem. This is responsible for 50% of all acute liver failures.[1] The developing countries are facing difficulties in the prevention and management of anti-tuberculosis drugs induced hepatotoxicity. The standard first line drugs used for tuberculosis therapy such as isoniazid and rifampicin are potentially hepatotoxic (2.6%) and lead to deaths from liver necrosis.[2] In the absence of satisfactory drugs to cure liver diseases in the conventional medicine, herbal drugs are used to treat liver diseases.
Thespesia lampas Dalz. & Gibs (Malvaceae) is a traditional medicinal plant used in the treatment of liver disorders in India. While studying the hepato-protective principles of this plant, among other things, we have observed high concentration of ellagic acid in the alcohol extract of the plant root.
Ellagic acid is known to have anti-carcinogenic and anti-oxidant properties.[3] It is used as a food additive for its antioxidant property. Animal experiments have shown its protective action against liver toxicity induced by carbon tetrachloride,[3] paracetamol[4] and cisplatin (a chemotherapeutic agent).[5] In a sub-chronic toxicity study using rats, no toxic symptoms were observed, when ellagic acid (up to 5%) was included in the diet.[6] Thus it appears that this compound is useful to abrogate drug induced hepatic damages. In this connection, further studies are useful to establish its hepato-protective activity against different drugs which induce liver damage and to carry out clinical trials. Therefore, we have studied the effect of ellagic acid on liver damage induced by anti-tuberculosis drugs, isoniazid and rifampicin in rats.
Isonicotinic acid hydrazide and rifampicin were purchased from Sigma chemicals, Bangalore. Silymarin was obtained from IPCA labs, Madhya Pradesh. Either gender of albino rats of Wistar strain weighing 100 – 150 g were used for the study. They were maintained under standard conditions and fed with standard pellet diet (SaiDurga Feeds and Foods, Bangalore, India) and water ad-libitum. The protocol was approved by Institutional Animal Ethics Committee constituted as per the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals).
The plant roots were collected from Attapadi hills in Western Ghats located in Palakkad district in the month of June 2006. Ethanol extract of the plant root powder was prepared as described.[7] The yield was 4.4% of root powder. Ellagic acid was isolated from the extract. Briefly, ethanol extract (20 g) on column chromatography (500 × 30 mm) using Merck silica gel (60-120 mesh) and eluting with increasing polarity of solvents (petroleum ether, chloroform, ethyl acetate and methanol) in different ratios, yielded several sub fractions (50 fractions). Again, fractions 11-15 was subjected to column chromatography and eluted with (10 ml each) methanol: ethyl acetate (10:90; 15:85; 20:80; 25:75; 30:70). This yielded ellagic acid which moved as a single band (Rf: 0.65) on silica gel TLC (solvent system: toluene-ethyl acetate-formic acid, 1:2:1 v/v) and the yield was 0.15% of the root powder. It was obtained as a creamy light yellow crystalline powder with a melting point of 317°C and identified by IR, NMR and mass spectra. Figure 1 shows chemical structure of ellagic acid.
Figure 1.
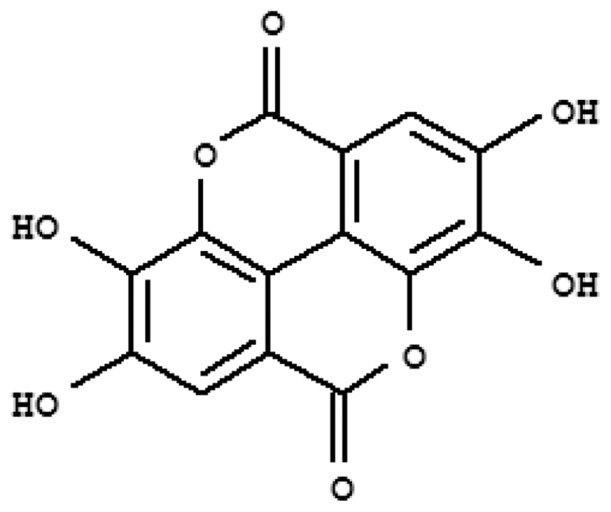
Chemical structure of ellagic acid isolated from Thespesia lampas
Hepatoprotective activity of ellagic acid on isoniazid-rifampicin-induced hepatic damage was evaluated in rats.[7] Briefly, 30 healthy albino rats of either sex, weighing 100-150 g, were randomly divided into five groups of six animals each. Normal control (group 1) rats received orally pure water and drug control (group 2) animals were injected (i. p.) with isoniazid and rifampicin (each at a dose of 50 mg/kg dissolved in sterile distilled water) for 15 days. The positive control (group 3) rats received silymarin (50 mg/kg/day) orally 1hr prior to injection (i. p.) of isoniazid and rifampicin for 15 days. Similarly, test groups (groups 4 and 5) received ellagic acid (EA) 25 and 50 mg/kg respectively, orally 1hr prior to isoniazid and rifampicin administration. The rats were sacrificed on the fifteenth day by cervical dislocation and blood was collected by ocular puncture of retro orbital plexus and serum was separated by centrifugation and used for estimation of biochemical parameters. Liver pieces were fixed in buffered formalin for histological studies. Commercial kits from SPAN Diagnostics Ltd, Surat, India were used to measure the hepatic marker enzymes and serum bilirubin.
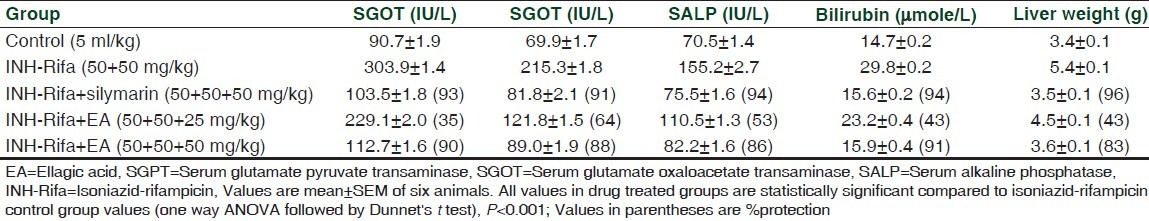
As shown in Table 1, isoniazid-rifampicin induced severe liver damage in rats as evidenced from increased liver weight, elevated levels of serum markers for liver damage (transaminases, alkaline phosphatase and bilurubin). Ellagic acid showed concentration dependent hepato-protection against the anti-tuberculosis drugs-induced liver damage. Ellagic acid (50 mg/kg) showed better activity compared to 25 mg/kg dose. The efficacy of 50 mg/kg ellagic acid was found to be almost comparable to that of the standard herbal drug silymarin (50 mg/kg). Histopathological pictures confirmed the hepatoprotective activity of ellagic acid (Figures not shown).
Table 1.
Effect of ellagic acid on isoniazid-rifampicin-induced liver injury in rats
Elagic acid was isolated for the first time in a pure form from T. lampas. The hepato-protective efficacy of ellagic acid against isoniazid-rifampicin induced liver damage in rats was comparable to that of silymarin, a commonly used hepato-protective fraction from Sylibum marianum.
Ellagic acid is a natural phenolic compound found in numerous fruits and vegetables including strawberries and pomegranates. Experiments on animals point out its several health benefits[3] as well as non-toxic nature even at relatively high concentrations.[7] Therefore, ellagic acid may be used in combination with isoniazad-rifampicin (first-line therapy for tuberculosis) in the treatment of tuberculosis to prevent liver damage.
In light of the above points, clinical studies are warranted to determine the likely use of ellegic acid as an attractive alternative medicine to silymarin to prevent the drug induced liver damage. However, anti-oxidant interactions of catechin, caffic acid and ellagic acid on human low density lipoprotein (LDL) oxidation has been reported.[8] So drug interaction studies may have to be done.
T. lampas root contains another unidentified major active principle also (unpublished observations of the authors). So, an active fraction from this plant can also be considered as an effective and safe medicine against drug induced liver damage.
ACKNOWLEDGEMENT
The authors are thankful to Dr. Rangamoopan Vaithyan, Goolikadavu in Attapadi hills in Kerala for his help in plant collection, Dr. Mathew Dan for authentication of plant specimens and Indian Institute of Technology, Chennai for recording Spectra.
REFERENCES
- 1.Mumoli N, Cei M, Cosimi A. Drug-related hepatotoxicity. N Engl J Med. 2006;354:2191–3. [PubMed] [Google Scholar]
- 2.Tasduq SA, Peerzada K, Koul S, Bhat R, Johri RK. Biochemical manifestation of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol Res. 2005;31:132–5. doi: 10.1016/j.hepres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Vattem DA, Shetty K. Biological function of ellagic acid: A review. J Food Biochem. 2005;29:234–66. [Google Scholar]
- 4.Girish C, Koner BC, Jayanthi S, Ramachandra Rao K, Rajesh B, Pradhan SC. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol. 2009;23:735–45. doi: 10.1111/j.1472-8206.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 5.Yuce A, Atessahin A, Ceribasi AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007;101:345–9. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 6.Tasaki M, Umemura T, Maeda M, Ishii Y, Okamura T, Inoue T, et al. Safety assessment of ellagic acid, a food additive, in a sub-chronic toxicity study using F344 rats. Food Chem Toxicol. 2008;46:1119–24. doi: 10.1016/j.fct.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Subramoniam A, Evans DA, Rajasekharan S, Pushpangadan P. Hepatoprotective activity of Trichopus zeylanicus extract against paracetamol-induced hepatic damage in rats. Indian J Exp Biol. 1998;36:385–9. [PubMed] [Google Scholar]
- 8.Meyer AS, Heinonen M, Frankel EN. Anti-oxidant interactions of catechin, cyaniding, caffeic acid, quercetin and ellagic acid on human LDL oxidation. Food Chem. 1998;6:71–5. [Google Scholar]


