Abstract
A 75-year-old man was diagnosed as having pancreatic ductal carcinoma containing remarkable lymphocytic and plasma cell infiltration, as revealed by the cytological examination of endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) specimen. The EUS-FNA specimen showed small amounts of atypical epithelium with noticeable lymphocytes and plasma cells. A pancreatic resection was performed, and the histopathological features showed an invasive pancreatic ductal carcinoma with autoimmune pancreatitis (AIP) lymphoplasmacytic sclerosing pancreatitis (LPSP)-like lesions. Most of the plasma cells were immunoreactive to anti-IgG4 antibody. EUS-FNA may be necessary for the differential diagnosis of AIP and pancreatic cancer, and close attention should be given to the presence of marked lymphoplasmacytic cells in EUS-FNA specimens while making the diagnosis.
Keywords: Adenocarcinoma, autoimmune pancreatitis, endoscopic ultrasound guided fine-needle aspiration, IgG4
Introduction
Autoimmune pancreatitis (AIP) and pancreatic cancer have recently been reported.[1–5] In such cases, cytological examination can be difficult due to sclerosis, inflammation and reactive atypia in the corresponding tissue. In cytological examinations, some authors have reported that when a smear preparation exhibits a predominance of inflammatory cells (lymphocytes and plasma cells) with sparse epithelial cells lacking atypia, a cytological diagnosis of AIP may be possible.[6]
Here, we report, a case of invasive pancreatic ductal carcinoma with marked lymphoplasmacytic infiltration. Although ductal cancer was suggested based on endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) cytology findings, other medical examinations indicated chronic pancreatitis or AIP.
Case Report
A 75-year-old man without a history of malignancy was referred to our institution because of weight loss and a mass lesion in the pancreas tail. The laboratory studies were unremarkable. The patient's CEA level was elevated (12.7 ng/mL; normal, 0-6 ng/mL), but the CA 19-9 level was within the normal range (25.6 U/mL; normal, 0-37.0 U/mL). No increase was noted in the serum amylase level (51 IU/L; normal value, 25-120 IU/L) or the lipase level (22 U/L; normal value, 13-49 U/L). Dynamic-enhanced computed tomography (CT) imaging showed a mass measuring 20 mm in the tail of the pancreas with no dilation of the main pancreatic duct, anomalous arrangement of the pancreaticobiliary ducts, or obstructive jaundice. Endoscopic retrograde cholangiopancreatography (ERCP) revealed a narrowing of the main pancreatic duct at the tail. A cytology analysis of the pancreatic juice was negative for tumor cells. These results suggested that the mass lesion in the pancreas was caused by chronic pancreatitis or autoimmune pancreatitis, rather than a pancreatic carcinoma. Further laboratory tests showed a normal IgG level (1100 mg/mL; normal, 800-1800 mg/mL), a normal IgG4 level (67.0 mg/mL; normal, 4-180 mg/mL) and the absence of antinuclear antibody. The EUS-FNA specimens showed a mixture of abnormal columnar epithelia and abundant lymphocytes. A diagnosis of pancreas cancer was made, and a pancreatosplenectomy was performed.
Cytological and histopathological findings
Smear preparations of the EUS-FNA materials from the pancreas tumor were stained using Diff-Quik solution. Cytoblocks were made by allowing the aspirated materials to clot in 10% buffered formalin, followed by treatment in alginic acid. The smear preparations showed numerous lymphocytes including plasma cells with necrotic material [Figure 1a] and small clusters of atypical epithelial cells [Figure 1b]. The atypical cells had a high nuclear to cytoplasmic ratio; the cells were hyperchromatic and contained prominent nucleoli. The Papanicolaou-stained smear preparations and the cytoblock preparations showed similar findings. In the cytoblock specimens, some plasma cells were immunoreactive to anti-IgG4 antibody.
Figure 1.
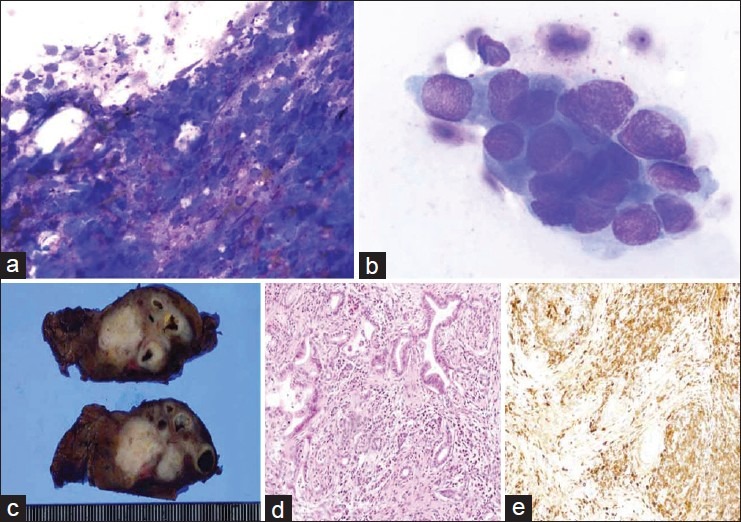
(a) Numerous lymphocytes and plasma cells with necrotic material and fibrosis are visible (Diff-Quik, ×400); (b) Atypical epithelial cells (Diff-Quik, ×1000); (c) The cut surface of the resected specimen show an elastic, hard, white mass that was located in the pancreas tail; (d) Ductal adenocarcinoma with lymphoplasmacytic infiltration was visible (H and E, ×100); (e) Immunohistochemical findings showed abundant IgG4-positive plasma cells around the pancreatic duct and cancer cells (IHC, ×100)
The cut surface of the resected pancreas showed an elastic, hard, white mass in the pancreas tail [Figure 1c]. In the histopathologic examination, intralobular and interlobular fibrosis was observed, with numerous lymphocyte and plasma cell infiltrations. No granulocytic epithelial lesions were found, so these histological features were compatible with the characteristics of type 1 autoimmune pancreatitis-lymphoplasmacytic sclerosing pancreatitis (Type 1 AIP-LPSP). Invasive ductal adenocarcinoma with lymphoplasmacytic infiltration was also observed in these lesions [Figure 1d]. Immunohistochemical staining, revealed abundant IgG4-positive plasma cells (>50 cells per high-power field [HPF]) around the pancreatic duct and tumor cells [Figure 1e]. The pathological diagnosis was moderately differentiated tubular adenocarcinoma with abundant lymphoplasmacytic infiltration and AIP-LPSP features.
Discussion
In the current report, we were able to make a correct diagnosis of pancreatic carcinoma based on the presence of atypical epithelial cells in EUS-FNA specimens with concomitant AIP-LPSP features.
The differentiation of pancreatic ductal adenocarcinoma from focal chronic pancreatitis, such as autoimmune pancreatitis, is particularly difficult in some cases. Indeed, although AIP can be well controlled with corticosteroids, some patients undergo a pancreatic resection. This diagnostic and management dilemma has been discussed in detail. Several reports of criteria for distinguishing AIP from pancreas ductal adenocarcinoma have been made. The proposed criteria for diagnosing AIP include radiological studies, laboratory examinations, histopathological features, and response to steroid therapy.[7–9] Because a definitive diagnosis of AIP, based on its characteristic histopathology, can only be made using surgically resected tissues, establishing preoperative criteria for AIP using biopsy specimens has not been practical.
Some recent reports of pancreas adenocarcinoma associated with AIP have been made.[1,4,5] Moreover, Kamisawa et al.,[10] reported the possibility that pancreatic cancer may develop in AIP patients. They proposed that K-ras mutations may be frequently detected in AIP patients, and AIP may be a risk factor for gastric and colonic cancer.
EUS-FNA cytology improves the diagnosis of pancreatic masses, providing a sensitivity of 80%-90%, a specificity of 95%-100%, and an accuracy of 90%-95%.[9] EUS-FNA cytology is often a helpful indicator for determining therapeutic strategies for patients with pancreatic masses, possibly leading to either resection or careful observation in patients in whom diagnosis, resectability, or operability are in question. In our case, the radiology findings suggested either AIP or chronic pancreatitis, and only the EUS-FNA cytology findings indicated pancreatic adenocarcinoma. Thus, EUS-FNA was a very useful tool for the preoperative differential diagnosis of pancreatic adenocarcinoma and AIP.
If patients partially meet the criteria for the diagnosis of AIP and/or the mass lesion in the pancreas remains unchanged during steroid therapy, resection should be considered because of the possibility of pancreatic cancer.[4] Even if the cytological findings are negative for malignancy and the patient is diagnosed as having AIP, a possible association with adenocarcinoma should be considered. Indeed, our EUS-FNA findings showed numerous lymphocytes and plasma cells with associated necrosis. These findings seemed to indicate AIP, but atypical epithelial cells were present; thus, we were able to diagnose the patient as having pancreatic ductal adenocarcinoma accurately. EUS-FNA findings may be useful in guiding therapeutic management and may prevent the misdiagnosis of pancreatic tumors. EUS-FNA cytology should be further improved to enable pancreatic carcinomas to be distinguished from AIP, thereby preventing opportunities for potentially curative resections from being lost while avoiding unnecessary surgical interventions for those with AIP.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Inoue H, Miyatani H, Sawada Y, Yoshida Y. A case of pancreas cancer with autoimmune pancreatitis. Pancreas. 2006;33:208–9. doi: 10.1097/01.mpa.0000232329.35822.3a. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, et al. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006;12:6225–8. doi: 10.3748/wjg.v12.i38.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghazale A, Chari S. Is autoimmune pancreatitis a risk factor for pancreatic cancer? Pancreas. 2007;35:376. doi: 10.1097/MPA.0b013e318073ccb8. [DOI] [PubMed] [Google Scholar]
- 4.Witkiewicz AK, Kennedy EP, Kennyon L, Yeo CJ, Hruban RH. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol. 2008;39:1548–51. doi: 10.1016/j.humpath.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Iida H, Kubota K, Mawatari H, Yoneda M, Goto A, Abe Y, et al. A case of autoimmune pancreatitis developed pancreatic tail cancer. J Jpn Pancreas Soc. 2008;23:608–4. [Google Scholar]
- 6.Kamisawa T, Imai M, Yui Chen P, Tu Y, Egawa N, Tsuruta K, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas. 2008;37:e62–7. doi: 10.1097/MPA.0b013e318175e3a0. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–63. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 8.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–53. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 9.Levy MJ, Wiersema MJ, Chari ST. Chronic pancreatitis: focal pancreatitis or cancer? Is there a role for FNA/biopsy? Autoimmune pancreatitis. Endoscopy. 2006;38:S30–5. doi: 10.1055/s-2006-946648. [DOI] [PubMed] [Google Scholar]
- 10.Kamisawa T, Horiguchi S, Hayashi Y, Yun X, Yamaguchi T, Tsuruta K, et al. K-ras mutation in the major duodenal papilla and gastric and colonic mucosa in patients with autoimmune pancreatitis. J Gastroenterol. 2010;45:771–8. doi: 10.1007/s00535-010-0211-y. [DOI] [PubMed] [Google Scholar]


