Abstract
The growing worldwide epidemic of obesity and diabetes portends a significant increase in cardiovascular disease. Obesity is associated with insulin resistance, and there is growing evidence that these conditions independently increase the risk of heart failure. Changes in myocardial substrate utilization develop in obesity and insulin resistance, and are characterized by increased fatty acid oxidation and utilization, and decreased glucose utilization. This paper will review the evidence for altered myocardial fatty acid utilization in obesity and insulin resistance, review mechanisms that are responsible, and discuss the relative contributions of systemic and myocardial insulin resistance in the regulation of fatty acid utilization in the heart.
Keywords: Fatty acid oxidation, insulin resistance, myocardial substrate utilization, obesity
Introduction
Recent statistics indicate an inexorable increase in the prevalence of obesity and insulin resistance in the developed world and emerging economies. These demographic changes are fueling an increase in the incidence of obesity-related disorders such as cardiovascular disease (CVD), diabetes, and sleep-disordered breathing [1,2]. The clustering of obesity, insulin resistance, and increased CVD risk has been termed the metabolic syndrome, and is defined by a clustering of abdominal obesity, increased triglyceride, decreased high-density lipoprotein cholesterol, glucose intolerance, and hypertension [3]. The increased prevalence of CVD in individuals with the metabolic syndrome is multifactorial and results from increased atherosclerosis, leading to: increased coronary artery disease (CAD) and myocardial ischemia; hypertension – an important risk factor for left ventricular hypertrophy and heart failure; increased hypercoagulability; sleep-disordered breathing that increases the risk of cardiac hypertrophy and atrial fibrillation; and glucose intolerance and diabetes, which amplify the aforementioned risks and which may have direct adverse consequences on the myocardium [1]. Obesity and diabetes are also independently associated with heart failure, even after adjusting for underlying CAD, and potential mechanisms have been reviewed in recent years [4,5]. This review will focus on the potential role of altered myocardial fatty acid utilization.
Pathophysiology of insulin resistance
Although genetic predisposition to insulin resistance and obesity exists, it is widely accepted that a major contribution to the increasing prevalence of obesity and insulin resistance is caloric excess and an increasingly sedentary lifestyle [2]. As body weight increases, there is expansion of the adipose tissue mass, particularly visceral adipose tissue. Insulin signaling is downregulated in adipose tissue, skeletal muscle, and the liver [6]. Because of the central role of insulin signaling in suppressing lipolysis, insulin resistance in adipose tissue is associated with increased release of free fatty acids, which in turns fuels increased hepatic generation of triglycerides. Expansion of adipose tissue is associated with increased release of adipokines such as leptin, and various inflammatory cytokines such as tumor necrosis factor-α. Moreover, there is reduced release of adiponectin. Insulin resistance also reduces adipocyte glucose transport, which might directly lead to increased release of the adipokine, retinol binding protein 4. These humoral and metabolic changes have all been implicated as potential mediators of changes in myocardial fatty acid utilization [1]. Insulin resistance also develops in skeletal muscle and liver. Several mechanisms are responsible for hepatic and muscle insulin resistance. These include: (1) lipotoxicity, arising from increased lipid deposition in these organs, which impairs insulin action as a consequence of increased accumulation of triglycerides and lipid intermediates such as ceramide and diacylglycerol (DAG) [7]; (2) increased activation of nutrient-sensing pathways such as the hexosamine biosynthetic pathway*, which directly impairs insulin signaling [8]; (3) increased activation of inflammation-mediated signaling cascades such as Toll-like receptor (TLR)* signaling, and jun N-terminal kinase (JNK)* signaling pathways [9]; (4) mitochondrial dysfunction, which has also been implicated in insulin resistance, although there is controversy regarding whether or not these are primary or secondary changes [10]. Many of these changes may also occur in cardiac muscle, and could potentially contribute to changes in cardiac metabolism. Although accumulating evidence suggests that myocardial insulin resistance develops in obesity and diabetes, as characterized by impaired insulin-stimulated glucose utilization, evidence from animals and humans suggests that proximal insulin signaling in the heart might be maintained, meaning that certain intracellular signaling pathways might actually be hyperactivated in the heart as a result of the hyperinsulinemia that inevitably accompanies systemic insulin resistance [11,12].
Myocardial fatty acid utilization in obesity and insulin resistance: insights from human and animal studies
The overwhelming majority of studies in this area have been performed in individuals or animal models of type 2 diabetes; they have been the subject of many review papers [1,4,13]. However, some recent studies have examined fatty acid utilization in humans and animals with obesity before the onset of significantly impaired glucose tolerance or diabetes. In a study of obese and insulin-resistant females, Peterson and colleagues [14] observed increased rates of myocardial fatty acid uptake, utilization, and oxidation, and increased myocardial oxygen consumption (mVO2), which increased in proportion to body mass index or to the degree of glucose intolerance. Independent studies in obese individuals have also described an association between obesity and increased myocardial concentrations of triglyceride [7]. Buchanan et al [15], reported that myocardial fatty acid oxidation and mVO2 were increased and cardiac efficiency was decreased in 4-week-old ob/ob and db/db mice (both of which develop obesity and insulin resistance, on the basis of leptin deficiency or resistance, respectively) at a time when these animals were obese and before the onset of hyperglycemia. Recent studies in rodents placed on high-fat and highcarbohydrate diets have also revealed increased myocardial fatty acid uptake and rates of fatty acid oxidation that precede the development of significant obesity or glucose intolerance [12,16]. Taken together, the findings of these studies support the conclusion that increased myocardial fatty acid utilization is a characteristic response of the heart to obesity or caloric excess and occurs independently of or before the onset of diabetes or impaired glucose tolerance.
Molecular mechanisms
PPARa signaling, glucose uptake, and fatty acid uptake
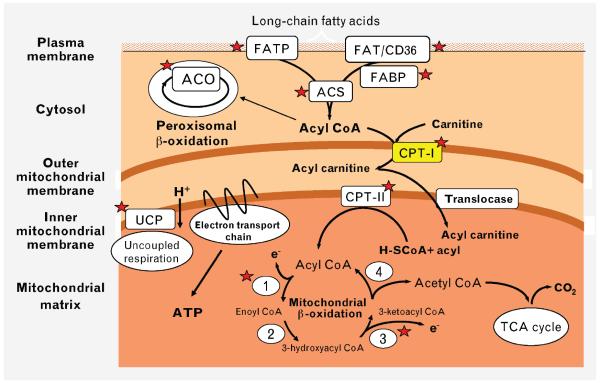
The classical view of the mechanism leading to increased fatty acid oxidation in obesity has been that obesity increases circulating concentrations of fatty acids, which in turn activates PPARα. Indeed, pharmacological activation of PPARα or transgenic overexpression of PPARα in the heart increases myocardial fatty acid oxidation [17,18]. As summarized in Figure 1, PPARα transactivates most genes involved in myocardial fatty acid utilization, including those involved in fatty acid uptake at the sarcolemma, generation of fatty acyl coenzyme A (CoA), mitochondrial uptake of fatty acyl CoA, and mitochondrial β-oxidation. An important regulatory node is mitochondrial fatty acyl CoA uptake via carnitine palmitoyl transferase-I (CPT-I), which is under allosteric inhibition by malonyl CoA. Steady-state concentrations of malonyl CoA are determined by the balance of synthesis by acetyl CoA carboxylase and degradation via malonyl CoA decarboxylase. Acetyl CoA carboxylase is inhibited by AMP kinase (AMPK), and malonyl CoA decarboxylase is a target of PPARα. Thus activation of AMPK or PPARα would decrease malonyl CoA concentrations by decreasing synthesis or by increasing malonyl CoA degradation. Decreasing concentrations of malonyl CoA will increase CPT-I activity. Recent studies have suggested that PPARα activation might not be an early event leading to increased fatty acid utilization in the heart in obesity, but may be a later event that sustains the increase. Four-week-old ob/ob and db/db mice exhibit significantly increased fatty acid oxidation before any increase in the expression of PPARα-regulated target genes, the levels of which increase only as the animals age [15]. Moreover, after short-term high-fat feeding, an increase in fatty acid oxidation was clearly evident as early as 2 weeks, and occurred in the absence of significant changes in the expression of PPARα target genes and even in the absence of changes in circulating concentrations of free fatty acids and triglycerides. Evidence for activation of PPARα target genes was seen only after 5 weeks of high-fat feeding. Hyperinsulinemia was present, however, and the ability of insulin to increase myocardial glucose uptake and the translocation of GLUT4 was impaired, despite normal insulin-mediated activation of Akt/protein kinase B (PKB) [12]. These studies point to a primary defect in glucose transport and GLUT4 translocation as an early change in the evolution of the metabolic adaptation of the heart to high-fat feeding. Because GLUT4 translocation accounts for the bulk of myocardial glucose utilization, we proposed a model wherein an initial reduction in myocardial glucose utilization leads to a secondary increase in fatty acid oxidation as a consequence of the Randle cycle. A recent study using cardiac tissue obtained from humans with diabetes also confirmed a defect in glucose uptake and plasma membrane GLUT4 content, despite an increase in proximal insulin signaling [11].
Figure 1.
PPARa targets within cardiomyocytes. Activation of PPARa by increased availability of fatty acid ligands increases the expression of several genes (indicated by red stars) that mediate increased fatty acid uptake at the sarcolemma, increased generation of fatty acyl CoA, increased mitochondrial fatty acid uptake, and increased b-oxidation. ACO, acyl coenzyme A (CoA) oxidase; ACS, acyl CoA synthetase; CPT, carnitine palmitoyl transferase; FAT, fatty acid translocase; FABP, fatty acid binding protein; FATP, fatty acid transport protein; TCA, tricarboxylic acid; UCP, uncoupling proteins 2 and 3.
Further evidence that argues against a role for malonyl CoA in the regulation of fatty acid oxidation in diet-induced obesity comes from recent studies demonstrating reduced activity of AMPK after highfat feeding, which would be predicted to increase malonyl CoA as a result of disinhibition of acetyl CoA carboxylase activity [19]. Moreover, increased concentrations of malonyl CoA, and normal CPT-I activity, were noted in the hearts of db/db mice [20]. An additional mechanism that might drive fatty acid utilization early in the evolution of high-fat feeding or obesity-related cardiac dysfunction is increased plasma membrane translocation of the fatty acid transporter, CD36. This was recently described in the hearts of db/db mice, and in Zucker (fa/fa) rats and rats fed a high-fat diet [16,20,21]. It is interesting to note that CD36 translocation in the heart is stimulated by insulin signaling in an Akt/PKB-dependent manner. It is well established that insulin acutely suppresses fatty acid oxidation, thus the simultaneous increase in CD36 translocation might seem to be paradoxical. However, in light of a recent report that most of the fatty acids that are oxidized in the heart arise from the endogenous triacylglycerol (TAG) pool [22], the possibility exists that, under physiological conditions, insulin may serve to replenish the cardiac TAG pool. It is further postulated that, under conditions of chronic hyperinsulinemia, because proximal insulin signaling to Akt remains intact in the heart in the evolution of diet-induced obesity, the associated hyperinsulinemia could increase CD36 translocation to the plasma membrane, thereby contributing to further expansion of the TAG pool and increased likelihood of accumulation of potential toxic intermediates of lipid metabolism.
Mitochondrial mechanisms
Recent studies in mouse models of obesity and insulin resistance such as ob/ob, db/db, and UCP-DTA (Uncoupling Protein-Diphtheria Toxin A) transgenic mice suggest that mitochondrial uncoupling accompanies the metabolic changes that develop in the heart [23–25]. It is not known if mitochondrial uncoupling contributes to the changes in fatty acid metabolism per se, but it might contribute in part to the associated increase in mVO2 and reduction in cardiac efficiency. A proposed mechanism for increased mitochondrial uncoupling in obesity and insulin resistance is an increase in mitochondrial superoxide generation that leads to activation of uncoupling proteins. Increased production of reactive oxygen species (ROS) is probably the consequence of an imbalance between increased generation of reducing equivalents from β-oxidation and impaired function of the electron transport chain [23,24]. A recent study using atrial appendages derived from humans with diabetes also demonstrated mitochondrial dysfunction and increased ROS generation. The strongest data to support mitochondrial uncoupling have been obtained in mouse models of obesity and type 2 diabetes. Hyperglycemia alone might not be sufficient to induce mitochondrial uncoupling, as we and others have observed no evidence for mitochondrial uncoupling or increased ROS generation in mitochondria of models of type 1 diabetes [26]. Thus ROS-induced mitochondrial uncoupling might be unique to diabetes that is associated with insulin resistance. Of interest, genetic disruption of insulin signaling in cardiomyocytes leads to mitochondrial dysfunction that is characterized by increased ROS generation and mitochondrial uncoupling [27].
Myocardial insulin resistance
Impaired myocardial insulin-stimulated glucose uptake has been described in many studies of humans and animal models with obesity and insulin resistance [1,28]. As discussed above, this might be the result of decreased translocation of GLUT4, leading in turn to increased fatty acid utilization. Although insulin signal transduction might be relatively preserved at early stages, studies performed in some models of diet-induced obesity and in genetic models of obesity and insulin resistance (often with associated diabetes), suggest that insulin signal transduction might also be impaired [29–32]. Thus the question arises regarding the direct effects of myocardial insulin resistance on myocardial fatty acid utilization. The best data have come from mouse models with genetic defects in insulin action that are restricted to cardiomyocytes. These models are not confounded by secondary metabolic consequences such as altered circulating concentrations of lipids, glucose, insulin, or cytokines, which could have a secondary effect on cardiac metabolism. We have examined fatty acid metabolism in mice with genetic deletion of insulin receptors (cardiomyocyte-selective insulin receptor knockout [CIRKO]) and in mice that express a dominant negative phosphatidyl inositol 3-kinase transgene (PI3K) [33,34]. In both these models, impaired insulin signaling to PI3K is associated with decreased rates of myocardial fatty acid oxidation that is attributable to reduced expression of PPARα and β-oxidation genes, and mitochondrial dysfunction. In CIRKO mice, we also confirmed, using proteomic analyses, that the mitochondrial content of fatty acid oxidation proteins was reduced. This contrasts with models of type 1 diabetes, which are also insulin deficient, but in which there is an increase in mitochondrial fatty acid oxidation proteins. Thus impaired myocardial insulin signaling directly regulates the capacity for mitochondrial fatty acid oxidation by repressing PPARα, whereas in diabetes, and in long-standing insulin resistance and obesity, PPARα is activated as a result of increased concentrations of fatty acid ligands, leading to an increased fatty acid oxidation capacity of mitochondria. Thus it is important, when considering the impact of insulin resistance on myocardial fatty acid oxidation, to distinguish between direct effects of insulin resistance on myocardial metabolism and indirect effects that arise from changes in systemic metabolism or changes in myocardial glucose utilization.
Conclusion
An increase in myocardial fatty acid oxidation is an early and consistent finding in obesity and insulin resistance. The mechanisms for the increase in fatty acid utilization are multifactorial, and are summarized in Figure 2. Recent studies have highlighted an important role for changes in glucose utilization as a potential initial inciting event that leads to increased fatty acid oxidation. In addition, preservation of proximal insulin signaling (despite increased concentrations of DAG) promotes plasma membrane translocation of CD36. Later changes are sustained and driven by increased activation of PPARα targets. Increased fatty acid oxidation is associated with decreased cardiac efficiency, which is in part the result of the increased oxygen costs of oxidizing fatty acids, with contributions from mitochondrial uncoupling in insulin-resistant models with diabetes and impaired glucose tolerance.
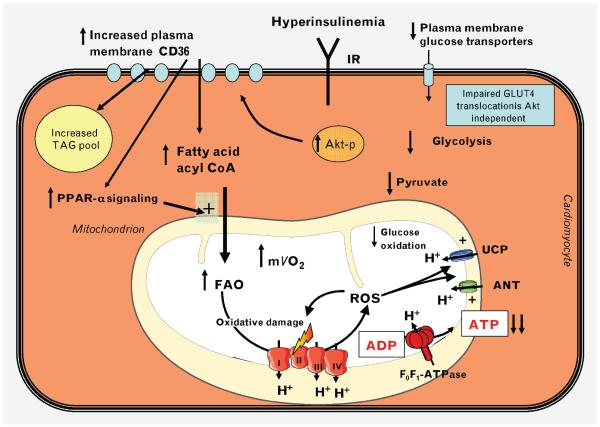
Figure 2.
Mechanisms leading to increased myocardial fatty acid oxidation in insulin-resistant states. In the evolution of diet-induced obesity, or in type 2 diabetes, hyperinsulinemia activates insulin receptors (IR) and Akt (protein kinase B), leading to increased plasma membrane translocation of CD36, which leads to increased fatty acid uptake. Decreased expression and translocation of glucose transporter-4 (GLUT4) in insulin resistance leads to decreased glucose uptake and decreased glycolysis, which further increase fatty acid utilization. Reduced GLUT4 translocation precedes significant downregulation of insulin signal transduction to Akt. Mechanisms for reduced GLUT4 translocation are incompletely understood. Increased lipid availability activates PPARa, which leads to increased expression of proteins involved in fatty acid utilization, and increased pyruvate dehydrogenase kinase-4 (PDK4), which increase fatty acid oxidation (FAO) and decrease glucose oxidation, respectively. Increased FAO is associated with increased myocardial oxygen consumption (mVO2). As insulin resistance progresses and diabetes ensues, reactive oxygen species (ROS)-mediated mitochondrial uncoupling develops, which further increases mVO2, decreases ATP generation, and decreases cardiac efficiency. I–IV, Mitochondrial electron transport chain complexes I-IV; Akt-p, phospho-Akt/protein kinase B; ANT, adenine nucleotide translocase; CoA, coenzyme A; TAG, triacylglycerol; UCP, uncoupling proteins 2 and 3.
Acknowledgments
Work in the Abel laboratory has been supported by grants from the National Institutes of Health, the American Heart Association, The American Diabetes Association, and the Juvenile Diabetes Research Foundation.
Footnotes
see glossary for definition of these terms.
Conflicts of interest: None.
REFERENCES
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1–7. doi: 10.1016/j.gtc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 6.Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109:137–148. doi: 10.1097/ALN.0b013e3181799d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Ongusaha PP, Miles PD, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 9.Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2009;11:761–765. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31:25–51. doi: 10.1210/er.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and leftventricular dysfunction. Eur Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JJ, Kim J, Buchanan J, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 14.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 16.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 17.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafstad AD, Khalid AM, Hagve M, et al. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and postischaemic functional loss. Cardiovasc Res. 2009;83:519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- 19.Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carley AN, Atkinson LL, Bonen A, et al. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem. 2007;113:65–75. doi: 10.1080/13813450701422617. [DOI] [PubMed] [Google Scholar]
- 21.Ouwens DM, Diamant M, Fodor M, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–1948. doi: 10.1007/s00125-007-0735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banke NH, Wende AR, Leone TC, et al. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARα. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudina S, Sena S, O’Neill BT, et al. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 24.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 25.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bugger H, Boudina S, Hu XX, et al. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57:2924–2932. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:219–226. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 29.Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK, Clarke K. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res. 2004;61:288–296. doi: 10.1016/j.cardiores.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Mazumder PK, O’Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 31.Ouwens DM, Boer C, Fodor M, et al. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 33.Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neill BT, Kim J, Wende AR, et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]