Abstract
Adipose tissue is an alternative source of mesenchymal stem cells and human adipose-derived stem cells (ASCs) display an attractive and substantial therapeutic potential when transplanted in animal models. To this end, an understanding of ASC biology is necessary and the knowledge of mechanisms that maintain ASCs in an undifferentiated state with no loss of differentiation potential during ex vivo expansion represents a crucial step. However, these mechanisms remain to be identified because appropriate human cellular models are scant. In this review we will describe a cellular model isolated from human adipose tissue displaying all the features of stem cells. Then, we will focus on the identification of intrinsic and extrinsic factors regulating the balance between human ASC proliferation and differentiation. We will point out the role of factors secreted by undifferentiated ASCs, such a FGF2, activin A, BMP4, Hedgehog molecules and secreted by adipose tissue macrophages. Finally, we will outline the role of miRNAs in these processes.
Keywords: Adipose-derived stem cells, proliferation, adipocyte differentiation, FGF2, activin A, miR30, Hedgehog
Adipose tissue is a source of stem cells and human Multipotent Adipose-Derived Stem (hMADS) cells is a cellular model for investigating factors regulating their self-renewal
The increase in adipose mass in normal development and in obesity is the result of an increase in size and number of adipocytes. As mature adipocytes do not divide in vivo, regeneration of adipocytes and the increase in adipocyte number depend on the self-renewal capacity of a pool of adipocyte progenitors which remains present during adult life [1,2]. It is established that adipocyte progenitors are located in the stromal fraction (SVF) of adipose tissue. But the SVF is a heterogeneous mixture of cells, and recently Zuk et al [3] reported the first evidences that subpopulations of adipocyte progenitors display stem cell features in the SVF of human adipose tissue. Thereafter, and because the advantage of being available in much larger quantities than cord blood or bone marrow, adipose tissue quickly appeared as an alternative source of mesenchymal stem cells. Several groups demonstrated that human adipose-derived stem cells (ASCs) could have a therapeutic potential when transplanted in animal models (See for recent reviews [4,5]). In this context, identification of hASC self-renewal factors is essential to determine the mechanisms that maintain cells in an undifferentiated state with no loss of differentiation potential during ex vivo expansion. The goal of this review is to focus on the identification of intrinsic and extrinsic factors regulating the balance between human ASC proliferation and differentiation.
Characterization of factors controlling ex vivo expansion of functional human ASCs is at its infancy partly due to the absence of appropriate cellular models. Therefore, the first challenge has been to isolate human ASCs and to establish culture conditions to expand them. Recently, culture conditions have been set up allowing isolation and maintenance of human ASC lines derived from the SVF of infant adipose tissues. These stem cells were termed human Multipotent Adipose-Derived Stem (hMADS) cells [6]. They exhibit the characteristics of mesenchymal stem cells, i.e., the capacity to self-renew and to differentiate into several cell types at the clonal level. Cells can be expanded ex vivo for more than 160 population doublings (i.e., around 30 passages) while maintaining a normal diploid karyotype. Expanded hMADS cells are then able to differentiate under serum-free adipogenic condition into cells able to exhibit characteristics of human fat cells. More recently, hMADs cells have been described as a faithful model to study human fat cell metabolism [7,8]. These data indicate that expanded hMADS cells enter the adipose lineage at a high rate and differentiate into cells that display a unique combination of properties similar, if not identical, to those of native human adipocytes. Thus, they provide a unique model to analyse human adipose tissue physiopathology. In this regard, they were further used to assess the molecular mechanisms underlying drug-induced adipose tissue disorders such as lipodystrophy as observed in HIV-infected patients receiving HAART therapy [9,10]. After ex vivo expansion, hMADS cells endow the ability to undergo differentiation into adipocytes, osteoblasts, and chondrocytes at the single cell level [6,11] without any loss of their therapeutic potential. Actually, transplantation of hMADS cells into mdx mouse, an animal model for Duchenne muscular dystrophy, results in substantial expression of human dystrophin on a long-term basis and engraftment takes place in non-immunocompromised animals [6]. When transplanted with a scaffold, hMADS cells are able to form ectopic bone in mouse [12].
Altogether hMADS cells appear to be a powerful cellular model to investigate human ASC self-renewal and differentiation.
Regulators of proliferation and differentiation of human ASCs
FGF2, activin A and BMP4
The morphology of hMADS cells changes from a spindle-shaped morphology to a flat one during ex vivo expansion. This morphological change is accompanied by a change in cell proliferation ability (at passage 15 the doubling time is around 2 days, and becomes 4 days at passage 20) and by a loss of differentiation potential. Interestingly, Zaragosi et al. reported that the increase in doubling time was concomitant with a decrease of FGF2 secreted by undifferentiated hMADS cells [11]. As cells express also FGF type 1 receptor, the high-affinity receptor for FGF2, it has been proposed that FGF pathway plays an autocrine/paracrine role in ASC self-renewal. In support of this hypothesis the addition of exogenous FGF2 was able to sustain proliferation and adipogenic potential over passages. It is interesting to note that EGF, PDGF or FGF10 were not able to mimic FGF2 effects. Activation of ERK1/2 pathway is required to mediate FGF2 effects on hMADS cell proliferation. However, inhibition of MEK1 reduced the clonogenic potential of hMADS cells but did not affect their differentiation potential, indicating that the ERK1/2 signalling pathway is partly involved in FGF2-mediated self-renewal. FGF1 has also been reported to stimulate proliferation of human adipose progenitors and subsequently to increase their capacity to undergo differentiation [13]. The role of FGF pathway in the maintenance of ASC pool in adipose tissue remains to be investigated, but recently it has been proposed that FGFs could play a role in expansion of adipose tissues in obese patients [14].
Activin A, a member of the TGFβ family, is secreted by human undifferentiated ASCs isolated from various fat depots of donors of different ages. Its expression dramatically decreases as ASCs undergo differentiation into adipocytes. Activin A is not only a marker of undifferentiated cells but plays also a functional role in differentiation and proliferation. Sustained activation of activin A pathway promotes hMADS cell proliferation and impaires adipocyte differentiation, whereas its inhibition decreases proliferation and promotes differentiation. These effects are mediated via C/EBPβ and Smad2 pathways in an autocrine/paracrine manner [15].
Therefore, it has been proposed a model in which FGF2 and activin A regulate ASC proliferation and differentiation. However, it was surprising to observe that in contrast to FGF2 expression, activin A expression increases during inhibition of hMADS cell self-renewal (Figure 1). Therefore, a critical question remains opened: does expansion of ASCs with FGF2 or with activin A maintain their differentiation potential?
Figure 1.
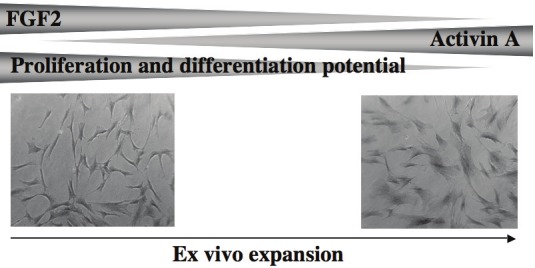
Cell morphology and potential of ASC to proliferate and to differentiate change during ex vivo expansion. In the same time, secretion of FGF2 and of activin A decreases and increases respectively.
Indeed, treatment of FGF2-expressing hMADS cells only during proliferation with PD173074, a specific FGF receptor inhibitor, decreases dramatically their differentiation potential, indicating that FGF pathway is required for both the maintenance of proliferation and of differentiation potential. In contrast, expansion of hMADS cells with activin A induces a myofibroblast-like phenotype. In addition, exposure of hMADS cells to activin A promotes osteogenic differentiation at the expense of adipogenic differentiation (unpublished data). This observation needs to be further investigated and suggests that activin A pathway could change the fate of undifferentiated ASCs. Altogether, these data indicate that both FGF2 and activin A promote ASC proliferation but might impact the differentiation potential of expanded hMADS cells in different ways (Figure 2). These data point out the importance to analyse in details the cellular and molecular events induced by factors regulating proliferation of ASCs and the consequences on their behaviour.
Figure 2.
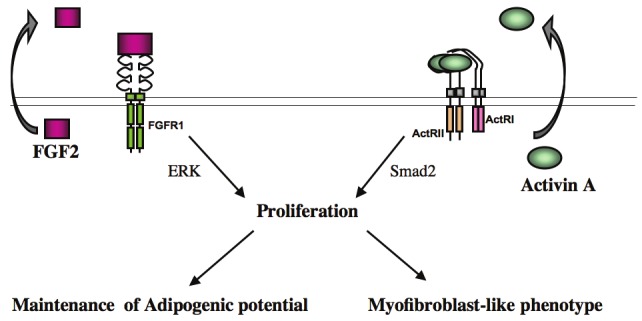
Autocrine/paracrine loops regulating proliferation and differentiation of human ASCs. FGF2 and activin A are secreted by undifferentiated ASCs and promote proliferation of ASCs via ERK and Smad2 pathways respectively. FGF2 maintains adipogenic potential of undifferentiated ASCs whereas activin A change their phenotype.
BMP4, which is as activin A another member of TGFβ super-family, regulates hASC proliferation in an autocrine and dose-dependent manner while maintaining their multipotent property [16]. Therefore, there is an emerging role of TGFβ pathway in self-renewal and differentiation of ASCs, as recently reviewed by Zamani and Brown [17].
Adipose tissue macrophage-secreted factors
Investigating FGF2 and activin A gene expression in the obesity context revealed new regulators of ASC proliferation and differentiation. Obesity is associated with the presence of a higher number of hypertrophic adipocytes, with new macrophages recruited into adipose tissue [18,19], and with an increased proportion of ASCs exhibiting proliferative potential [20]. This latter observation strongly suggests an important contribution of obese microenvironment is inducing ASC self-renewal. Interestingly, macrophages isolated from obese adipose tissues secrete factors that both inhibit differentiation of ASCs and stimulate expression of activin A and FGF2 genes in hMADS cells [15]. These observations fit well with a model proposing that macrophages play a key role in self renewal of ASCs in part through activin A and FGF2. Macrophage factors involved in the regulation of hMADS cell self renewal are unknown so far. However, TNF-α could be one of them because it mimics the effects of macrophage-conditioned medium on ASC proliferation and differentiation. In addition, TNFa stimulates expression of FGF2 and of activin A (Wdziekonski, Villageois and Dani, unpublished data). Besides TNFa other macrophages factors, such as inflammatory cytokines or Wnt molecules [21,22] are likely to be involved in proliferation and differentiation of human ASCs. Although the phenotype of ASCs expanded in the presence of macrophages factors remains to be investigated in details, these observations suggest that macrophages constitute determinant niche components for ASC self-renewal.
Hedgehog pathway
Hedgehog (Hh) pathway affects also self-renewal and differentiation of ASCs. Indeed, Hh signalling decreases during adipocyte and osteoblast differentiation of hMADS cells. Moreover, its activation inhibits both adipocyte maturation, producing ill-differentiated adipocytes that are insulin resistant [23], and osteogenic differentiation [24]. Interestingly, there is a basal level of Hh signaling in undifferentiated cells that appears necessary for the maintenance of hMADS cell proliferation and clonogenic capacity, probably through regulation of pRB phosphorylation and cyclin A expression [25]. However, in contrast with FGF pathway, inhibition of Hh signalling during proliferation did not alter the differentiation potential of hMADS cells.
miRNAs
MicroRNAs also emerged as important players in adipogenesis (see for recent review [26]) and in ASC self-renewal and differentiation. Dicer is an RNase III-family nuclease critical for miRNA generation. Knockdown of this enzyme using RNA interference, compromises severely hMADS cell proliferation and differentiation (Figure 3, unpublished data). This observation is in agreement with the impairment of ASC survival consequent to disruption of the miRNA processing machinery reported recently by Sun Kim et al.[27]. Deep sequencing of small RNAs expressed in undifferentiated and differentiating hMADS cells indicated an up-regulation on the miR-30 family during adipogenic differentiation and a down regulation during osteogenesis. Functional analysis revealed that inhibition of the miR-30 family blocks adipogenesis while it promotes osteogenesis. Then, it has been additionally showed that Runx2 targeting is, at least in part, responsible for miR30 positive effects on adipocyte differentiation. In this context it is interesting to remind that the transcription factor Runx2 is expressed in undifferentiated hMADS cells and that represents the major regulator of osteogenesis [28]. Altogether, these data support a model in which miR30 regulates the fate of hMADS cells via the modulation Runx2.
Figure 3.
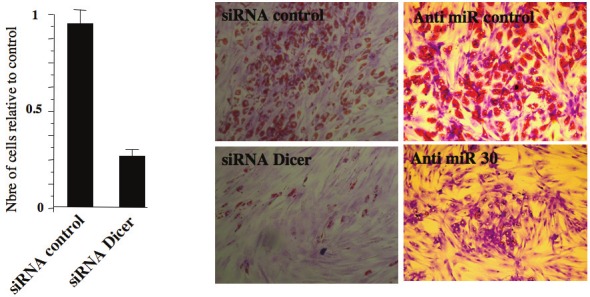
Role of miRNAs in proliferation and differentiation of ASCs. Knockdown of Dicer reduces the proliferation and adipocyte differentiation of hMADS cells. Similar effects were observed with the knockdown of miR30. Adipocytes are stained with Oild Red O for lipid droplets.
In conclusion, all together these observations reveal the complexity of ASC maintenance and behaviour. FGF2 and activin A appear as common regulators governing self-renewal of human embryonic and adult stem cells [29,30]. Other factors such as Wnt, Hh and BMPs, more readily implicated in self-renewal of neural stem cells [31,32] and bone-marrow mesenchymal stem cells [33] play also a key role in ASC biology. Therefore, ASCs seem to combine regulatory signalling pathways of embryonic and tissue-specific adult stem cell self-renewal. The influence of the niche is illustrated by the susceptibility of these cells to macrophage-secreted factors that are present in the adipose tissue of obese patients. A better knowledge of these factors and of their impact in a long-term exposure to ASCs is crucial to properly maintain these cells ex vivo for regenerative medicine and also to investigate their role in the pathological expansion of the adipose mass.
Acknowledgements
Dani’s team is supported by ANR (09-GENO-036), ARC (grant 5017), CNRS and Inserm.
References
- 1.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 5.Locke M, Feisst V, Dunbar PR. Concise Review: Human Adipose-Derived Stem Cells (ASC): Separating Promise from Clinical Need. Stem Cells. 2011;29:404–11. doi: 10.1002/stem.593. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez A-M, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne J-Y, Wdziekonski B, Villageois A, Bagnis C, Breittmayer J-P, Groux H, Ailhaud G, Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poitou C, Divoux A, Faty A, Tordjman J, Hugol D, Aissat A, Keophiphath M, Henegar C, Commans S, Clement K. Role of serum amyloid a in adipocyte-macrophage cross talk and adipocyte cholesterol efflux. J Clin Endocrinol Metab. 2009;94:1810–1817. doi: 10.1210/jc.2008-2040. [DOI] [PubMed] [Google Scholar]
- 8.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernochet C, Azoulay S, Duval D, Guedj R, Cottrez F, Vidal H, Ailhaud G, Dani C. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J Biol Chem. 2005;280:2238–2243. doi: 10.1074/jbc.M408687200. [DOI] [PubMed] [Google Scholar]
- 10.Djedaini M, Peraldi P, Drici MD, Darini C, Saint-Marc P, Dani C, Ladoux A. Lopinavir co-induces insulin resistance and ER stress in human adipocytes. Biochem Biophys Res Commun. 2009;386:96–100. doi: 10.1016/j.bbrc.2009.05.148. [DOI] [PubMed] [Google Scholar]
- 11.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- 12.Elabd C, Chiellini C, Massoudi A, Cochet O, Zaragosi LE, Trojani C, Michiels JF, Weiss P, Carle G, Rochet N, Dechesne CA, Ailhaud G, Dani C, Amri EZ. Human adipose tissue-derived multipotent stem cells differentiate in vitro and in vivo into osteocyte-like cells. Biochem Biophys Res Commun. 2007;361:342–348. doi: 10.1016/j.bbrc.2007.06.180. [DOI] [PubMed] [Google Scholar]
- 13.Widberg CH, Newell FS, Bachmann AW, Ramnoruth SN, Spelta MC, Whitehead JP, Hutley LJ, Prins JB. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocrinol Metab. 2009;296:E121–131. doi: 10.1152/ajpendo.90602.2008. [DOI] [PubMed] [Google Scholar]
- 14.Mejhert N, Galitzky J, Pettersson AT, Bambace C, Blomqvist L, Bouloumie A, Frayn KN, Dahlman I, Arner P, Ryden M. Mapping of the fibroblast growth factors in human white adipose tissue. J Clin Endocrinol Metab. 2010;95:2451–2457. doi: 10.1210/jc.2009-2049. [DOI] [PubMed] [Google Scholar]
- 15.Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, Scheideler M, Trajanoski Z, Takashima Y, Amri EZ, Lacasa D, Sengenes C, Ailhaud G, Clement K, Bouloumie A, Kirkland JL, Dani C. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicente Lopez MA, Vazquez Garcia MN, Entrena A, Olmedillas Lopez S, Garcia-Arranz M, Garcia-Olmo D, Zapata A. Low doses of bone morphogenetic protein 4 increase the survival of human adipose-derived stem cells maintaining their stemness and multipotency. Stem Cells Dev. 2011;20:1011–1019. doi: 10.1089/scd.2010.0355. [DOI] [PubMed] [Google Scholar]
- 17.Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maumus M, Sengenes C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, Galitzky J, Bouloumie A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 21.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilkovski R, Schulte DM, Oberhauser F, Mauer J, Hampel B, Gutschow C, Krone W, Laudes M. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obes (Lond) 2011;35:1450–4. doi: 10.1038/ijo.2011.6. [DOI] [PubMed] [Google Scholar]
- 23.Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells. 2008;26:1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- 24.Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009;27:703–713. doi: 10.1634/stemcells.2008-0888. [DOI] [PubMed] [Google Scholar]
- 25.Plaisant M, Giorgetti-Peraldi S, Gabrielson M, Loubat A, Dani C, Peraldi P. Inhibition of hedgehog signaling decreases proliferation and clonogenicity of human mesenchymal stem cells. PLoS One. 2011;6:e16798. doi: 10.1371/journal.pone.0016798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGregor RA, Choi MS. microRNAs in the regulation of adipogenesis and obesity. Curr Mol Med. 2011;11:304–316. doi: 10.2174/156652411795677990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS, Jung JS, Jang JH, Kang KS, Kang SK. Nuclear Argonaute 2 regulates adipose tissue-derived stem cell survival through direct control of miR10b and selenoprotein N1 expression. Aging Cell. 2011;10:277–291. doi: 10.1111/j.1474-9726.2011.00670.x. [DOI] [PubMed] [Google Scholar]
- 28.Zaragosi LE, Wdziekonski B, Le Brigand K, Villageois P, Mari B, Waldmann R, Dani C, Barbry P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12:R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 31.Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476–490. doi: 10.1002/neu.20160. [DOI] [PubMed] [Google Scholar]
- 32.Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satija NK, Gurudutta GU, Sharma S, Afrin F, Gupta P, Verma YK, Singh VK, Tripathi RP. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]


