Abstract
The predominant X-linked form of Dyskeratosis congenita results from mutations in dyskerin, a protein required for ribosomal RNA modification that is also a component of the telomerase complex. We have previously found that expression of an internal fragment of dyskerin (GSE24.2) rescues telomerase activity in X-linked dyskeratosis congenita (X-DC) patient cells. Here we have generated F9 mouse cell lines expressing the most frequent mutation found in X-DC patients, A353V and investigate the effect of expressing the GSE24.2 cDNA or GSE24.2 peptide on telomerase activity, mTERT and mTR expression. Expression of GSE24.2 increases mTR and to a lesser extent mTERT RNA levels and leads to recovery of telomerase activity. Point mutations in GSE24.2 residues known to be highly conserved and crucial for the pseudouridine-synthase activity of dyskerin abolished the effect of the peptide. Recovery of telomerase activity and increase in mTERT levels were found when the GSE24.2 peptide purified from bacteria was introduced into the cells Moreover mTR stability was also rescued by transfection of the peptide GSE24.2. These data indicate that supplying GSE24.2, either from a cDNA vector, or as a peptide, can reduce the pathogenic effects of Dkc1 mutations and could form the basis of a novel therapeutic approach.
Keywords: Dyskeratosis congenital, dyskerin, TER, GSE24.2
Introduction
Telomeres are nucleoprotein complexes located at the ends of linear chromosomes and consist of tandem repeats of simple DNA sequences (TTAGGG in humans) and proteins that interact directly or indirectly with these sequences [1]. Sequence erosion of terminal repeats takes place in each round of genome replication. The replenishment of telomeric repeats is accomplished by extension of their 3’ends, through a reaction mediated by the telomerase complex [2]. In humans, the active telomerase complex consists of a minimum of three essential components: hTERT, hTR and dyskerin [3]. Besides forming part of the telomerase complex, dyskerin is a pseudouridine-synthase, a component of H/ACA small nuclear RNPs [4], complexes that mediate the conversion of specific uridines (U) to pseudouridine in newly synthesized ribosomal RNA [5, 6, 7]. Point mutations in dyskerin cause a rare X-linked recessive disease named X-Dyskeratosis congenita [8]. Individuals with X-DC display features of premature ageing, as well as nail dystrophy, mucosal leukoplakia, interstitial fibrosis of the lung, bone marrow failure and increased cancer susceptibility, form head and neck and gastrointestinal tumors [9]. The tissues affected by X-DC, such as bone marrow and skin, are characterized by the high turnover of their progenitor cells.
We previously isolated a peptide GSE24.2, in a screen of cDNAs for those that confer survival ability on cells treated with cisplatin. Intriguingly GSE24.2 turned out to be a short dyskerin fragment containing 2-highly conserved motifs implicated in pseudouridine-synthase catalytic activity. GSE24.2 prevents telomerase inhibition mediated by different drugs including cisplatin and telomerase inhibitors. In X-DC and WI-38-VA13 cells, GSE24.2 induces an increase in hTERT and hTR levels and recovery of telomerase activity [10]. To obtain more information on the biological activity of this dyskerin fragment we have generated a mouse-F9 cell line carrying the A353V mutation in the Dkc1 gene. This is the mutation most frequently found in patients with X-DC [11, 12] and is localized in the PUA RNA-binding domain, the putative site for interaction with hTR. This mutation in mouse ES cells leads to severe destabilization of mTR, a reduction in telomerase activity and a significant continuous loss of telomere length during in vitro cell culture [13]. In addition, causes a decrease in the accumulation of a subset of H/ACA small nucleolar RNAs, and a significant decrease in site-specific pseudouridylation efficiency [13]. Here we show that expression of GSE24.2 is able to induce a recovery in telomerase activity in F9-X-DC mouse cell line model, by increasing mTR and mTERT RNA levels. Moreover a peptide encoding GSE24.2, when introduced into mutant cells, is able to rescue telomerase activity and also destabilization of mTR induced by the Dkc1 mutation suggesting that expression of GSE24.2, could prolong the lifespan of X-DC cells and protect from associated malignancies.
Materials and methods
Constructs, generation of F9 A353V cells and cell culture
GSE24.2, DKC, motif I and motif II were cloned as previously described [10]. The hTERT promoter construct was a gift from Dr. T. Kim [14]; the hTR promoter construct was a gift from Dr. N.Keith [15]; PGATEV protein expression plasmid [16] was obtained from Dr. G. Montoya. PGATEV-24.2 was obtained by subcloning the 24.2 fragment into the NdeI/XhoI sites of the pGATEV plasmid.
F9 cells were transfected with A353V targeting vector and selected as previously described for constructing the mouse ES cell line containing the A353V mutation [13]. F9A353V cells were cultured in Dulbecco modified Eagle medium (DMEM) 10% fetal bovine serum, 2 mM glutamine (Gibco) and Sodium bicarbonate (1,5 gr/ml)
Cell transfections and analysis of gene expression
F9 cells were transfected with 16 µg of DNA/106 cells, using lipofectamine plus (Invitrogen, Carslbad, USA), according to the manufacturer’s instructions. Protein extracts were prepared and luciferase activity determined using a commercial kit (Promega, Madison, USA). Each assay was performed in triplicate, and repeated in three different experiments. Transfection efficiencies were corrected by co-transfection of p-CMV-Renilla. 293T cells were transfected by the calcium phosphate procedure and protein extracts were obtained 24 hours later as described above. Transfection of GSE24.2 peptide, was performed by using the Transport Protein Delivery Reagent (50568; Lonza) transfection kit. Routinely from 6 to 15µg were used per 35 mm dish.
Antibodies
Anti-dyskerin and anti topoisomerase IIb (Ab7471) were from Abcam, (Cambridge. UK).
Site-directed mutagenesis
Mutation of single nucleotides of the GSE24.2 was performed using the Quickchange X-L site-directed mutagenesis kit (Stratagene, Santa Clara, USA) according to the manufacture’s instructions.
Telomeric repeat amplification protocol (TRAP) assay
Telomerase activity was measured using the TRAPeze kit [17] (Intergen, Purchase, USA) (Serologicals Norcross, GA) according to the manufacturer’s recommendations. TRAP assay activity was normalized for total protein concentration [10].
GSE24.2 peptide production and purification
E. coli Rosetta-gami 2 competent cells were transformed with pGATEV GSE24.2 and lysates prepared as described [16]. The fusion protein was purified with glutathion-sepharose and purity analyzed by gel electrophoresis. GSE24.2 was obtained and purified as described [16].
Western Blot
Whole-cell extracts were prepared essentially as described previously [18]. Nuclear extracts were obtained as previously reported [10]. Western blotting was performed using standard methods [18]. Protein concentration was measured by using the Bio-Rad protein assay.
cDNA preparation and RT-PCR
Total cellular RNA was extracted using Trizol (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. In each RT reaction, 2 µg of total RNA were reverse transcribed into cDNA using M-MLV reverse transcriptase (Promega, Madison, USA). Primers used are listed below.
mTERT-S 5’-AGATCAAGAGCAGTAGTCGCCAG-3’
mTERT-AS 5’- TTTACAGCACACCGACCCAGAG-3’.
Program: 94°C 60s, 60°C 60s, 72° 60s, 25 cycles
mTR-S 5’-CTGGTCTTTTGTTCTCCGC-3’
mTR-AS 5’-TGCACTTCCCACAGCTCA-3’
Program: 94°C 45s, 53°C 60s, 72°C 60s, 25 cycles
β-actin-S 5’-GGTATGGAATCCTGTGGCATCCATGAAA-3’.
β-actin-AS 5’-GTGTAAAACGCAGCTCAGTAACAGTCCG-3’
Program: 94°C 60s, 60°C 60s, 72°C 60s, 25 cycles
qRT-PCR
For qRT-PCR, 1µg of total RNA was reverse transcribed using Random Primer and M-MLV Reverse Transcriptase (Promega, Madison, USA).
qRT-PCR for mTR was done using Power SYBR Green (Applied Biosystems, Madrid, Spain) containing ROX to normalize to emissions. Primer used were:
m-TR-S: 5’-GCTGTGGGTTCTGGTCTTTTGTTC-3’
m-TR-AS: 5’-CGTTTGTTTTTGAGGCTCGGG-3’
m-c-myc-S: 5’-GAGCTGTTTGAAGGCTGGATTT-3’
m-c-myc-AS: 5’-TCCTGTTGGTGAAGTTCACGTT-3’
Primers β-actin and mTERT have been described above.
Program used for m-TR is 40 cycles at 95°C /15 s, 60°C /30 s acquiring 80°C/15 s.
Program used for m-TERT and c-myc is 40 cycles at 95°C/15 s, 60°C/30 s, 72°C/30s acquiring 75°C/15 s.
Each cDNA sample was analyzed in triplicate using StepOne Plus Real Time PCR System (Applied Biosystems, Madrid, Spain) and relative gene expression quantification was calculated according to the comparative threshold cycle method (2 −ΔΔCt), where ΔΔCt = ΔCt gene expression - ΔCt control, using β-actin as endogenous control.
Results
Induction of the Dkc1 A353V mutation in F9 cells
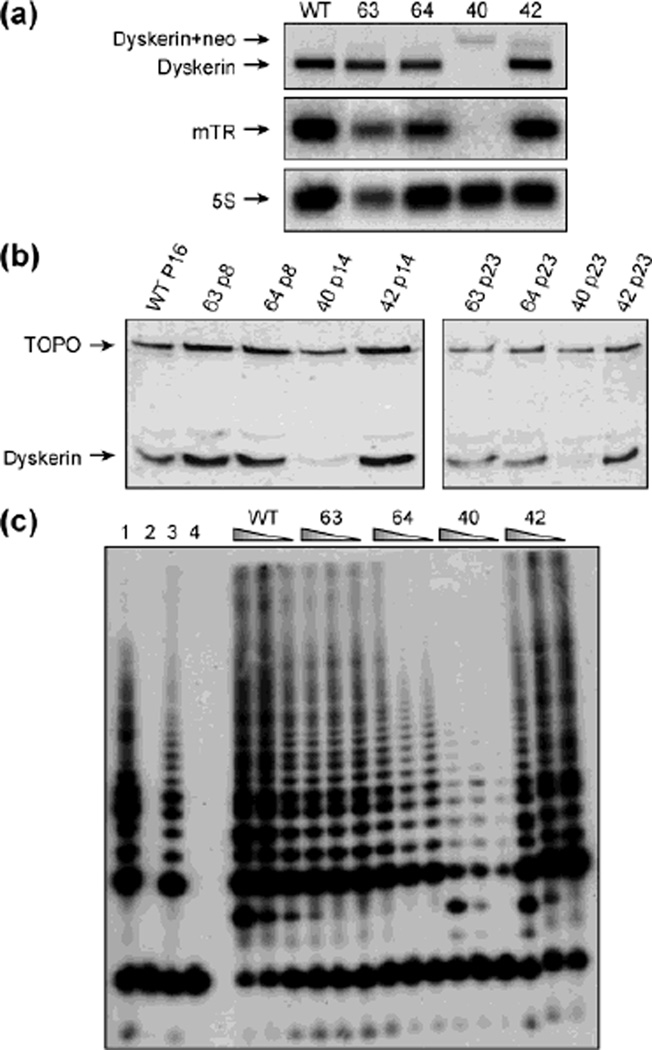
We chose to create mouse F9 cells containing the recurrent Dkc1-A353V mutation in order to study the molecular consequences of this mutation and the puzzling effects of the GSE24.2 peptide on cells with Dkc1 mutations. From the targeting experiment a single F9 cell line was isolated that was successfully targeted. This cell line was shown to contain a single Dkc1 gene, which contains the mutation causing the A353V change, by Southern blot, PCR and sequencing. Figure 1 shows that the correctly targeted cell line, cell line 40, has decreased dyskerin mRNA (Fig. 1a) and protein (Fig. 1b) and dramatically decreased amounts of mTR (Fig. 1a), compared with control cell lines. Cell line 40 also contains reduced telomerase activity (Fig. 1c). We therefore used this cell line, hereafter called F9A353V, for the experiments described in this manuscript.
Fig. 1.
Characterization of F9 derived cell lines: (a) Northern blots of different cell lines obtained from the targeting experiment. 1.6% agarose formaldehyde gels were run and blotted and probed for dyskerin, mTR and 5S RNA as a loading control. The cell lines were WT, the parental F9 cells; 63, a neomycin resistant cell line not targeted at the Dkc1 locus; 64, a neomycin resistant cell line lacking the A353V mutation that had contained and had lost the neomycin resistance cassette from the 3’UTR of Dkc1; 40, the correctly targeted A353V cell line; 42, a cell line not correctly targeted but containing multiple inserted targeting constructs. (b) Western blots of nuclear proteins extracted from different cell lines from the targeting experiment and probed with a mouse anti-dyskerin and a topoisomerase IIb antibody as a loading control. The cell lines were as in the legend of panel a. P indicates the passage number. (c) Telomerase activity was measured in cell extracts from different cell lines from the targeting experiment using the TRAPEZE protocol. The cell lines were as described in panel a. Control lanes: 1, TRS8 positive control, 2, negative control (Chaps buffer), 3, HeLa positive control, 4, inactivated HeLa extracts.
GSE24.2 expression in F9 and F9A353V cells results in stimulation of telomerase catalytic activity
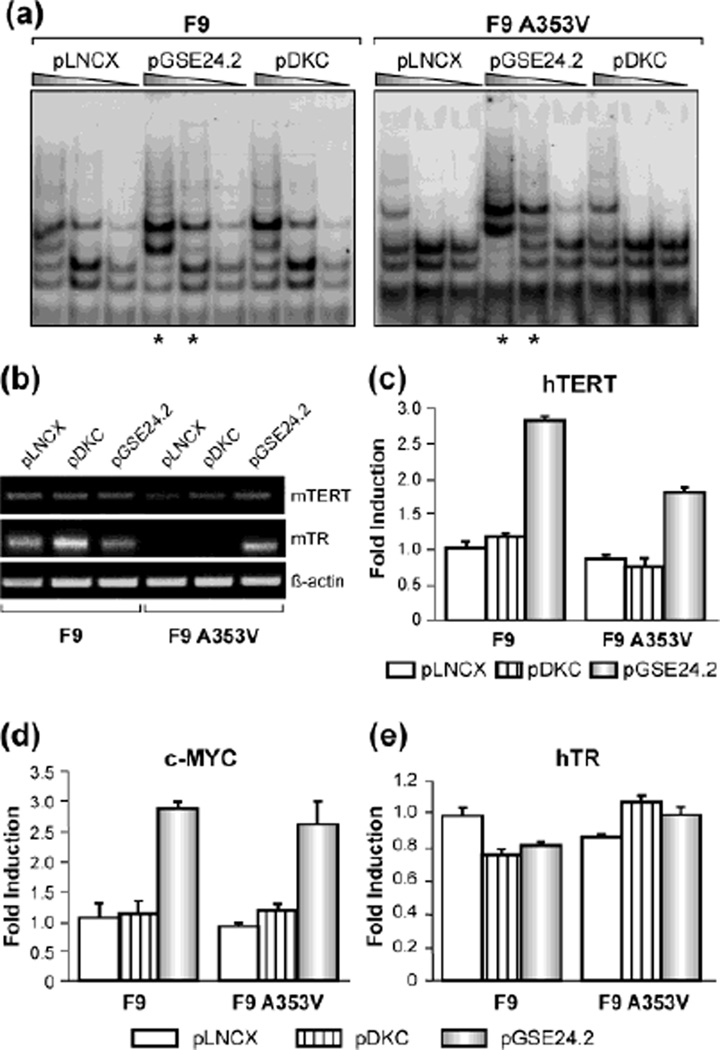
We did not observe any obvious difference in the growth rate or any morphological changes in the F9A353V cells as compared with wild-type F9 cells (Data not shown). We previously reported that expression of GSE24.2 increases telomerase activity in X-DC human derived fibroblasts [10]. To extent this observation to A353V mutation we transfected F9A353V and control cells with a plasmid expressing GSE24.2. Remarkably we found that GSE24.2 expression significantly increased telomerase activity in both F9A353V and F9 cells. (Fig. 2a, see asterisks). It has been previously described that overexpression of dyskerin in cells lacking telomerase activity does not stimulate telomerase activity [10, 19], so we were interested to see the effect of dyskerin overexpression in F9 cells. Surprisingly, expression of dyskerin in F9 cells significantly increases telomerase activity (Fig. 2a) but the increment is not observed in F9A353V (Fig. 2a), perhaps indicating a dominant negative effect of the A353V mutation. Expression of GSE24.2 increases mTERT mRNA and mTR levels in F9A353V cells and this correlates with the increment observed in telomerase activity indicating that telomerase complexes although lacking wild type dyskerin, may be more stable. Overexpression of dyskerin induces a mild increase in mTR levels in F9 cells, which could correlate with the small increase observed in telomerase activity (Fig. 2a, b). We next studied the activity of hTERT promoter in F9 and F9A353V cells using a luciferase reporter construct containing the hTERT promoter. hTERT transcription is activated by GSE24.2, suggesting that the increase in mTERT levels could to be due to activation of mTERT transcription (Fig. 2c). Over-expression of dyskerin does not have any effect on hTERT promoter activity. As the promoter of hTERT-telomerase component was constitutively activated in GSE24.2 expressing cells in a c-myc expression-dependent manner [10], we studied the effect of GSE24.2 expression on c-Myc promoter activity in F9 and F9A353V cells. We measured the activation of this promoter using a construct containing 3.2 Kb of the c-Myc regulatory region, followed by the luciferase gene (Fig. 2d). GSE24.2 also activates c-myc promoter in both cell lines. We also performed a luciferase assay using a construct that contained the hTR promoter followed by the luciferase gene. No activation of hTR promoter mediated by the GSE24.2 was observed in any of the cell lines, in agreement with previous results (Fig. 2e) suggesting that the increment in mTR levels should be due to stabilization of the RNA.
Fig. 2.
Consequences of GSE24.2 expression on telomerase activity of F9 and FA353V cells. (a) F9 and F9A353V cells were transfected with pLNCX, pDKC and pGSE24.2 (10 µg DNA per 106 cells). Telomerase activity was determined with the TRAP assay kit. Different extract dilutions are presented for each TRAP assay as indicated by the triangles. Asterisks indicate the samples that showed important differences in relation to the pLNCX transfected cells. The experiments were repeated 3 times, with similar results. (b) Levels of telomerase complex components in F9 and F9A353V cells: F9 and F9A353V were transfected with either pLNCX, pDKC or pGSE24.2. mTERT mRNA and mTR levels were determined by RT-PCR. β-actin mRNA was used as control. The experiments were repeated 3 times, with similar results. (c) F9 and F9A353V cells were co-transfected with the hTERT-luc reporter vector (1 µg per million cells) and pLNCX, pDKC and pGSE24.2 expression vectors. After 24h, the cells were processed and the luciferase activity determined. The activity obtained is represented as fold induction over the pLNCX control transfection (d) F9 and F9A353V cells were co-transfected with the pXP3.2myc promoter (1 µg per 106 cells) and pLNCX, DKC and GSE24.2. After 24h, the cells were processed and the luciferase activity determined. (e) F9 and F9A353V cells were co-transfected with the hTR-luc reporter vector (1 µg per 106 cells) and pLNCX, pDKC and pGSE24.2 expression vectors. After 24h, the cells were processed and the luciferase activity determined as described in panel d. The experiments were repeated 3 times, with similar results.
Recovery of telomerase activity by GSE24.2 requires a complete and intact pseudouridine synthase domain
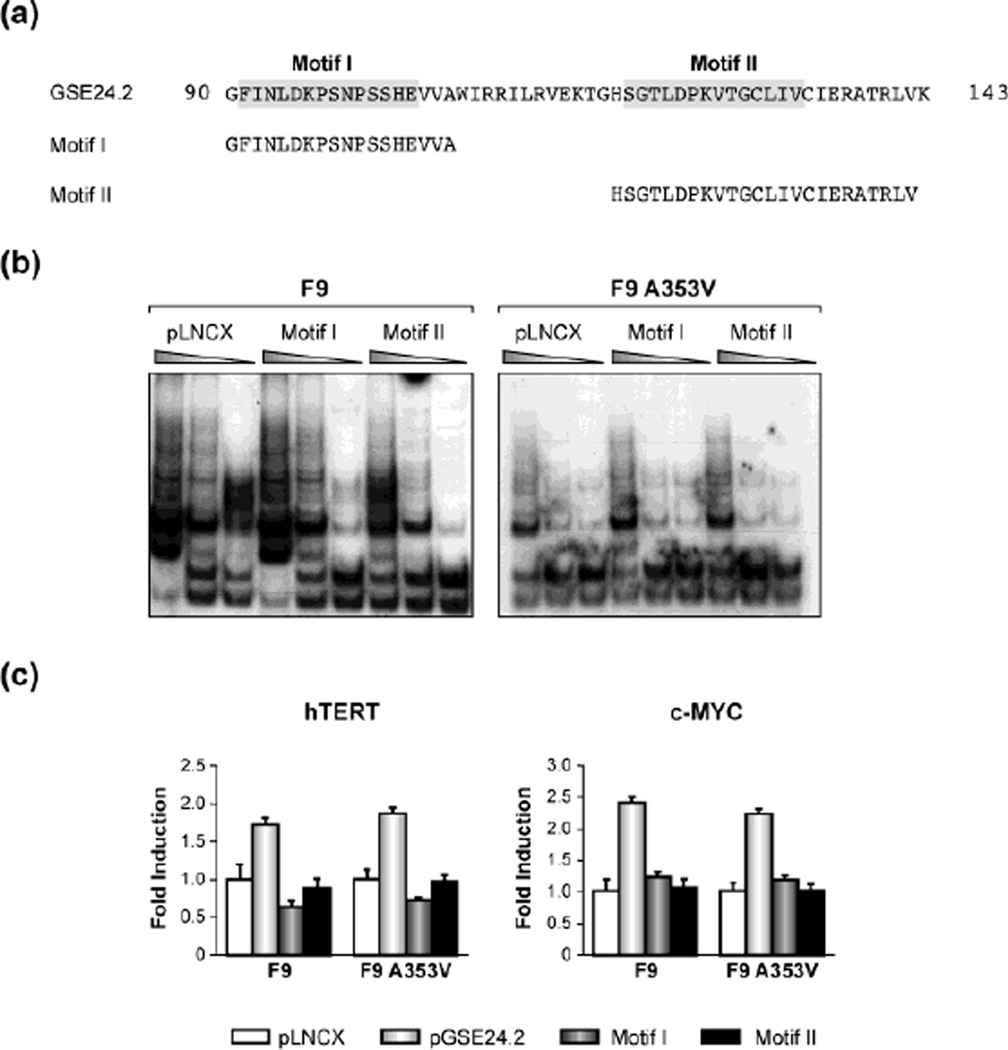
The GSE24.2 peptide corresponds to an internal fragment of dyskerin, which contains the pseudouridine-synthase domain TRUB. This comprises two highly conserved motifs; motif I is essential for the maintenance of the structural stability of the protein while motif-II contains at least one residue essential for the enzymatic activity [20, 21, 22]. We asked which of these motifs was responsible for the activation of telomerase activity in Dkc1 mutant cells, or whether both were required. We cloned sequences encoding the motifs independently and transiently transfected both F9 and F9A353V cells with the individual expression vectors (Fig. 4a). Twenty-four hours after transfection, measurement of telomerase activity was performed using TRAP assay. We observed that neither of these motifs alone induced a recovery of telomerase (Fig. 4b). We could not observe activation of hTERT or c-MYC transcription in contrast to our results with the whole domain (Fig. 4c) [10].
Fig. 4.
Point mutations of GSE24.2 impair its ability to activate telomerase. (a) Amino acid sequence of the GSE24.2 peptide indicating the point mutations generated in bold. (b) F9 and F9A353V cells were transfected with pLNCX or GSE24.2 K96V- and D125V-pLNCX derived plasmids (10 µg DNA per 106 cells). Telomerase activity was determined by using the TRAP assay kit. Different extract dilutions were used for each TRAP assay as indicated. The experiments were repeated 3 times, with similar results. (c) F9 and F9A353V cells were co18 transfected with the hTERT or cMYC luciferase reporter vectors (1 µg per million cells) and pLNCX, pGSE24.2 pK96V- and pD125V-pLNCX derived plasmids. After 24h, cells were processed and the luciferase activity determined. The experiments were repeated 3 times, with similar results.
We next asked if substitution of specific amino acid residues, affected the activity of GSE24.2 by performing site directed mutagenesis to alter TRUB domain amino-acid residues that are essential for pseudouridine-synthase activity and highly conserved through evolution. Therefore K96 in motif-I and D125 in motif-II were substituted for valine by site-directed mutagenesis (Fig. 5a). First of all, we studied the effect of these mutations on GSE24.2-mediated telomerase activation. We transiently transfected F9 and F9A353V cells with both mutant expression vectors and 24 hours after transfection we performed a TRAP assay. We observed that neither of these mutants activated telomerase (Fig. 5b) suggesting that the residues mutated that are essential for dyskerin activity are also essential for GSE24.2 activity. In order to determine the effect of the mutations on the activation of hTERT and c-MYC promoters, we transiently transfected F9 and F9A353V cells with the pLNCX, GSE24.2, GSE24.2(K96V) or GSE24.2(D125V) together with either hTERT or c-MYC luciferase reporter vectors (Fig. 5c). Both mutations impaired the activation of these promoters mediated by the GSE24.2, suggesting that these residues are very important for GSE24.2 activity. In agreement with previous results, no activation of hTR promoter by the GSE24.2 or any of the mutants was observed (Fig. 5c), but we did not detect accumulation of mTR when we used the mutated GSE24.2 expression plasmids (not shown) suggesting that stabilization of mTR may also contribute to the increased telomerase activity caused by GSE24.2.
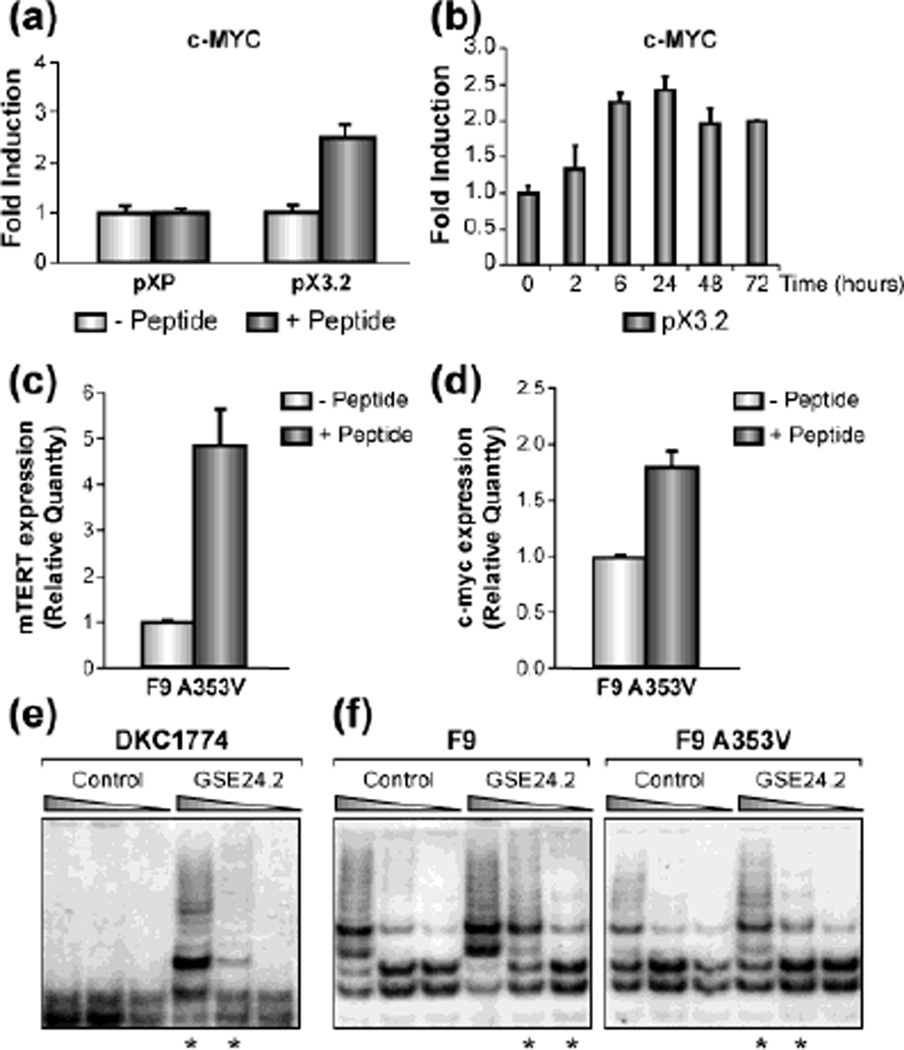
Fig. 5.
GSE24.2 peptide induces a recovery of telomerase activity and c-myc promoter activation. (a) 293T cells were transfected with the pXP (empty vector) or the pXP3.2myc promoter (0.5 µg per 35 mM dish), when indicated cells were transfected 24 hours later with the GSE24.2 peptide (15 µg per 35 mm dish). Luciferase assay was performed six hours later. The experiments were repeated 3 times, with similar results (b) Experiment was carried out as in A, but the cells were lysed after peptide transfection at the indicated times. (c, d) F9A353V cells were transfected with the GSE24.2 peptide (15 µg per 35 mm dish). Total RNA was extracted 24 hours later and the levels of mTERT (c) and c-myc (d) measured by qRT-PCR. (e) DKC1774 a human XDC cell line was transfected either with a control peptide or GSE24.2 peptide (15 µg per 35 mm dish) and telomerase activity was determined four hours later using the TRAP assay kit. Different dilutions of extracts used for each TRAP assay are indicated. Asterisks indicate the samples that showed important differences with respect to the control. The experiments were repeated 3 times, with similar results. (f) F9 and F9A353V cells were transfected either with a control peptide or GSE24.2 peptide (15 µg per 35 mm dish). Telomerase activity was determined four hours later with the TRAP assay kit. Different extract dilutions were used for each TRAP assay as indicated. Asterisks indicate the samples that showed important differences with respect to the control. The experiments were repeated 3 times, with similar results.
Transfection of purified GSE24.2 peptide stimulates telomerase activity
Since GSE24.2 codes for an internal dyskerin peptide, we tested if GSE24.2 peptide purified from bacteria, would have the same effect on telomerase activity as expression of the plasmid containing GSE24.2 cDNA. Initially, we used 293T cells, because of their high transfection efficiency. Cells were transfected with the construct containing the c-MYC promoter and 24 hours later transfected with the GSE24.2 peptide and luciferase activity was assayed six hours later. The results showed activation of c-MYC promoter in cells transfected with GSE24.2 peptide, indicating that the induction observed when we transfected GSE24.2 cDNA (Fig. 5a) is indeed due to its translation into a protein. We assayed the stability of the transfected peptide by testing its activity on the c-myc promoter, and found that the activity of the peptide lasted until 72 hours after transfection (Fig. 5b). This correlated with an increase in mTERT and c-myc transcription estimated by q-RT-PCR (Figs. 5c, d). Then, X-DC dermal human fibroblast were transfected with GSE24.2 peptide and 4 hours later assayed for telomerase activity. The peptide was efficient in activating telomerase activity in X-DC cells (Fig. 5e) as also happened when cells were transfected with GSE24.2 cDNA [10]. We then transfected F9 and F9A353V cells with GSE24.2 peptide and measured telomerase activity using the TRAP assay. We observed that there was an increment in telomerase activity after the transfection with GSE24.2 peptide in both cell lines (Fig. 5f, see asterisks). Heat inactivated GSE24.2 peptide did not induce a recovery in telomerase activity (data not shown) indicating that GSE24.2 peptide could be used for a transient recovery of telomerase activity.
GSE24.2 peptide increases mTR stability
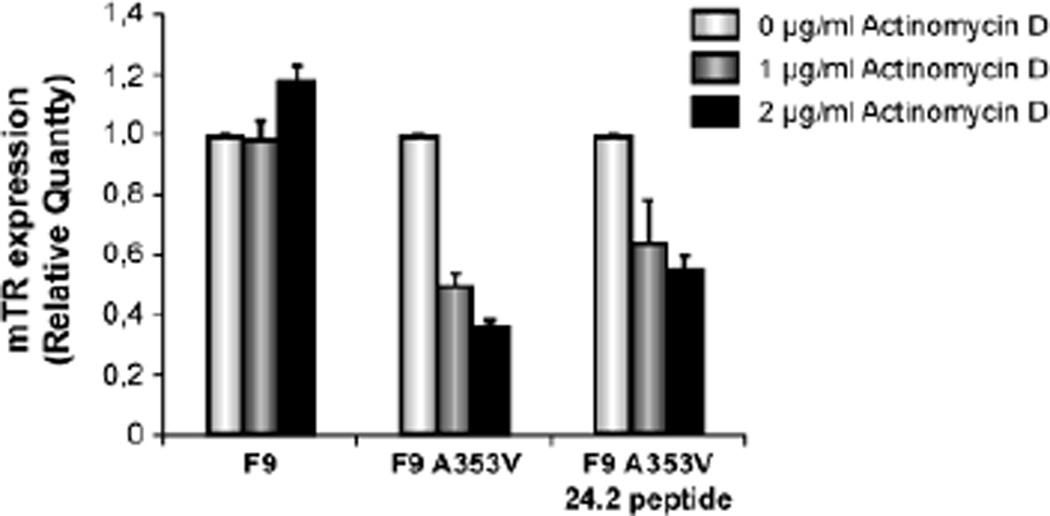
One of the key events affected by DKC A353V mutation was stability of mTR. However, we observed that hTR promoter activity was not changed when GSE24.2 was expressed. We wanted to investigate if this peptide was able to increase mTR stability, by assaying its effect on mTR half-life. For that purpose F9 and F9A353V cells were transfected with GSE24.2 peptide and treated with two doses of actinomycin D. Total RNA was obtained after 2 hours of treatment and expression of mTR assayed by qRT-PCR. The result showed that mTR-RNA levels did not change in F9 cells after actinomycin treatment, while in F9A353V cells a decrease in mTR expression levels up to 60% was observed, as expected (Fig. 6). F9A353V cells transfected with GSE24.2 peptide showed a more stable expression of mTR even after treatment with the higher dose of actinomycinD demonstrating that GSE24.2 peptide was able to increase mTR stability in the presence of a mutated Dkc1.
Fig. 6.
GSE24.2 peptide is able to increase mTR stability. F9, F9A353V and F9A353V cells transfected with GSE24.2 peptide (15 µg per 35 mm dish) were incubated two hours with actinomycin D at two concentrations (1 and 2 µg/ml). Total RNA was extracted and expression levels of mTR determined by qRT-PCR. Results are expressed relative to expression in the absence of drug.
Discussion
The predominant X-linked form of DC results from mutations in DKC1 encoding dyskerin, a protein required for ribosomal RNA-pseudouridine modification that is also a component of the telomerase complex. Telomerase activation in X-DC cells allows telomere elongation, telomere length maintenance and allows the cells to avoid premature senescence. This activation can be mediated by the expression of both TERT and TR that forms part of the telomerase complex. Expression of wt-DKC is not able to rescue either telomerase activity or premature senescence [23]. We have previously reported that a dyskerin domain (GSE24.2) reactivates telomerase activity in cells that are deficient in this activity by increasing hTERT and hTR levels. We have also previously reported that GSE24.2 activates hTERT transcription by modulating c-MYC expression and also increases TR levels. Supporting our previous findings, we here describe that expression of this dyskerin domain is able to recover telomerase activity in a X-DC mouse cell model carrying the mutation A353V. This mutation is the most frequent in X-DC patients [24] and is located within the highly conserved archeosine-transglycosylase (PUA) RNA binding domain of the protein [25]. Here we have found that, this mutation causes a marked decrease of mTR levels, a smaller decrease in mTERT levels and lowered telomerase activity in F9 cells. All these defects are reverted by GSE24.2 expression, as previously shown for the T66A mutation in X-DC cells [23]. The fact that GSE24.2 has the same effect on mouse and human cells [10], and with different DKC mutations, reinforces the proposal that this highly conserved peptide is functionally active [11, 26]. Deletion and mutagenesis studies have shown that the whole structure of this domain is essential for its action on telomerase. Neither of the two motifs that form the pseudouridylation domain of dyskerin recovered telomerase activity when individually expressed. In addition, we have shown that two residues that are highly conserved among different species are essential for GSE24.2 activity. Interestingly one of these residues, D125, is essential for the pseudouridine-synthase activity of dyskerin. Therefore an intact pseudouridine-synthase domain is required for GSE24.2 activity.
The GSE24.2 peptide purified from bacteria, once transfected into cells reactivates telomerase activity through the same mechanism that we have found when we transfect the cDNA and its activity lasted at least 72 hours after transfection. Interestingly, the peptide once transfected is able to increase, mTERT and c-myc levels together with increased mTR stability in F9-DKC mutant cells. These data support the idea that TR stabilization, mediated by GSE24.2 contributes efficiently to the recovery of telomerase activity in mutant cells. The fact that this peptide is able to rescue telomerase activity with the same efficiency as the cDNA does indicate that could be used to recover telomerase activity in human cells as an alternative transient, and chronic therapeutic tool to the cDNA for example for skin in X-DC patients.
Recently it has been reported that steady state levels of TR in X-DC with different DKC mutations are lower that WT-control fibroblasts. [27]. Lower levels of TR were found in these isoforms with mutations at the PUA domain such as A353V mutation and stable expression of TR transgene rescues for telomerase activity. In agreement with these findings, our observation that expression of A353V mutation in F9 cells strongly decreases mTR expression and its reversion by GSE24.2 expression as a cDNA or as a peptide, make us propose that the decrease in decreased levels of mTR in this mouse model could be the more important cause of the defects observed in mutant cells. By reactivating telomerase activity throughout an increase of mTR stability, GSE24.2 should attenuate the impact of A353V mutation on telomere metabolism and therefore on the ability of cells to divide. Indeed, we have previously shown that expression of GSE24.2 rescues dermal X-DC cells from senescence, indicating that expression of GSE24.2 may form the basis of a useful therapeutic strategy for X-DC patients either as a permanent or as a temporary telomerase activator both for systemic and topic uses. These will also results in decreased genomic instability by preventing telomere fusions and therefore cancers associated to X-DC.
Fig. 3.
Transfection of GSE24.2 motifs I or II do not induce reactivation of telomerase activity. (a) Amino acid sequence of dyskerin motifs I and II. B) F9 and F9A353V cells were transfected with Motif I, Motif II, pLNCX derived plasmids or pLNCX (10 µg DNA per 106 cells). Telomerase activity was determined with the TRAP assay kit. Different extract dilutions were used for each TRAP assay as indicated. Experiments were repeated 3 times, with similar results. (c) F9 and F9A353V cells were co-transfected with hTERT-luc or c-MYC-luc reporter plasmids (1 µg per 106 cells) and pGSE24.2 Motif I, Motif II, pLNCX derived plasmids or pLNCX. After 24h, cells were processed and the luciferase activity determined. The experiments were repeated 3 times, with similar results.
Acknowledgments
Funding sources
This work was supported by grants: 08/1485 from FIS, Fundación Genoma and BFU-05-0138 and Fundación Ramón Areces and R01 CA 106995 from the NIH. R.M-P was supported by Fundación Genoma. C. Manguan-Garcia and J Carrillo were supported by CIBER de Enfermedades Raras
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
References
- 1.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 2.Osterhage JL, Friedman KL. Chromosome end maintenance by telomerase. J Biol Chem. 2009;284:16061–16065. doi: 10.1074/jbc.R900011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SB, Graham ME, Lovrecz GO, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 4.Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- 6.Ni J, Tien AL, Fournier MJ. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Isaac C, Wang C, et al. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol Biol Cell. 2000;11:567–577. doi: 10.1091/mbc.11.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 9.Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet. 2008;73:103–112. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 10.Machado-Pinilla R, Sanchez-Perez I, Murguia JR, Sastre L, Perona R. A dyskerin motif reactivates telomerase activity in X-linked dyskeratosis congenita and in telomerase-deficient human cells. Blood. 2008;111:2606–2614. doi: 10.1182/blood-2007-04-083261. [DOI] [PubMed] [Google Scholar]
- 11.Knight SW, Heiss NS, Vulliamy TJ, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vulliamy TJ, Marrone A, Knight SW, et al. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci U S A. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S, Song YH, Kim UJ, Yim J, Kim TK. In vivo and in vitro analyses of Myc for differential promoter activities of the human telomerase (hTERT) gene in normal and tumor cells. Biochem Biophys Res Commun. 1999;263:361–365. doi: 10.1006/bbrc.1999.1366. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JQ, Hoare SF, McFarlane R, et al. Cloning and characterization of human and mouse telomerase RNA gene promoter sequences. Oncogene. 1998;16:1345–1350. doi: 10.1038/sj.onc.1201892. [DOI] [PubMed] [Google Scholar]
- 16.Kalinin A, Thoma NH, Iakovenko A, et al. Expression of mammalian geranylgeranyltransferase type-II in Escherichia coli and its application for in vitro prenylation of Rab proteins. Protein Expr Purif. 2001;22:84–91. doi: 10.1006/prep.2001.1423. [DOI] [PubMed] [Google Scholar]
- 17.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 20.Ramamurthy V, Swann SL, Paulson JL, Spedaliere CJ, Mueller EG. Critical aspartic acid residues in pseudouridine synthases. J Biol Chem. 1999;274:22225–22230. doi: 10.1074/jbc.274.32.22225. [DOI] [PubMed] [Google Scholar]
- 21.Spedaliere CJ, Hamilton CS, Mueller EG. Functional importance of motif I of pseudouridine synthases: mutagenesis of aligned lysine and proline residues. Biochemistry. 2000;39:9459–9465. doi: 10.1021/bi001079n. [DOI] [PubMed] [Google Scholar]
- 22.Zucchini C, Strippoli P, Biolchi A, et al. The human TruB family of pseudouridine synthase genes, including the Dyskeratosis Congenita 1 gene and the novel member TRUB1. Int J Mol Med. 2003;11:697–704. [PubMed] [Google Scholar]
- 23.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 25.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 26.Marrone A, Mason PJ. Dyskeratosis congenita. Cell Mol Life Sci. 2003;60:507–517. doi: 10.1007/s000180300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng XL, Thumati NR, Fleisig HB, et al. The accumulation and not the specific activity of telomerase ribonucleoprotein determines telomere maintenance deficiency in X-linked dyskeratosis congenita. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr504. Pub. ahead of prinitng Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]