Abstract
Myoepithelioma of the breast is very rare. Breast myoepithelioma can develop in women from their early 20s right up to their 80s, but it is most common in women over 50. We report a case of 20-year-old female, who presented with a well-defined breast lump of 3 × 3 cm in size. Fine needle aspiration was performed. The cytological findings revealed good cellularity comprising monomorphic loosely cohesive sheets of plasmacytoid, round to polygonal cells with round to ovoid eccentrically placed nuclei, finely dispersed chromatin, and moderate amount of cytoplasm. On the basis of cytological findings, a diagnosis of benign myoepithelioma (plasmacytoid type) was made which was confirmed on histopathologic examination. The breast is a very rare localization for this type of tumor. The benign character of the disease in conjunction with its slow progression could delay its detection and diagnosis. A detailed pathology examination is a prerequisite for avoidance of misleading diagnosis.
Keywords: Breast, myoepithelioma, fine needle aspiration
Introduction
Myoepithelioma/adenomyoepithelioma is a rare salivary gland tumor arising from proliferation of myoepithelial cells. These tumors represent 1%-1.5% of all salivary gland tumors, and are distributed 48% in parotids, 42% in the small salivary glands and the remaining in glandula submandibularis and seromucous glands of the nose and larynx.[1] Other localizations reported are the skin, breast, chest, lung and pancreas.[1,2] Incidence of myoepithelioma in breast could not be ascertained due rarity of the lesion and paucity of literature on the subject with few case reports.
The combined proliferation of epithelial and myoepithelial cells is common with in breast, e.g., in papillomas and sclerosing adenosis. The myoepithelial proliferation in myoepithelioma is usually more marked than in other disorders. Although there are no agreed diagnostic criteria, it is common practice to restrict the diagnosis of myoepithelioma to cases falling outside the spectrum of well recognized and common benign conditions.[3]
Here, we present a case of myoepithelioma of breast diagnosed on cytopathology.
Case Report
A 20-year-old female presented to us with slowly growing breast lump for last 1 year, localized in upper outer quadrant. The lump was well defined, freely mobile and measured 3 × 3 cm in greatest dimension. The overlying skin was unremarkable. Axillary palpation was negative for lymph nodes.
Fine needle aspiration was performed. The cytological findings revealed cell rich smears comprising monomorphic loosely cohesive sheets of plasmacytoid cells, round to polygonal cells with round to ovoid eccentrically placed nuclei, finely dispersed chromatin, and moderate amount of cytoplasm. Overall findings suggested myoepithelioma, breast – plasmacytoid type [Figure 1].
Figure 1.
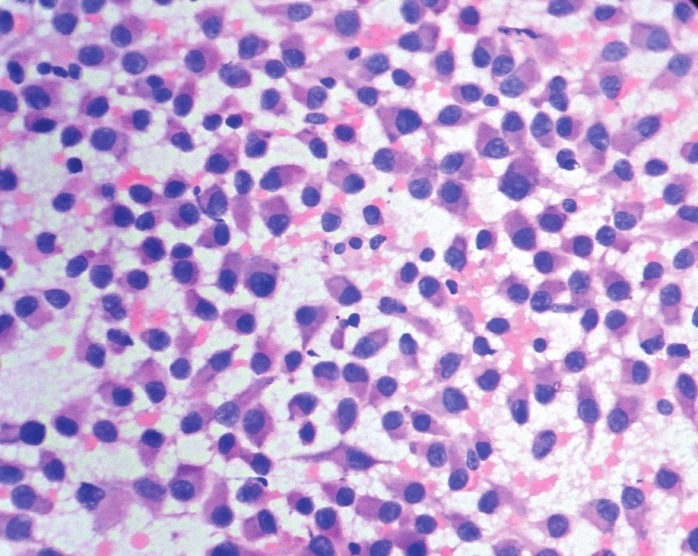
FNAC smears showing cells having eccentric nuclei and moderate amount of eosinophilic cytoplasm (H and E, ×500)
On the basis of above findings lumpectomy was done and the tissue was sent for histopathological examination. Grossly, the tissue was well circumscribed greyish white measuring 3 × 3 cm. Microscopic examination revealed tumor cells arranged in sheets with abundant myxoid stroma. The individual cells were round to polygonal, having prominent eosinophillic cytoplasm and eccentrically placed round nuclei. The diagnosis of myoepithelioma, plasmacytoid type was made on the basis of the above findings.
On immunohistochemistry cells were positive for S-100 protein and actin.
Discussion
Myoepithelioma of the breast is a very rare tumor, originating from the myoepithelial cells of normal duct lining of breast. The two cells types, epithelial and myoepithelial are found in the linings. The epithelial cell layer, which is right next to the open space or lumen of the duct, largely has a secretary function. The myoepithelial cells are further back, away from the lumen and are attached to the basement membrane.
The myoepithelial cells containing myofilaments in their cytoplasm show contractility, they support the parenchyma, and contribute to the production of laminin, collagen type-IV, and fibronectin to maintain the “basal lamina.”[4] The increasing myoepithelial cells are arranged in bound, nests, or mandel like structures. The presence of spindle cells, plasmacytoid, epithelioid, “clear” cells, or combinations determines the histological classification of the tumor, respectively.[2,5,6] Myoepitheliomas are slowly growing, well encapsulated, and defined masses.[1]
A large majority of myoepitheliomas are of benign character. Malignant tumors (10%) show a destructive-infiltrative growth pattern with necroses, rough chromatin, marked cellular pleomorphism, and high mitotic activity.[2,7]
Some cases could present unusual difficulties in macro and microscopic diagnosis. Electron microscopy could be of importance for the identification of cytoplasmic myofilaments and pinocytic vesicles.[4,7] Further immunohistochemical diagnostic assays include positive p63, S-100 protein, actin smooth muscle specific, cytokeratin 14, vimentin, and fibrous glycoproteins.[5]
The most frequent types of myoepithelioma are the plasmacytoid, and the spindle-cell form. Tubular and lobulated variants have also been described.[8] The tubular variety is indistinguishable from tubular adenoma. Electron microscopy and immunohistochemistry could help resolve the dilemma.[3]
Pure spindle cell myoepithelial tumors may resemble spindle cell carcinoma when the tumor cells are large and pleomorphic, leiomyoma, or fibromatosis if cells are small and regular. Distinction from leiomyoma rests on identification of epithelial lined luminal spaces.
Fibromatosis has a characteristically infiltrative pattern with uniform spindle cells and interlacing fascicles. Pure spindle cell myoepithelioma cells are usually larger and less uniform than those seen in fibromatosis.
Myoepithelial cells are plumper and often have eosinophilic cytoplasm in lobulated type. Cells are arranged in clusters which give lobular architecture.[3]
Plasmacytoid type of myoepithelioma has close resemblance with pleomorphic adenoma. Tumors which are devoid of epithelial ductular differentiation are labelled as myoepithelioma.[9]
The wide variety of patterns described in myoepithelial tumors is due to ability of myoepithelial cells to differentiate along different cell lines.[9] These patterns have led to a number of problems of distinction between other benign entities including papilloma, ductal adenoma, tubular adenoma, sclerosing adenosis, and complex sclerosing lesion. Distinction is arbitrary and rests on subjective opinion. It is common practice to restrict the diagnosis of myoepithelioma to cases falling outside the spectrum of well recognized and common benign conditions.[3]
The recommended treatment is complete surgical excision. Benign myoepithelioma can undergo malignant transformation, especially in long standing tumors with multiple recurrences.[10]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Koenigsberg RA, Vakil N, Noronha B. Undifferentiated metastatic carcinoma and myoepithelioma: two rare causes of hypervascular masses of the parapharyngeal space. Ear Nose Throat J. 2007;86:402–5. [PubMed] [Google Scholar]
- 2.Darvishian F, Lin O. Myoepithelial cell-rich neoplasms: Cytologic features of benign and malignant lesions. Cancer. 2004;102:355–61. doi: 10.1002/cncr.20642. [DOI] [PubMed] [Google Scholar]
- 3.Ellis IO, Pinder SE, Lee AH. Tumours of the breast. In: Fletcher CD, editor. Diagnostic histopathology of tumours. 3rd ed. Philadelphia: Churchill Livingstone; 2007. pp. 951–2. [Google Scholar]
- 4.Martínez-Madrigal F, Santiago Payán H, Meneses A, Domínguez Malagón H, Rojas ME. Plasmacytoid myoepithelioma of the laryngeal region: A case report. Hum Pathol. 1995;26:802–4. doi: 10.1016/0046-8177(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 5.Cuadra Zelaya F, Quezada Rivera D, Tapia Vazquez JL, Paez Valencia C, Gaitán Cepeda LA. Plasmacytoid myoepithelioma of the palate. Report of one case and review of the literature. Med Oral Patol Oral Cir Bucal. 2007;12:E552–5. [PubMed] [Google Scholar]
- 6.Dardick I, Thomas MJ, Van Nostrand AW. Myoepithelioma-new concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol. 1989;13:187–224. doi: 10.3109/01913128909057442. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim R, Bird DJ, Sieler MW. Malignant myoepithelioma of the larynx with massive metastatic spread to the liver: An ultrastructural and immunocytochemical study. Ultrastruct Pathol. 1991;15:69–76. doi: 10.3109/01913129109021305. [DOI] [PubMed] [Google Scholar]
- 8.Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15:554–68. doi: 10.1097/00000478-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Dardick I. Myoepithelioma: Definitions and diagnostic criteria. Ultrastruct Pathol. 1995;19:335–45. doi: 10.3109/01913129509021906. [DOI] [PubMed] [Google Scholar]
- 10.Ellis GL, Auclair PL. Fascicle 17. 3rd series. Washington DC: AFIP; 1996. Tumors of the salivary gland, Atlas of pathology; pp. 80–94. [Google Scholar]


