Abstract
Adenomatoid tumor is a benign neoplasm of the male and female genital tracts arising from mesothelial cells. Fine needle aspiration cytology (FNAC) plays a pivotal role in its preoperative diagnosis. Therefore, it is imperative that pathologists should be well aware of its cytological features so as to avoid erroneous diagnosis and hence prevent unnecessary surgical interventions. We hereby, present a case of adenomatoid tumor of testis in a 41 year male diagnosed by FNAC and later confirmed by histopathological examination.
Keywords: Adenomatoid tumor, cytology, testis
Introduction
Adenomatoid tumor (AT) is a benign neoplasm of mesothelial cell origin occurring both in the male and female genital tracts.[1] The most common site is epididymis in males.[2,3] Fine needle aspiration cytology (FNAC) plays very crucial role in its preoperative diagnosis. It is rapid and a conclusive diagnostic tool obviating unnecessary surgical interventions.[4] Few cases of AT diagnosed by FNAC have been recorded in the literature. Testis being an uncommon site, we hereby, report a case of AT of testis in a 41 year old male diagnosed preoperatively by FNAC and later confirmed by histopathology.
Case Report
A 41 year old male presented to our department with left sided scrotal swelling and pain for fifteen days. There was no history of trauma or surgery. Local examination revealed a firm, tender nodule 2.5 cm in diameter. The contralateral testis was normal. Clinical diagnosis of testicular neoplasm/epididymal cyst was made. Investigations revealed normal serum α-fetal protein, human chorionic gonadotropin and lactate dehydrogenase levels. Scrotal ultrasonography revealed 2.5 cm well defined, heterogeneous, hypo echoic solitary mass in the left testis. Guided aspiration was done using 10 mL syringe and 24 gauge needle. Scanty, grayish white aspirate was obtained. Smears were stained with hematoxylin and eosin (H and E), Papanicolaou and May–Grόnwald-Giemsa (MGG) stains.
Cytological findings
Moderately cellular smears consisted of tumor cells arranged in monolayered sheets and loosely cohesive clusters. Cells were round to oval with indistinct cell borders and moderate to abundant pale vacuolated cytoplasm. The monomorphic nuclei were eccentrically placed with fine granular chromatin and small inconspicuous nucleoli. Occasional binucleated cells were seen. Background showed pink amorphous material along with bare nuclei and few lymphocytes [Figure 1]. These cytological features suggested the diagnosis of AT. The patient underwent conservative testis sparing surgery, with excision of nodule.
Figure 1.
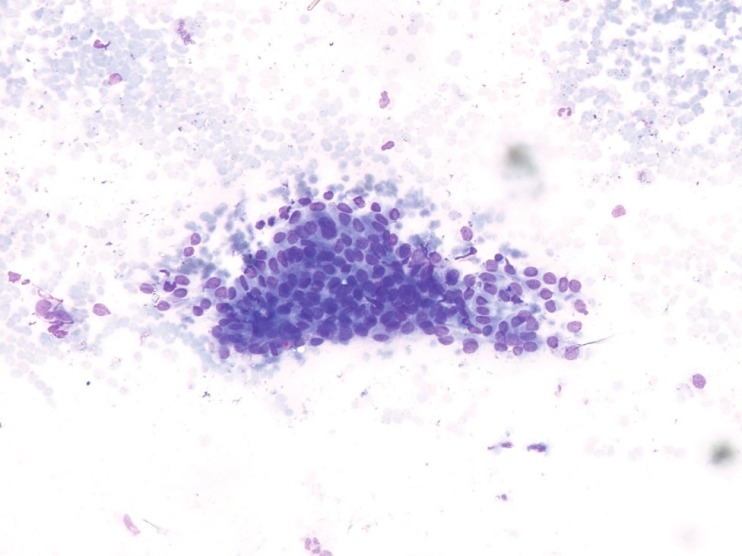
Photomicrograph of FNAC smear showing moderate cellularity with sheets of monotonous round to oval cells showing indistinct cell borders and moderate to abundant pale vacuolated cytoplasm. The nuclei are eccentric and monomorphic with fine granular chromatin and small inconspicuous nucleoli. Background shows bare nuclei and few lymphocytes (MGG, ×200)
Histopathological findings
Grossly, an encapsulated grayish white mass measuring 2.5 × 2 × 1.8 cm was obtained. Microscopic examination revealed cuboidal to flat epithelial cells in solid cords, tubules and cystic spaces, some showing cytoplasmic vacuolation. Intervening stroma showed fibrocollagenous tissue with sparse lymphocytic infiltrate. Epithelial cells showed periodic acid schiff (PAS) positivity, the features consistent with adenomatoid tumor.
Discussion
FNAC has become the preliminary preoperative diagnostic tool for all kinds of neoplastic processes, benign or malignant, in any organ or tissue of body, guiding towards a rational treatment. Advancements in FNAC for primary diagnosis of tumors in last 30 years have been enormous. Accuracy of the technique approaches that of histopathology in providing an unequivocal diagnosis. FNAC has an important role in the preoperative diagnosis of adenomatoid tumor and poses a diagnostic challenge for the pathologists, especially when the tumor arises from sites other than epididymis because they can be mistaken for intra-testicular tumors.
AT is rare benign neoplasm of male genital tract. In 1945, Godman and Ash[5] coined this term. Mostly AT presents as slow growing, small, firm, asymptomatic intrascrotal lump peaking in third to fifth decade of life.[3] AT of epididymis constitute 30% of paratesticular neoplasms.[3,6] In females it is seen in uterus, fallopian tube, ovary and paraovarian tissue.[7] Extra genital sites of involvement include heart, lymph node, adrenal gland, intestinal mesentery, omentum and retroperitoneum.[8] Histogenesis has been argued for years; proposed cells of origin being endothelial, mesothelial, mesonephric, coelomic, mullerian and epithelial cells. Evidence suggests mesothelial cell origin by immunohistochemical and ultra structural studies.[1,3,5] Cytological features of AT have been described very briefly in literature, with many textbooks on FNAC not even mentioning this entity. Initial cytological description of AT was described by Perez-Guillemro et al,[3] followed by few random case reports.[2,7,9] Smears contain sheets of epithelial cells and clusters of monomorphic cells with round or oval nuclei and inconspicuous nucleoli. Cytoplasm is clear and vacuolated in Papanicolaou stain and stains pink with Giemsa stain. Naked nuclei and fragments of stroma along with few lymphocytes can also be seen, features which are identical to those noted in our case.
Macroscopically, these tumors are firm, smooth, grayish white and usually less than 2 cm in size. Microscopically, tumor is composed of two major elements; epithelial like cells and fibrous stroma. The epithelial like cells are arranged in network of tubules, cords, channels and microcystic spaces. Fibrous stroma may be hyalinized and can contain smooth muscle. Immunohistochemically, they are strongly positive for high and low molecular weight cytokeratins, EMA and primary mesothelial monoclonal antibody HBME-1. Adenomatoid tumor of testis are positive for WT1 and Calretinin like all genital tract adenomatoid tumors as both of them are markers of mesothelial differentiation.[10]
Cytological differential diagnosis of AT include reactive mesothelial hyperplasia, papillary cystadenoma, spermatic granuloma, malignant meothelioma and metastatic adenocarcinoma.[3,7,9] Pathologists should be aware of the cytological features of such lesions, so as to avoid diagnostic pitfalls. Reactive mesothelial hyperplasias are associated with hydrocele and unlike ATs do not have definite cytological arrangement. Singly placed or small clusters of mesothelial cells show moderate anisonucleocytosis with moderate pale cytoplasm which can mimic malignancy. Nuclear groove, lobulation and mitosis may be seen.[7,9] Papillary cystadenomas show papillary structures of uniform round to polygonal cells without nuclear atypia; single isolated cells with cytoplasmic vacuoles in mucoid background. Psammoma bodies can be seen within papillary structures as well as in background.[11] Spermatic granuloma, tuberculous and chronic epididymitis are clinical differential diagnosis and can be ruled out by microscopy.[7] Malignant mesotheliomas show diffuse cell pattern. Individual cells show ruffled cell borders with dense cytoplasm. Unlike AT, mesotheliomas have complex architectural pattern, cellular and nuclear pleomorphism, macronucleoli, multinucleation and high mitotic index. Cellular crowding with compression of adjacent nuclei is seen in mesothelioma but not in adenomatoid tumor. Metastatic adenocarcinoma shows cytological features of malignancy and cells are positive for mucicarmine stain.[7] Treatment involves excision or enucleation of the tumor without orchidectomy. Complete excision of this benign tumor has good prognosis without recurrence.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mardi K, Sharma J. Cytodiagnosis of adenomatoid tumor of the epididymis: A case report. J Cytol. 2006;23:26–7. [Google Scholar]
- 2.Perez-Campos A, Jimenez-Hefferman JA, Perez F, Vicandi B. Cytologic features of paratesticular adenomatoid tumor. Acta Cytol. 2004;48:457–8. [PubMed] [Google Scholar]
- 3.Perez-Guillermo M, Thor A, Lowhagen T. Paratesticular adenomatoid tumors. The cytologic presentation in fine needle aspiration biopsies. Acta Cytol. 1989;33:6–10. [PubMed] [Google Scholar]
- 4.Kalyani R, Das S. Adenomatoid tumor: Cytological diagnosis of two cases. J Cytol. 2009;26:30–2. doi: 10.4103/0970-9371.54865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu K, Pai RR, Pai RC. Diagnosis of adenomatoid tumor by fine needle aspiration cytology. J Cytol. 1999;16:51–3. [Google Scholar]
- 6.Kolgesiz AI, Kantarci F, Kodiglu A, Mihmanli I. Adenomatoid tumor of the tunica vaginalis testes: A special maneuver in diagnosis by ultrasonography. J Ultrasound Med. 2003;22:303–5. doi: 10.7863/jum.2003.22.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Rege JD, Amarapurkar AD, Pathak AM. Fine needle aspiration cytology of adenomatoid tumor. A case report. Acta Cytol. 1999;43:495–7. doi: 10.1159/000331108. [DOI] [PubMed] [Google Scholar]
- 8.Isotalo PA, Nascimento AG, Trastek VF, Wold LE, Cheville JC. Extragenital adenomatoid tumor of a mediastinal lymph node. Mayo Clin Proc. 2003;78:350–4. doi: 10.4065/78.3.350. [DOI] [PubMed] [Google Scholar]
- 9.Manjunath GV, Nandini NM, Sunila Fine needle aspiration cytology of adenomatoid tumor: A case report with review of literature. Indian J Pathol Microbiol. 2005;48:503–4. [PubMed] [Google Scholar]
- 10.Schwartz J, Longacre TA. Adenomatoid tumors of the female and male genital tracts express WT1. Int J Gynecol Pathol. 2004;23:123–8. doi: 10.1097/00004347-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Nepka C, Kipouros A, Amplianitis I, Kangas A, Papalakis A, Kafanas A. Cytologic features of papillary cystadenoma of the epididymis associated with hydrocoele. Acta Cytol. 2004;48:467–9. [PubMed] [Google Scholar]


