Abstract
Secretory diarrhea remains a major health challenge worldwide. Excessive fluid secretion in the intestine caused by enterotoxins results in activation of luminal Cl− channels on enterocytes. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is the major cyclic adenosine monophosphate (cAMP)-regulated Cl− channel activated in cholera as well as in diarrheas caused by other bacterial enterotoxins. Small-molecule screens have yielded CFTR inhibitors with half-maximal inhibitory concentration (IC50) values as low as 4 nmol/l. The data from proof-of-concept studies in animal models support the development of CFTR inhibitors for antidiarrheal therapy.
Secretory diarrhea is the second leading cause of mortality globally in children under the age of 5 years, accounting for an estimated 15% of childhood deaths. In addition, repeated hypovolemia from diarrheal episodes has been linked to malnutrition, stunting, and impaired physical and mental development. 1 The intestine normally absorbs and secretes fluid across the epithelium, resulting in net fluid absorption in order to maintain adequate overall hydration. In secretory diarrheas such as cholera this balance is perturbed such that fluid secretion predominates.
The mainstay of diarrheal therapy is the administration of oral rehydration solution (ORS) to promote absorption of intestinal fluid and maintain hydration. The use of ORS has reduced mortality from diarrhea fourfold over the past 30 years. However, the effectiveness of ORS has diminished over the past decade, perhaps because of the practical difficulties involved in consistently administering large quantities of fluid and the consequent reduction in its use. Although ORS administration remains the first-line therapy for diarrheal disease, the use of antisecretory drugs that reduce diarrhea volume and duration may be useful as adjunctive therapy, and perhaps as first-line therapy when ORS is not available. In addition to achieving further reduction in overall mortality, potential benefits of antisecretory therapy include reduction in long-term sequelae such as impaired growth and development, increased use of ORS, and use in emergencies such as natural disasters, when the logistics of ORS administration are difficult.
INTESTINAL FLUID TRANSPORT MECHANISMS
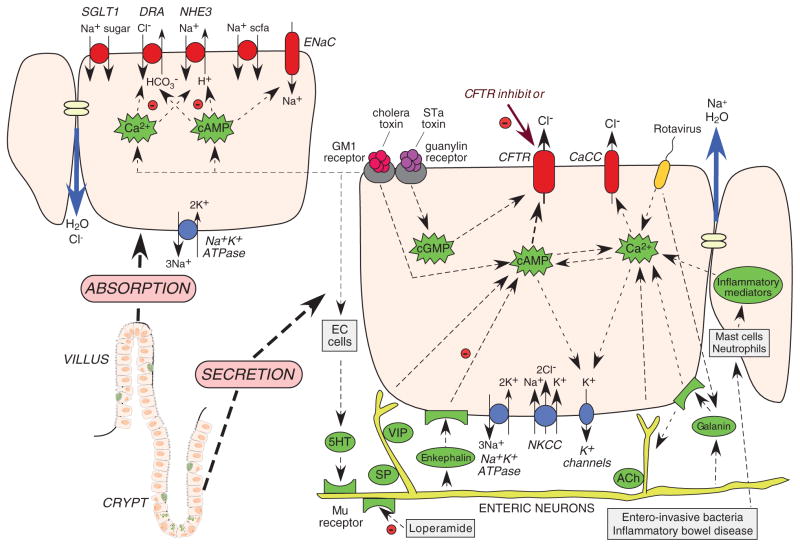
Fluid transport in the intestine, as in other epithelia, occurs secondary to active salt transport across the epithelium. Anatomically, the intestinal epithelium is composed of long, finger-like projections (villi) adjacent to cylindrical glands (crypts). Both absorption and secretion occur throughout the crypt–villus axis, with absorption predominating in villi and secretion in crypts (Figure 1). Fluid absorption in the small intestine is driven by Na+-coupled transport mechanisms at the luminal membrane, including Na+/H+ exchange and Na+-glucose cotransport, as well as luminal Cl−/HCO3 − exchange. The electrochemical driving force for absorption is established by the basolateral Na+K+-ATPase pump. These solute transporters are constitutively active, although they can be modulated by second messengers, including cAMP and Ca2+. In the colon, absorption is also facilitated by the epithelial Na+ channel and short-chain fatty acid transporters.
Figure 1.
Intestinal fluid transporting mechanisms. Lower left: crypt–villus unit in the small intestine, comprising basal crypt stem cells, enterocytes, enterochromaffin cells (EC cells), and goblet cells. Right: crypt secretory cell with luminal (top) and basal (bottom) transporters, ion channels, and second messengers. Left: villus absorptive cell with luminal (top) and basal (bottom) transporters. cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; CaCC, Ca2+-activated Cl− channel; CFTR, cystic fibrosis transmembrane conductance regulator; STa, heat-stable.
Fluid secretion in the intestine is driven by active Cl− transport from the basolateral to the apical side of enterocytes (Figure 1). Cl− is transported into the cell at the basolateral membrane by the Na+/K+/2Cl− cotransporter, which is driven by Na+ and Cl− concentration gradients produced by the Na+K+-ATPase and basolateral K+ channels. The electrochemical gradient drives Cl− secretion across the cell apical membrane through CFTR as well as Ca2+-activated Cl− channels (CaCCs). Paracellular Na+ secretion follows, creating the osmotic driving force for water secretion.
A variety of stimuli can cause enterocyte Cl− secretion (Figure 1). For example, secretory neuronal pathways cause release of 5-hydroxytryptamine from enterochromaffin cells, resulting in activation of cholinergic and vasoactive intestinal peptide neurons, and increases in cAMP and Ca2+. Inflammatory mediators such as prostaglandins and interleukins are also involved in Cl− secretion, as are nucleotides and purinergic signaling. Bacterial enterotoxins such as cholera toxin from Vibrio cholerae and heat-stable enterotoxin from Escherichia coli activate Cl− secretion though multiple convergent signaling pathways. Elevations in the levels of cAMP, cyclic guanosine monophosphate (cGMP), and Ca2+ activate apical Cl− channels (CFTR and CaCC) and basolateral K+ channels (KCNQ1/KNE3, KCNN4).
THE ROLES OF CFTR AND CaCC IN SECRETORY DIARRHEA
There is compelling evidence to implicate CFTR as the major Cl− channel responsible for fluid secretion in diarrheas caused by bacterial enterotoxins.2 The contribution of CaCCs to fluid secretion in various diarrheas is currently not clear. Some studies support a role for CaCCs in viral diarrheas, such as that caused by rotavirus, via the putative enterotoxin NSP4 acting through galanin receptors. CaCCs may also be involved in certain druginduced diarrheas and may contribute to cyclic nucleotide–dependent chloride secretion through cross-talk in intestinal signaling pathways. At present, the molecular identities of intestinal CaCCs remain unknown, making it difficult to evaluate their relative contribution to toxin-induced and viral diarrheas. Phenotype-based high-throughout screening has identified small-molecule inhibitors of intestinal CaCCs2; however, their utility in diarrhea therapy remains to be proven.
CFTR INHIBITORS
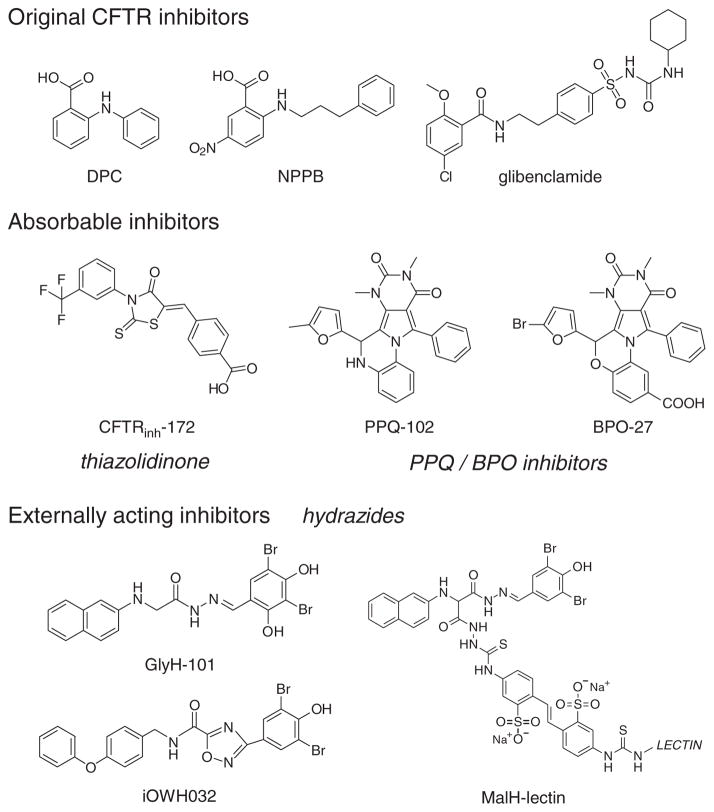
Before small-molecule screening, the available inhibitors of CFTR Cl− conductance included diphenylamine-2-carboxylate, 5-nitro-2-(3-phenylpropyl-amino)benzoate, and glibenclamide (Figure 2), all of which are nonspecific in their action and have low potency. High-throughput screening of small-molecule collections has yielded several new chemical classes of CFTR inhibitors. Screening has been carried out in epithelial cells expressing human CFTR and a genetically encoded I−-sensing fluorescent protein, in which cAMP-induced CFTR activity is deduced from the kinetics of intracellular fluorescence following the extracellular addition of I−.2
Figure 2.
Chemical structures of small-molecule CFTR inhibitors. Top: original (pre-high–throughput screening) CFTR inhibitors. Center: absorbable CFTR inhibitors acting at the CFTR cytoplasmic surface. Bottom: externally acting hydrazide-class CFTR inhibitors. BPO, benzopyrimido-pyrrolo-oxazine-dione; CFTR, cystic fibrosis transmembrane conductance regulator; DPC, diphenylamine-2-carboxylate; NPPB, 5-nitro-2-(3-phenylpropyl-amino)benzoate; PPQ, pyrimido-pyrrolo-quinoxalinedione.
The original class of CFTR inhibitors includes the thiazolidinone CFTRinh-172 (Figure 2), which has been widely used in cystic fibrosis research to investigate the involvement of CFTR in various cellular processes. Patch-clamp and mutagenesis studies indicate that CFTRinh-172 stabilizes the CFTR channel closed-state by binding at or near arginine-347 on the CFTR cytoplasmic surface. The IC50 for inhibition of CFTR Cl− current by CFTRinh-172 is ~300 nmol/l, although this value rises to several μmol/l in epithelial cells because of their strongly negative interior membrane potential. CFTRinh-172 has low toxicity and is excreted mainly by the kidney with minimal metabolism. CFTRinh-172 appears to accumulate in the intestine by enterohepatic recirculation, as shown by the high concentrations in bile. Studies in mouse models of cholera and heat-stable enterotoxin–induced intestinal fluid secretion have demonstrated the efficacy of CFTRinh-172.3 Structure–activity studies have identified thiazolidinone CFTR inhibitors with greater water solubility as compared with CFTRinh-172. These include an analog containing a 4-tetrazolophenyl in place of the 4-carboxyphenyl in CFTRinh-172. The tetrazolo analog reduced kidney cyst progression in mouse models of polycystic kidney disease, in which fluid secretion into cysts is CFTR-dependent.
Additional screening identified a second class of absorbable CFTR inhibitors—the PPQ/BPO inhibitors—that have a cytoplasmic site of action (Figure 2). PPQ-102 inhibits CFTR Cl− conductance, with an IC50 of ~ 90 nmol/l. Structure–activity analysis and follow-on medicinal chemistry efforts yielded the improved compound BPO-27, with structural changes that greatly increase its metabolic stability, inhibitory potency, and aqueous solubility.4 The IC50 for CFTR inhibition by (racemic) BPO-27 is ~8 nmol/l. We recently separated and determined the crystal structure of BPO-27 enantiomers; one enantiomer had an IC50 of ~ 4 nmol/l and the other was inactive. PPQ-102 and BPO-27 have shown efficacy in models of polycystic kidney disease but have not yet been tested in models of diarrhea.
The hydrazides (Figure 2) are an interesting class of CFTR inhibitors. They were discovered using a screen biased to identify compounds that act at the external surface of CFTR. Patch-clamp analysis of glycine hydrazide GlyH-101 showed an altered CFTR current–voltage relationship that changed from linear to inwardly rectifying, as well as rapid single-channel flicker. These findings, together with additional biophysical data, suggested an external pore-blocking inhibition mechanism. The lumen-facing site of action of the hydrazides provided an interesting opportunity to develop nonabsorbable compounds for antisecretory therapy. An inhibitor that is in early-phase clinical trials is iOWH032, an analog of GlyH-101(Figure 2). It has been shown to inhibit CFTR with an IC50 of ~ 8 μmol/l. However, several concerns raise doubts about the utility of small-molecule hydrazide inhibitors. These include (i) predicted rapid washout (by convection) of an externally bound inhibitor, (ii) multiple off-target effects of hydrazides, and (iii) poor inhibitory potency of hydrazides at interior-negative membrane potentials. The first concern is particularly critical; we have computed that, because of fluid convection across the luminal surface during secretion, the intestinal concentration of an externally acting inhibitor needs to be >100-fold its IC50 value to maintain inhibitory efficacy.
In attempting to overcome these concerns, we discovered from structure–activity studies that substitutions on the glycyl methylene group of the glycine hydrazide scaffold were tolerated, allowing the synthesis of nonabsorbable polyethylene glycol conjugates containing a malonic acid hydrazide (MalH) CFTR-inhibiting moiety. These macromolecular conjugates fully inhibited CFTR when added at the extracellular surfaces of cells. Various MalH–macromolecular conjugates were synthesized, including a MalH–lectin conjugate (Figure 2), the goal being to improve CFTR inhibitory potency and resist dissociation from the luminal surface of CFTR. MalH–lectin conjugates were generated with IC50 values lowered to 50 nmol/l for inhibition of CFTR Cl− conductance; these remained bound to CFTR for many hours, as compared with only a few seconds in the case of small-molecule hydrazides.5 The improved potency of the MalH–lectin conjugate and its resistance to washout is probably due to trapping in the enterocyte glycocalyx. Despite the advantages they possess as compared with the hydrazides, MalH–lectins are probably not practical development candidates for antisecretory therapy in developing countries because, as protein-containing conjugates, they are relatively costly to synthesize and may have limited stability during storage.
New pharmacologic therapies aimed at reducing the volume and duration of diarrheal episodes should prove valuable in reducing the mortality and morbidity associated with infectious diarrheas. The burden of diarrheal disease lies predominantly in the developing world, and therefore new antidiarrheal drugs should be very inexpensive to manufacture and distribute. To be widely available and not cost-prohibitive at the point of care, the development of antisecretory drugs will probably require the support of international health organizations and charities, as well as of governments and the pharmaceutical industry. CFTR is an attractive target for the development of drugs aimed at reducing secretion, although inhibition of CaCC may also be required in treating some diarrheas. At present, because of concerns about the potentially low in vivo efficacy of the externally acting hydrazides due to convective washout, the absorbable CFTR inhibitors of the thiazolidinone and PPQ/BPO classes are the most promising candidates for antisecretory therapy.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Petri WA, Jr, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Snyder D, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable pyrimido-pyrrolo-quinoxolindione CFTR inhibitor for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]