Abstract
Transmembrane protein 16A (TMEM16A) channels are recently discovered membrane proteins that functions as a calcium activated chloride channel (CaCC). CaCCs are major regulators of various physiological processes, such as sensory transduction, epithelial secretion, smooth muscle contraction and oocyte fertilization. Thirty novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids (B01–B30) were synthesized and evaluated for their TMEM16A inhibitory activity by using short circuit current measurements in Fischer rat thyroid (FRT) cells expressing human TMEM16A. IC50 values were calculated using YFP fluorescence plate reader assay. Final compounds, having free carboxylic group displayed significant inhibition. Eight of the novel compounds B02, B13, B21, B23, B25, B27, B28, B29 exhibit excellent CaCCs inhibition with IC50 value <6 μM, with compound B25 exhibiting the lowest IC50 value of 2.8 ± 1.3 μM. None of the tested ester analogs of final benzofuran derivatives displayed TMEM16A/CaCCs inhibition.
Keywords: Calcium activated chloride channel, Transmembrane protein TMEM16A, Short circuit current, 5-Substituted benzyloxy-2-arylbenzofuran-3-carboxylic acid, CFTR
1. Introduction
Formation of a transmembrane-conductive pathway for anions is a common functional characteristic of a structurally heterogeneous group of channel proteins known as anion channels. Cl– ion being the most abundant permeable anion under physiologic conditions, these channels mostly mediate Cl– currents. It is well established that Cl– channels have fundamental roles in many physiological functions.1–6 Members of one group of these channels are activated by intracellular Ca2+, and accordingly are collectively referred to as Ca2+ activated chloride channels (CaCCs).7–10 CaCCs are present in various tissues and are fundamental mediators in numerous physiological processes including cardiac and neuronal excitation, sensory transduction, transepithelial secretion, smooth muscle contraction, fertilization, etc.11–13 CaCCs are potential drug targets for hypertension, secretory diarrheas, asthma and pain.14,15 Notwithstanding the important role played by CaCCs in various physiologic functions, understanding the structure, function, and regulation of CaCCs is still far from complete owing to the uncertainty about the underlying channel protein and a lack of specific and potent chemical modulators of CaCCs.1 Recent studies have shown that transmembrane protein 16A (TMEM16A),16–18 also called anoctamin 1 (ANO1), is a valid molecular counterpart of the CaCCs that is activated by intracellular Ca+2 and Ca+2-mobilizing stimuli. Owing to poor understanding about the intricacies of the CaCCs, these channels are still studied using pharmacological agents as main tools. The agents include structurally diverse chemical classes and most of the compounds available to inhibit CaCCs are either non-specific or the minimum inhibition concentration is rather high.19–21 Thus efforts are essentially required in developing more specific inhibitors so as to enhance our understanding of the functional role and nature of these channels. Benzofuran derivatives has been shown a wide spectrum of the biological activities such as antiviral, anti-inflammatory, antimicrobial, dopamine D2 receptor antagonists, protein tyrosine phosphatase 1B inhibitors, etc.22–26 Appreciation of these findings led us to investigate 5-benzyloxy-2-arylbenzofuran-3-carboxylic acids as TMEM16A/CaCC inhibitors. We focused our attention on benzofuran derivatives following a recent report from Verkman and co-workers27 detailing the potential of benzofuran core as TMEM16A inhibitors. This is an exploratory work in search for new drugs targeting CaCCs.
2. Results
Novel compounds synthesized in present investigation were evaluated for their Calcium activated chloride channels inhibition using Fischer rat thyroid (FRT) cells transfected with human TMEM16A and the halide sensor YFP-F46L/H148Q/I152L. The IC50 values calculated from YFP fluorescence plate reader assay are given in Table 1.
Table 1.
Calcium activated chloride channels inhibitory assay data of benzofuran derivatives (B01–B30). IC50 was determined from fluorescene plate reader assay (IC50 mean ± S.E., n = 3)
| Compound | Ar | R1 | R2 | R3 | R4 | IC50 (μM) |
|---|---|---|---|---|---|---|
| B01 | 4-OCH3C6H4 | H | F | H | H | 28.7 ± 3.6 |
| B02 | 4-OCH3C6H4 | H | I | H | H | 5.9 ± 1.9 |
| B03 | 4-OCH3C6H4 | H | H | Br | H | 16.3 ± 2.6 |
| B04 | 4-OCH3C6H4 | H | Cl | H | H | 29.2 ± 4.1 |
| B05 | 4-OCH3C6H4 | F | H | H | F | 27.0 ± 2.5 |
| B06 | 4-OCH3C6H4 | H | CF3 | H | H | NA |
| B07 | 4-OCH3C6H4 | H | F | F | H | 23.5 ± 3.3 |
| B08 | 4-OCH3C6H4 | H | H | F | H | 21.2 ± 3.6 |
| B09 | 4-OCH3C6H4 | Br | H | H | H | 11.2 ± 2.4 |
| B10 | 4-OCH3C6H4 | H | C6H5 | H | H | 25.9 ± 4.0 |
| B11 | 4-CH3C6H4 | H | F | H | H | 15.6 ± 3.1 |
| B12 | 4-CH3C6H4 | H | I | H | H | 10.8 ± 1.9 |
| B13 | 4-CH3C6H4 | H | H | Br | H | 3.3 ± 1.1 |
| B14 | 4-CH3C6H4 | H | Cl | H | H | 13.3 ± 2.9 |
| B15 | 4-CH3C6H4 | F | H | H | F | 23.0 ± 4.4 |
| B16 | 4-CH3C6H4 | H | CF3 | H | H | 13.1 ± 2.5 |
| B17 | 4-CH3C6H4 | H | F | F | H | 9.0 ± 2.9 |
| B18 | 4-CH3C6H4 | H | H | F | H | 17.3 ± 2.4 |
| B19 | 4-CH3C6H4 | Br | H | H | H | 6.3 ± 1.2 |
| B20 | 4-CH3C6H4 | H | C6H5 | H | H | 16.3 ± 3.5 |
| B21 | Naphthyl | H | F | H | H | 3.8 ± 1.7 |
| B22 | Naphthyl | H | I | H | H | 12.3 ± 2.1 |
| B23 | Naphthyl | H | H | Br | H | 4.9 ± 1.5 |
| B24 | Naphthyl | H | Cl | H | H | 7.7 ± 2.1 |
| B25 | Naphthyl | F | H | H | F | 2.8 ± 1.3 |
| B26 | Naphthyl | H | CF3 | H | H | 10.4 ± 3.4 |
| B27 | Naphthyl | H | F | F | H | 3.2 ± 1.6 |
| B28 | Naphthyl | H | H | F | H | 3.2 ± 1.9 |
| B29 | Naphthyl | Br | H | H | H | 5.2 ± 2.3 |
| B30 | Naphthyl | H | C6H5 | H | H | 26.9 ± 4.3 |
NA = Not active.
The short circuit current measured for the compound B25 which exhibits the highest inhibition of CaCC/TMEM16A, in FRT cells is presented in the form of Figure 1.
Figure 1.
Representative current traces showing dose-dependent inhibition of TMEM16A chloride current by compound B25. Short-circuit (apical membrane) current measured in TMEM16A-expressing FRT cells in the presence of a transepithelial chloride gradient. Inhibitors were added 5 min prior to TMEM16A activation by 100 μM ATP.
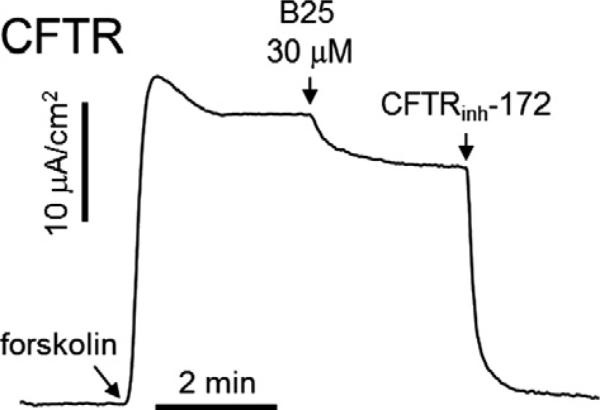
In Figure 2, we investigated effect of the compound B25 on cystic fibrosis transmembrane conductance regulator (CFTR, a cAMP-regulated chloride channel). The compound B25 (30 μM) had little effect on CFTR Cl– conductance (inhibited by <20%).
Figure 2.
Representative current traces showing inhibition of CFTR chloride current by compound B25. CFTR was activated by 10 μM forskolin in primary cultured human bronchial epithelial cells and 30 μM compound B25 added to apical bath as indicated. The remaining CFTR activity was blocked by 10 μM CFTRinh-172.
3. Discussion
3.1. Chemistry
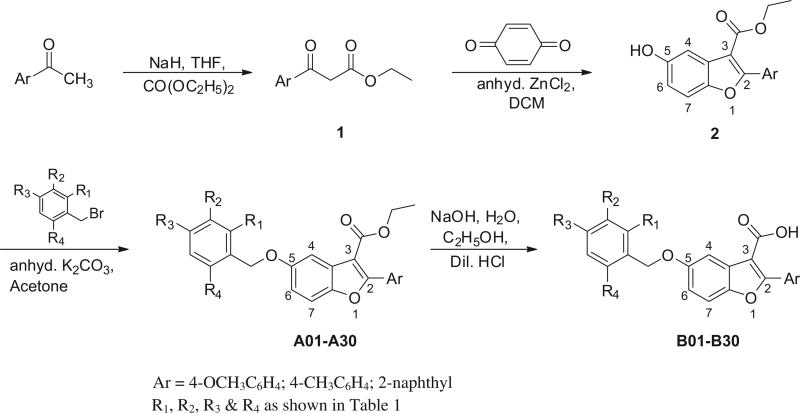
In the present investigation target compounds 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acid derivatives (B01– B30) were synthesized mainly in the lab of Late Professor Aaron D. Mills (Department of Chemistry, University of Idaho, Moscow, ID, USA), following the synthetic route as outlined in Scheme 1. Benzofuran core structure was constructed from the conveniently available benzoylacetates (1) as starting material which in turn was prepared by sodium hydride mediated carbethoxylation of the commercially available appropriately substituted acetophenones with diethyl carbonate.28 Zn-mediated condensation of the benzoylacetates (1) with p-benzoquinone in dichloromethane at 110 °C in a microwave reactor resulted in the formation of substituted ethyl 5-hydroxy-2-arylbenzofuran-3-carboxylates (2). Benzylation of hydroxyarylbenzofurans 2 with appropriately substituted benzyl bromides in acetone in the presence of potassium carbonate yielded ethyl 5-substituted benzyloxy-2-aryl-1-benzofuran-3-carboxylates (A01–A30) which were converted to the corresponding acids, 5-substituted benzyloxy-2-aryl-1-benzofuran-3-carboxylic acids (B01–B30) following basic hydrolysis of the ester group under microwave conditions.
Scheme 1.
The novel compounds synthesized in the present study were characterized by their IR, 1H NMR, 13C NMR, mass as well as elemental analysis and are in full agreement with the proposed structures. 1H NMR of ethyl 3-aryl-3-oxopropionates (1) exhibits characteristics of 1,3-diketoesters in the form of a quartet around δ 4.2, singlet around 4.0 and a triplet around δ 1.3–1.2 ppm. In 13C NMR, signals around δ 193.9–190.3 and δ 168.5–167.6 confirmed the presence of two carbonyl groups. A signal around δ 9.5–8.3 in the 1H NMR of substituted benzofurans 2 can be assigned to the hydroxy group present at C-5 position of the benzofuran ring. Further a quartet around δ 4.3–4.0 and triplet around δ 1.3 can be assigned to ethoxy group of ester moiety at C-3 position of benzofuran ring. Signal in the form of doublet of doublet around δ 6.9–6.8 can be ascribed to proton present at C-6 position of benzofuran ring. 1H NMR of compounds A01–A30 predictably differ from that of 2 by exhibiting the presence of a singlet for two benzylic protons around 5.2 ppm besides showing additional aromatic protons from the benzyloxy group. A quartet around δ 4.3 and a triplet around δ 1.3 confirms the presence of ethoxy group of ester moiety at C-3 position of benzofuran ring in A01–A30. A broad singlet around δ 13.3–13.0 due to carboxylic proton in 1H NMR spectra confirmed the presence of free carboxylic group in final compounds B01–B30. Another singlet around δ 5.3–5.1 can be assigned to –OCH2– group of benzyloxy group. In 13C NMR spectra of three compounds (B01, B11, B21), presence of fluorine at 3-position of benzyloxy group is evident from the presence of doublets around δ 163.5–162.2 (1JCF = 243 Hz), 140.1–130.4 (3JCF = 8 Hz) and 115.8–114.1 (2JCF = 28–22 Hz). The vicinal presence of fluorine at 3- and 4-position of the benzyloxy group in B07, B17 and B27 was evident from the occurrence of two doublet of doublets around δ 149.7–149.2 (1JCF = 251–244 Hz; 2JCF = 30 Hz) and 149.5–149.0 (1JCF = 245–244 Hz; 2JCF = 29 Hz).
3.2. Biology
The final compounds (B01–B30) were evaluated for their calcium activated chloride channel inhibition assay using short circuit current measurement following the method used previously.29 IC50 (μM) values calculated from YFP fluorescence plate reader assay are given for all the newly synthesized compounds in Table 1. Compound B25 with the lowest IC50 value of 2.8 ± 1.3 μM displayed highest potential as CaCC inhibitor in the present investigation amongst all the newly synthesized compounds. In general, compounds with a naphthyl group at postion-2 of benzofuran show better inhibition as compared to the compounds with a tolyl or anisyl group. Furthermore, compounds having 4-methylphenyl group at 2-position of benzofuran core displayed better inhibition than having 4-methoxyphenyl. Compounds B21, B23, B25, B27, B28 and B29 having naphthyl ring at 2-position of benzofuran ring and fluoro or bromo group at different positions of benzyloxy group exhibit enhanced inhibition of calcium activated chloride channels compared to compounds having other groups. Amongst the compounds having 4-methoxyphenyl group at 2-position of benzofuran ring, only B02 with an iodo group at 3-position of benzyloxy moiety exhibits strong inhibition with IC50 value of 5.9 ± 1.9 μM. Amongst the compounds with a 4-methylphenyl group at position-2 of the benzofuran ring, compounds B13 and B19 containing a bromo substituent at the benzyloxy moiety exhibit strong inhibition with IC50 value 3.3 ± 1.1 & 6.3 ± 1.2, respectively.
The short circuit current data for B25, the best compound of the present study, is given in the form of Figure 1. None of the tested ester derivatives of the synthesized benzofurans (A01–A030) in the present investigation show any inhibition against TMEM16A. It can be concluded from the results that a free carboxylic acid group is better than the corresponding ester group for such compounds to act as CaCC inhibitors. The selectivity of the best TMEM16A inhibitor (B25) was studied as depicted in Figure 2. Many CaCC inhibitors strongly block the CFTR activity at the concentration showing complete inhibition of CaCC activity but B25 does not significantly affect the CFTR activity. This result suggests that B25 could be used as a selective TMEM16A/CaCC inhibitor.
4. Conclusion
In the present investigation benzofuran derivatives B01–B30 were synthesized and evaluated for their calcium activated chloride channels inhibition using TMEM16A. Except one compound B06, all other novel benzofuran derivatives displayed inhibition of the calcium activated chloride channels. Some of the newly synthesized compounds exhibit excellent inhibition of TMEM16A as evident from their IC50 values given in Table 1. Eight of the final compounds B02, B13, B21, B23, B25, B27, B28, B29 showed TMEM16A inhibition with IC50 <6 μM. The best compound amongst all is 5-[(2,6-difluorobenzyl)oxy]-2-(2-naphthyl)benzofuran-3-carboxylic acid (B25), with IC50 value 2.8 ± 1.3 μM. Fifteen ester analogs of the 5-(substituted benzyloxy)-2-arylbenzofuran-3-carboxylic acid were also assayed for TMEM16A inhibition but surprisingly, none of the ester analogs of the final benzofuran compounds showed TMEM16A inhibition. From the IC50 data, it seems that compounds having fluoro or bromo groups exhibit better inhibition as compared to chloro, iodo, and phenyl. Moreover the presence of the trifluoromethyl group as substituent at 3-position of benzyloxy group does not have significant effect on the inhibitory power. From the biological assay data, it may be concluded that the presence of free carboxylic functionality is better as compared to an ester functionality to support the potency of compounds as TMEM16A inhibitors. Novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids can be used as potential research tools for pharmacological dissection of TMEM16A function.
5. Experimental
5.1. General
Melting points were taken in open capillaries using Thomas Hoover melting point apparatus and are uncorrected. IR spectra were recorded with MB3000 Horizon FTIR. 1H and 13C nuclear magnetic resonance spectra were recorded in deutrated chloroform (CDCl3) or dimethyl sulfoxide (DMSO-d6) using a 300 MHz Bruker spectrometer with Tetramethylsilane (TMS) as an internal standard. Mass spectrometry, DART-MS (Direct Analysis in Real Time) was done on a JEOL-AccuTOF JMS-T100LC Mass spectrometer.
5.2. General protocol for synthesis of ethyl 3-aryl-3-oxopropionates (1)
To a stirred mixture of sodium hydride (3 mol equiv) washed with hexane (3 × 15 ml), and diethyl carbonate (4 mol equiv) in 50 mL of tetrahydrofuran (THF) was added drop wise appropriately substituted acetophenone (1 mol equiv) over 30 min. Reaction mixture was refluxed for 3–4 h till color changed to dark brown and monitored by TLC (10% ethyl acetate/hexanes). The reaction mixture was cooled and acidified with 5 mL glacial acetic acid followed by addition 100 mL of ice cold dilute HCl solution. The aqueous layer was extracted with ethyl acetate (3 × 75 ml); combined organic phase was washed with saturated sodium bicarbonate, brine and water, dried over anhydrous Na2SO4 and evaporated in vacuo, yielded the desired product as viscous mass in excellent yield. The compounds were characterized on the basis of their 1H NMR and 13C NMR spectra as reported in the literature.
5.2.1. Ethyl 3-(4-Methoxyphenyl)-3-oxopropanoate (1a)30
Viscous oil, yield 95%; 1H NMR (300 MHz, CDCl3): δ 7.89 (d, J = 7 Hz,2H, Ar-H), 6.91 (d, J = 7 Hz, 2H), 4.17 (q, J = 7 Hz, 2H, OCH2CH3), 3.90 (s, 2H, COCH2CO), 3.83 (s, 3H, OCH3), 1.22 (t, 3H, J = 7 Hz, OCH2CH3); 13C NMR (75.5 MHz, CDCl3): 190.9, 167.6, 163.9, 130.8, 129.1, 113.9, 61.3, 55.4, 45.7, 14.0.
5.2.2. Ethyl 3-(4-methylphenyl)-3-oxopropanoate (1b)28
Viscous oil, yield 92%; 1H NMR (300 MHz, CDCl3): δ 7.85 (d, J = 8 Hz, 2H, Ar-H), 7.29 (d, J = 8 Hz, 2H, Ar-H), 4.22 (q, J = 7 Hz, 2H, OCH2CH3), 3.98 (s, 2H, COCH2CO), 2.43 (s, 3H, CH3), 1.26 (t, 3H, J = 7 Hz, OCH2CH3); 13C NMR (75.5 MHz, CDCl3): 192.1, 167.6, 144.7, 133.7, 129.4, 128.7, 61.4, 46.0, 21.7, 14.3.
5.2.3. Ethyl 3-(naphthalen-2-yl)-3-oxopropanoate (1c)31
Viscous oil, yield 90%; 1H NMR (300 MHz, CDCl3): δ 8.42 (s, 1H, Ar-H), 7.99 (dd, J = 2, 9 Hz, 1H, Ar-H), 7.93 (d, J = 8 Hz, 1H, Ar-H), 7.87 (d, J = 8 Hz, 1H, Ar-H), 7.82 (d, J = 8 Hz, 1H, Ar-H), 7.61–7.50 (m, 2H, Ar-H), 4.22 (q, J = 7 Hz, 2H, COOCH2CH3), 4.00 (s, 2H, COCH2CO), 1.30 (t, J = 7 Hz, 3H, COOCH2CH3); 13C NMR (75.5 MHz, CDCl3): 193.3, 168.5, 136.7, 134.3, 133.3, 131.5, 130.6, 129.8, 129.6, 128.6, 127.9, 124.7, 62.4, 47.0, 15.2.
5.3. General protocol for synthesis of ethyl 5-hydroxy-2-arylbenzofuran-3-carboxylates (2)
Appropriately substituted ethyl 3-aryl-3-oxopropionate (1 mol equiv), p-benzoquinone (1.1 mol equiv), anhydrous ZnCl2 (0.7 mol equiv) and 10 mL dichloromethane in a 20 mL microwavable sealed vial (with stand pressure up to 25 bars) were microwaved for 30 min at 110 °C in biotage initiator. Reaction was monitored by TLC (ethyl acetate/hexanes) and 1H NMR spectroscopy. The crystalline solid separated out in the vial was filtered, washed with dichloromethane and dried over vacuum to provide pure product. Furthermore crude left was recrystallized from ethyl acetate–hexane to provide the desired compound in overall good yield.
5.3.1. Ethyl 5-hydroxy-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylate (2a)
Mp 170–172 °C [lit. 172–173 °C],32 yield 84%; 1H NMR (300 MHz, DMSO-d6): δ 8.34 (s, 1H, OH), 7.91 (d, J = 8 Hz, 2H, Ar-H), 7.41 (s, 1H, Ar-H), 7.22 (d, J = 8 Hz, 1H, Ar-H), 6.90 (d, J = 8 Hz, 2H, Ar-H), 6.79 (dd, J = 2, 9 Hz, 1H, Ar-H), 4.30 (q, J = 7 Hz, 2H, COOCH2CH3), 3.79 (s, 3H, OCH3), 1.33 (t, 3H, J = 7 Hz, COOCH2CH3); 13C NMR (75.5 MHz, DMSO-d6): 164.1, 161.2, 160.9, 153.9, 147.9, 130.9, 128.0, 122.2, 113.7, 113.3, 110.9, 107.6, 107.4, 60.2, 55.2, 14.2.
5.3.2. Ethyl 5-hydroxy-2-(4-methylphenyl)-1-benzofuran-3-carboxylate (2b)
Mp 154–156 °C, yield 82%; 1H NMR (300 MHz, DMSO-d6): δ 8.82 (s, 1H, OH), 7.82 (d, J = 7 Hz, 2H, Ar-H), 7.75–7.72 (m, 4H, Ar-H), 6.83–6.80 (m, 1H, Ar-H), 4.31 (q, J = 6 Hz, 2H, COOCH2CH3), 2.36 (s, 3H, CH3), 1.34 (t, 3H, J = 6 Hz, COOCH2CH3); 13C NMR (75.5 MHz, DMSO-d6): 163.7, 160.3, 153.8, 147.6, 139.9, 130.6, 128.8, 127.6, 126.5, 113.7, 110.8, 108.6, 107.0, 60.0, 21.1, 13.9.
5.3.3. Ethyl 5-hydroxy-2-(naphthalene-2-yl)-1-benzofuran-3-carboxylate (2c)
Mp 168–170 °C, yield 86%; 1H NMR (300 MHz, DMSO-d6): δ 9.52 (s, 1H, OH), 8.57 (s, 1H, Ar-H), 8.07–7.96 (m, 4H, Ar-H), 7.64–7.60 (m, 2H, Ar-H), 7.54 (d, J = 9 Hz, 1H, Ar-H), 7.43 (d, J = 2 Hz, 1H, Ar-H), 6.89 (dd, J = 2, 9 Hz, 1H, Ar-H), 4.34 (q, J = 7 Hz, 2H, COOCH2CH3), 1.30 (t, J = 7 Hz, 3H, COOCH2CH3); 13C NMR (75.5 MHz, DMSO-d6)): 163.4, 160.4, 154.7, 147.8, 133.6, 132.4, 129.6, 128.9, 127.8, 127.7, 127.0, 126.8, 126.2, 115.9, 114.7, 112.0, 108.8, 106.8, 60.6, 14.2.
5.4. General protocol for synthesis of 5-aryloxy-2-arylbenzofuran-3-carboxylic acids (B01–B30)
Ethyl 5-aryloxy-2-arylbenzofuran-3-carboxylate
A stirred mixture of appropriate ethyl 5-hydroxy-2-arylbenzofuran-3-carboxylate (1 mol equiv), appropriately substituted benzyl bromide (1.2 mol equiv) and anhydrous potassium carbonate (3 mol equiv) in 10 mL acetone was refluxed for 6–8 h at 80 °C over oil bath. After evaporation of solvent under reduced pressure crude product so obtained was purified by column chromatography (ethyl acetate/hexane) 1:19 yielded the desired compound (A) as crystalline solid in excellent yield.
Hydrolysis of ester
A suspension of appropriate ethyl 5-aryloxy-2-arylbenzofuran-3-carboxylate (0.364 mmol) in 4 mL ethanol, 4 mL 5% NaOH and 4 mL water was microwaved in sealed tube for 10 min at 50 °C. Poured the reaction mixture in ice cold water and neutralization with dil. HCl solution resulted in precipitation of desired product as white solid. Solid so obtained was filtered, washed with water and dried to obtain the final compound (B) in excellent yield.
5.4.1. 5-[(3-Fluorobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B01)
Mp 185–186 °C, yield 84%; IR (cm–1): 1674, 1612, 1582, 1504, 1458, 1257, 1196, 1095, 1026, 933, 833, 771; 1H NMR (300 MHz, DMSO-d6): δ 13.02 (br s, 1H, COOH), 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.59 (d, J = 9 Hz, 2H, Ar-H), 7.49–7.42 (m, 2H, Ar-H), 7.36–7.32 (m, 2H, Ar-H), 7.20–7.15 (dt, J = 2, 8 Hz, 2H, Ar-H), 7.10–7.06 (m, 4H, Ar-H), 5.19 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 166.1, 163.5 (d, 1JCF = 243 Hz), 162.1, 156.5, 149.3, 141.5, 141.4, 132.3, 131.8 (d, 3JCF = 8 Hz), 129.4, 124.8, 124.8, 122.8, 115.8 (d, 2JCF = 23 Hz), 115.5, 115.4, 115.0, 113.1, 109.4, 107.4, 70.3, 56.7; DART MS m/z calculated for C23H17FO5 (M+) 392.11; found 392.14. Anal. Calcd for C23H17FO5: C, 70.40; H, 4.37. Found: C, 70.42; H, 4.34.
5.4.2. 5-[(3-Iodobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B02)
Mp 159–160 °C, yield 87%; IR (cm–1): 1674, 1612, 1574, 1504, 1458, 1366, 1304, 1257, 1180, 1095, 1018, 926, 818, 764, 694, 663; 1H NMR (300 MHz, DMSO-d6): δ 13.02 (br s, 1H, COOH), 7.99 (d, J = 9 Hz, 2H, Ar-H), 7.88 (s, 1H, Ar-H), 7.71 (d, J = 8 Hz, 1H, Ar-H), 7.60–7.57 (m, 2H, Ar-H), 7.23 (d, J = 8 Hz, 1H, Ar-H), 7.22 (t, J = 8 Hz, 1H, Ar-H), 7.10–7.0 (m, 3H, Ar-H), 5.15 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 164.7, 160.7, 155.1, 147.9, 139.8, 136.4, 136.0, 130.8, 130.5, 128.0, 126.9, 121.5, 115.8, 115.7, 114.0, 113.6, 111.7, 106.1, 94.7, 68.9, 55.3; DART MS m/z calculated for C23H17IO5 (M+) 500.01; found 500.05. Anal. Calcd for C23H17IO5: C, 55.22; H, 3.43. Found: C, 55.23; H, 3.40.
5.4.3. 5-[(4-Bromobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B03)
Mp 208–210 °C, yield 79%; IR (cm–1): 1674, 1605, 1558, 1504, 1458, 1242, 1180, 1095, 1049, 1011, 841, 810, 764; 1H NMR (300 MHz, DMSO-d6): δ 12.98 (br s, 1H, COOH), 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.61–7.55 (m, 4H, Ar-H), 7.45 (d, J = 8 Hz, 2H, Ar-H), 7.09–7.03 (m, 3H, Ar-H), 5.14 (s, 2H, OCH2), 3.85 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 164.6, 160.8, 160.5, 155.1, 147.9, 136.6, 131.2, 130.8, 129.7, 129.6, 128.0, 121.4, 120.8, 115.7, 114.0, 113.6, 111.7, 108.0, 106.1, 69.1, 55.3; DART MS m/z calculated for C23H17BrO5 (M+)/(M+2+) 452.03/454.03; found 452.05/454.05. Anal. Calcd For C23H17BrO5: C, 60.94; H, 3.78. Found: C, 60.97; H, 3.77.
5.4.4. 5-[(3-Chlorobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B04)
Mp 180–182 °C, yield 81%; IR (cm–1): 1674, 1612, 1582, 1458, 1358, 1304, 1265, 1180, 1095, 1018, 926, 841,787, 764, 687; 1H NMR (300 MHz, DMSO-d6): δ 12.98 (br s, 1H, COOH), 7.99 (d, J = 9 Hz, 2H, Ar-H), 7.60–7.57 (m, 2H, Ar-H), 7.46–7.41 (m, 3H, Ar-H), 7.10–7.07 (m, 3H, Ar-H), 5.19 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 167.4, 166.1, 162.1, 156.5, 149.4, 141.1, 134.4, 132.2, 131.6, 129.5, 129.0, 128.6, 127.5, 122.9, 115.4, 115.0, 115.0, 113.1, 109.6, 107.5, 70.4, 56.7; DART MS m/z calculated for C23H17ClO5 (M+)/(M+2+) 408.08/410.08; found 408.09/410.10. Anal. Calcd for C23H17ClO5: C, 67.57; H, 4.19. Found: C, 67.59; H, 4.19.
5.4.5. 5-[(2,6-Difluorobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B05)
Mp 214–215 °C, yield 86%; IR (cm–1): 1674, 1612, 1582, 1504, 1458, 1257, 1180, 1095, 1018, 941, 833, 787; 1H NMR (300 MHz, DMSO-d6): δ 13.05 (br s, 1H, COOH), 7.99 (d, J = 9 Hz, 2H, Ar-H), 7.62–7.50 (m, 3H, Ar-H), 7.19 (t, J = 8 Hz, 2H, Ar-H), 7.08 (d, J = 9 Hz, 2H, Ar-H), 7.05 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.19 (s, 2H, OCH2), 3.86 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 166.0, 162.6 (d, 1JCF = 249 Hz), 162.5 (d, 1JCF = 249 Hz), 161.9, 156.4, 149.6, 132.9, 132.3, 129.4, 122.8, 115.6, 115.0, 113.2, 113.2, 112.9, 109.5, 107.7, 60.0, 56.7; DART MS m/z calculated for C23H16F2O5 (M+) 410.10; found 410.13. Anal. Calcd for C23H16F2O5: C, 67.32; H, 3.93. Found: C, 67.35; H, 3.90.
5.4.6. 2-(4-Methoxyphenyl)-5-{[3-(trifluoromethyl)benzyl]oxy}-1-benzofuran-3-carboxylic acid (B06)
Mp 167–168 °C, yield 80%; IR (cm–1): 1674, 1612, 1582, 1504, 1466, 1327, 1257, 1173, 1119, 1026, 841, 810, 764, 702; 1H NMR (300 MHz, DMSO-d6): δ 13.05 (br s, 1H, COOH), 7.99 (d, J = 9 Hz, 2H, Ar-H), 7.87–7.81 (m, 2H, Ar-H), 7.73–7.58 (m, 4H, Ar-H), 7.10–7.07 (m, 2H, Ar-H), 5.27 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 166.1, 162.1, 161.9, 156.5, 149.4, 140.0, 133.0, 132.3, 130.9, 130.7, 130.3, 129.4, 125.8, 125.4, 122.8, 115.5, 115.0, 113.1, 109.5, 107.4, 70.3, 56.7; DART MS m/z calculated for C24H17F3O5 (M+) 442.10; found 442.13. Anal. Calcd for C24H17F3O5: C, 65.16; H, 3.87. Found: C, 65.17; H, 3.83.
5.4.7. 5-[(3,4-Difluorobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B07)
Mp 186–187 °C, yield 88%; IR (cm–1): 1674, 1612, 1582, 1512, 1458, 1257, 1180, 1095, 1034, 941, 833, 771; 1H NMR (300 MHz, DMSO-d6): δ 13.04 (br s, 1H, COOH), 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.60–7.45 (m, 5H, Ar-H), 7.08 (d, J = 9 Hz, 2H, Ar-H), 7.07 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.15 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 166.1, 162.1, 161.9, 156.4, 149.3, 148.7, 132.3, 129.4, 125.9, 125.8, 125.8, 122.8, 118.8 (d, 2JCF = 17 Hz), 118.1 (d, 2JCF = 17 Hz), 115.5, 115.0, 113.1, 109.4, 107.3, 69.9; DART MS m/z calculated for C23H16F2O5 (M+) 410.10; found 410.12. Anal. Calcd for C23H16F2O5: C, 67.32; H, 3.93. Found: C, 67.35; H, 3.94.
5.4.8. 5-[(4-Fluorobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B08)
Mp 200–201 °C, yield 83%; IR (cm–1): 1674, 1612, 1582, 1458, 1257, 1180, 1095, 1026, 833, 771; 1H NMR (300 MHz, DMSO-d6): δ 12.97 (br s, 1H, COOH), 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.60–7.53 (m, 4H, Ar-H), 7.23 (t, J = 9 Hz, 2H, Ar-H), 7.10–7.04 (m, 3H, Ar-H), 5.15 (s, 2H, OCH2), 3.86 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 165.1, 162.2 (d, 1JCF = 244 Hz), 161.0, 155.8, 148.6, 140.7, 133.8 (d, 4JCF = 3 Hz), 130.4 (d, 3JCF = 9 Hz), 129.6, 129.2, 128.3, 126.8, 115.7 (d, 2JCF = 21 Hz), 114.9, 112.3, 109.3, 106.5, 69.7, 21.5; DART MS m/z calculated for C23H17FO5 (M+) 392.11; found 376.15. Anal. Calcd for C23H17FO5: C, 70.40; H, 4.37. Found: C, 70.38; H, 4.34.
5.4.9. 5-[(2-Bromobenzyl)oxy]-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B09)
Mp 185–186 °C, yield 78%; IR (cm–1): 1674, 1612, 1582, 1512, 1458, 1257, 1180, 1095, 1034, 833, 771; 1H NMR (300 MHz, DMSO-d6): δ 12.99 (br s, 1H, COOH), 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.71–7.32 (m, 6H, Ar-H), 7.10–7.06 (m, 3H, Ar-H), 5.19 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 166.0, 162.2, 156.5, 149.4, 137.3, 133.9, 132.6, 131.5, 131.4, 129.4, 129.2, 124.1, 122.8, 115.5, 115.0, 113.2, 107.5, 71.1, 56.7; DART MS m/z calculated for C23H17BrO5 (M+) 452.03; found 452.04. Anal. Calcd For C23H17BrO5: C, 60.94; H, 3.78. Found: C, 60.95; H, 3.76.
5.4.10. 5-([1,1’-Biphenyl]-3-ylmethoxy)-2-(4-methoxyphenyl)-1-benzofuran-3-carboxylic acid (B10)
Mp 150–151 °C, yield 79%; IR (cm–1): 1674, 1612, 1582, 1512, 1458, 1257, 1180, 1095, 1034, 833, 771; 1H NMR (300 MHz, DMSO-d6): δ 7.98 (d, J = 9 Hz, 2H, Ar-H), 7.79 (s, 1H, Ar-H), 7.71–7.58 (m, 3H, Ar-H), 7.62 (d, J = 2 Hz, 1H, Ar-H), 7.59 (d, J = 9 Hz, 1H, Ar-H), 7.51–7.38 (m, 5H, Ar-H), 7.10 (dd, J = 3, 9 Hz, 1H, Ar-H), 7.09 (d, J = 9 Hz, 2H, Ar-H), 5.25 (s, 2H, OCH2), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, DMSO-d6): 165.2, 161.3, 160.9, 155.9, 148.4, 140.8, 140.4, 138.3, 131.4, 129.6, 129.4, 128.5, 128.0, 127.2, 126.6, 126.5, 122.0, 116.3, 116.2, 114.7, 114.2, 112.2, 108.6, 70.4, 55.8; DART MS m/z calculated for C29H22O5 (M+) 450.15; found 450.17. Anal. Calcd for C29H22O5: C, 77.32; H, 4.92. Found: C, 77.35; H, 4.90.
5.4.11. 5-[(3-Fluorobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B11)
Mp 202–203 °C, yield 86%; IR (cm–1): 1674, 1466, 1327, 1288, 1203, 1165, 1126, 1103, 818, 764; 1H NMR (300 MHz, DMSO-d6): δ 13.03 (br s, 1H, COOH), 7.89 (d, J = 8 Hz, 2H, Ar-H), 7.63–7.58 (m, 2H, Ar-H), 7.49–7.42 (m, 1H, Ar-H), 7.36–7.33(m, 2H, Ar-H), 7.20–7.14 (m,1H, Ar-H), 7.08 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.16 (s, 2H, OCH2), 2.40 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 164.5, 162.3 (d, 1JCF = 246 Hz), 160.4, 155.2, 148.1, 140.1 (d, 3JCF = 8 Hz), 130.4 (d, 3JCF = 8 Hz), 129.1, 128.7, 127.9, 126.4, 123.4 (d, 4JCF = 3 Hz), 114.6, 114.4, 114.3, 114.1 (d, 2JCF = 22 Hz), 111.8, 108.8, 106.0, 69.0, 21.0; DART MS m/z calculated for C23H17FO4 (M+) 376.11; found 376.12. Anal. Calcd for C23H17FO4: C, 73.40; H, 4.55. Found: C, 73.43; H, 4.53.
5.4.12. 5-[(3-Iodobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B12)
Mp 140–142 °C, yield 89%; IR (cm–1): 1674, 1597, 1558, 1458, 1412, 1381, 1288, 1196, 1095, 1026, 818, 779, 687, 663; 1H NMR (300 MHz, DMSO-d6): δ 7.99 (d, J = 8 Hz, 2H, Ar-H), 7.87 (s, 1H, Ar-H), 7.71 (d, J = 8 Hz, 1H, Ar-H), 7.60–7.51 (m, 3H, Ar-H), 7.30 (d, J = 8 Hz, 2H, Ar-H), 7.21 (t, J = 8 Hz, 1H, Ar-H), 7.03 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.13 (s, 2H, OCH2), 2.38 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 166.7, 154.8, 148.0, 139.9, 139.2, 136.4, 136.0, 130.5, 128.9, 128.6, 127.0, 126.9, 113.9, 111.4, 106.3, 94.7, 68.8, 20.9; DART MS m/z calculated for C23H17IO4 (M+) 484.02; found 484.05. Anal. Calcd for C23H17IO4: C, 57.04; H, 3.54. Found: C, 57.04; H, 3.50.
5.4.13. 5-[(4-Bromobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B13)
Mp 225–226 °C, yield 82%; IR (cm–1): 1674, 1582, 1458, 1366, 1281, 1188, 1095, 1011, 949, 849, 810, 764; 1H NMR (300 MHz, DMSO-d6): δ 13.02 (br s, 13.02, 1H, COOH), 7.88 (d, J = 8 Hz, 2H, Ar-H), 7.60 (d, J = 8 Hz, 2H, Ar-H), 7.58–7.57 (m, 2H, Ar-H), 7.46 (d, J = 8 Hz, 2H, Ar-H), 7.34 (d, J = 8 Hz, 2H, Ar-H), 7.08 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.16 (s, 2H, OCH2), 2.40 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.0, 161.0, 155.7, 148.6, 140.7, 137.1, 131.8, 130.2, 129.6, 129.2, 128.4, 126.9, 121.3, 114.9, 112.3, 109.4, 69.6, 21.5; DART MS m/z calculated for C23H17BrO4 (M+)/(M+2+) 436.03/438.03; found 436.05/438.05. Anal. Calcd for C23H17BrO4: C, 63.17; H, 3.92. Found: C, 63.20; H, 3.91.
5.4.14. 5-[(3-Chlorobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B14)
Mp 184–186 °C, yield 81%; IR (cm–1): 1674, 1605, 1574, 1458, 1366, 1281, 1196, 1095, 1026, 957, 818, 771, 679; 1H NMR (300 MHz, DMSO-d6): δ 13.02 (br s, 1H, COOH), 7.88 (d, J = 8 Hz, 2H, Ar-H), 7.62–7.57 (m, 3H, Ar-H), 7.47–7.42 (m, 3H, Ar-H), 7.34 (d, J = 8 Hz, 2H, Ar-H), 7.10 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.21 (s, 2H, OCH2), 2.41 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 164.5, 160.4, 155.1, 148.1, 140.1, 139.7, 133.0, 130.2, 129.4, 129.1, 128.7, 127.8, 127.6, 127.2, 126.4, 126.1, 114.4, 111.8, 108.8, 106.0, 68.9, 21.0; DART MS m/z calculated for C23H17ClO4 (M+) 392.08; found 392.09. Anal. Calcd for C23H17ClO4: C, 70.32; H, 4.36. Found: C, 70.35; H, 4.34.
5.4.15. 5-[(2,6-Difluorobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B15)
Mp 186–187 °C, yield 90%; IR (cm–1): 1674, 1597, 1543, 1458, 1265, 1203, 1095, 1011, 833, 748; 1H NMR (300 MHz, DMSO-d6): δ 13.07 (br s, 1H, COOH), 7.90 (d, J = 8 Hz, 2H, Ar-H), 7.62–7.48 (m, 3H, Ar-H), 7.34 (d, J = 8 Hz, 2H, Ar-H), 7.18 (t, J = 8 Hz, 2H, Ar-H), 7.06 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.19 (s, 2H, OCH2), 2.40 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.1, 161.8 (d, 1JCF = 249 Hz), 161.6 (d, 1JCF = 249 Hz), 161.0, 155.6, 148.8, 140.7, 132.1 (t, 3JCF = 11 Hz), 129.6, 129.2, 128.4, 126.9, 115.0, 113.1, 112.8, 112.4 (d, 2JCF = 25 Hz), 112.4, 112.2 (d, 2JCF = 25 Hz), 112.1, 109.5, 106.8, 59.1, 21.5; DART MS m/z calculated for C23H16F2O4 (M+) 394.10; found 394.11. Anal. Calcd for C23H16F2O4: C, 70.07; H, 4.09. Found: C, 70.05; H, 4.10.
5.4.16. 2-(4-Methylphenyl)-5-{[3-(trifluoromethyl)benzyl]oxy}-1-benzofuran-3-carboxylic acid (B16)
Mp 165–166 °C, yield 84%; IR (cm–1): 1674, 1605, 1535, 1504, 1466, 1327, 1288, 1203, 1126, 1165, 1103, 887, 818, 764; 1H NMR (300 MHz, DMSO-d6): δ 13.14 (br s, 1H, COOH), 7.89 (d, J = 8 Hz, 2H, Ar-H), 7.88 (s, 1H, Ar-H), 7.83 (d, J = 8 Hz, 1H, Ar-H), 7.73–7.59 (m, 4H, Ar-H), 7.34 (d, J = 8 Hz, 2H, Ar-H), 7.11 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.28 (s, 2H, OCH2), 2.39 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): 165.1, 160.8, 155.6, 148.7, 140.6, 132.1, 130.3, 130.0, 129.6, 129.4, 129.2, 129.0 128.5, 126.9, 124.9, 124.9, 124.7 (q, 1JCF = 272 Hz), 124.5, 124.4, 114.8, 112.3, 109.6, 106.6, 69.5, 21.5; DART MS m/z calculated for C24H17F3O4 (M+) 426.11; found 426.13. Anal. Calcd for C24H17F3O4: C, 67.60; H, 4.02. Found: C, 67.64; H, 4.00.
5.4.17. 5-[(3,4-Difluorobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B17)
Mp 201–203 °C, yield 88%; IR (cm–1): 1674, 1612, 1582, 1520, 1458, 1288, 1196, 1095, 1018, 941, 818, 779; 1H NMR (300 MHz, DMSO-d6): 7.87 (d, J = 8 Hz, 2H, Ar-H), 7.59 (d, J = 9 Hz, 2H, Ar-H), 7.56 (d, J = 3 Hz, Ar-H), 7.48–7.32 (m, 4H, Ar-H), 7.08 (dd, J = 3, 9 Hz, Ar-H), 5.15 (s, 2H, OCH2), 2.38 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.1, 161.0, 155.6, 149.7 (dd, 1JCF = 251, 2JCF = 30 Hz), 149.5 (dd, 1JCF = 245, 2JCF = 29 Hz), 148.7, 140.7, 135.4 (dd, 3JCF = 5, 4JCF = 4 Hz), 129.6, 129.2, 128.4, 126.9, 124.9 (dd, 3JCF = 7, 4JCF = 4 Hz), 117.9 (d, 2JCF = 17 Hz), 117.1 (d, 2JCF = 17 Hz), 114.9, 112.3, 109.3, 106.6, 69.1, 21.5; DART MS m/z calculated for C23H16F2O4 (M+) 394.10; found 394.12. Anal. Calcd for C23H16F2O4: C, 70.05; H, 4.09. Found: C, 70.06; H, 4.07.
5.4.18. 5-[(4-Fluorobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B18)
Mp 218–220 °C, yield 85%; IR (cm–1): 1674, 1605, 1582, 1512, 1458, 1366, 1281,1227, 1188, 1095, 1011, 941, 825, 779; 1H NMR (300 MHz, DMSO-d6): δ 7.86 (d, J = 8 Hz, 2H, Ar-H), 7.59–7.51 (m, 4H, Ar-H), 7.32 (d, J = 8 Hz, 2H, Ar-H), 7.22 (t, J = 9 Hz, 2H, Ar-H), 7.06 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.13 (s, 2H, OCH2), 2.38 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.09, 162.21 (d, 1JCF = 244 Hz), 161.0, 155.8, 148.6, 140.7, 133.8 (d, 4JCF = 3 Hz), 130.6 (d, 3JCF = 8 Hz), 129.6, 129.2, 128.3, 126.8, 115.7 (d, 2JCF = 21 Hz), 114.9, 112.3, 109.3, 106.5, 69.7, 21.5; DART MS m/z calculated for C23H17FO4 (M+) 376.11; found 376.13. Anal. Calcd for C23H17FO4: C, 73.40; H, 4.55. Found: C, 73.43; H, 4.55.
5.4.19. 5-[(2-Bromobenzyl)oxy]-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B19)
Mp 180–182 °C, yield 83%; IR (cm–1): 1666, 1612, 1450, 1366, 1257, 1180, 1095, 1011, 949, 833, 756; 1H NMR (300 MHz, DMSO-d6): δ 7.88 (d, J = 8 Hz, 2H, Ar-H), 7.69 (dd, J =1, 8 Hz, 1H, Ar-H), 7.65–7.57 (m, 4H, Ar-H), 7.44 (dt, J = 1, 8 Hz, 1H, Ar-H), 7.34 (d, J = 8 Hz, 1H, Ar-H), 7.30–7.28 (m, 1H, Ar-H), 7.09 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.19 (s, 2H, OCH2), 2.39 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.0, 161.0, 155.7, 148.7, 140.7, 136.4, 133.1, 130.7, 130.6, 129.6, 129.2, 128.4, 126.9, 123.3, 114.9, 112.4, 109.4, 106.5, 70.2, 21.5; DART MS m/z calculated for C23H17BrO4 (M+)/(M+2+) 436.03/438.03; found 436.06/438.05. Anal. Calcd for C23H17BrO4: C, 63.17; H, 3.92. Found: C, 63.20; H, 3.88.
5.4.20. 5-([1,1’-Biphenyl]-3-ylmethoxy)-2-(4-methylphenyl)-1-benzofuran-3-carboxylic acid (B20)
Mp 150–152 °C, yield 80%; IR (cm–1): 1674, 1605, 1551, 1504, 1458, 1288, 1196, 1095, 1018, 825, 795, 756, 702; 1H NMR (300 MHz, DMSO-d6): δ 7.94 (d, J = 8 Hz, 2H, Ar-H), 7.78 (s, 1H, Ar-H), 7.68 (d, J = 8 Hz, 2H, Ar-H), 7.64–7.61 (m, 2H, Ar-H), 7.56 (d, J = 9 Hz, 1H, Ar-H), 7.50–7.35 (m, 5H, Ar-H), 7.31 (d, J = 8 Hz, 2H, Ar-H), 7.07 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.23 (s, 2H, OCH2), 2.37 (s, 3H, CH3); 13C NMR (75.5 MHz, DMSO-d6): 165.7, 159.3, 155.7, 148.5, 140.8, 140.4, 140.1, 138.4, 129.5, 129.4, 129.3, 129.2, 129.0, 128.0, 127.3, 127.2, 126.6, 126.5, 114.7, 112.1, 111.3, 106.7, 70.3, 21.5; DART MS m/z calculated for C29H22O4 (M+) 434.15; found 434.17. Anal. Calcd for C29H22O4: C, 80.17; H, 5.10. Found: C, 80.21; H, 5.07.
5.4.21. 5-[(3-Fluorobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B21)
Mp 185–187 °C, yield 92%; IR (cm–1): 1674, 1597, 1551, 1474, 1265, 1188, 1095, 771; 1H NMR (300 MHz, DMSO-d6): δ 13.23 (br s, 1H, COOH), 8.60 (s, 1H, Ar-H), 8.10–7.98 (m, 4H, Ar-H), 7.67–7.58 (m, 4H, Ar-H), 7.50–7.43 (m, 1H, Ar-H), 7.37–7.33 (m, 1H, Ar-H), 7.19 (dd, J = 2, 8 Hz, 1H, Ar-H), 7.13 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.22 (s, 1H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 164.6, 162.2 (d, 1JCF = 244 Hz), 155.2, 148.4, 140.1, 133.3, 132.2, 130.4 (d, 3JCF = 8 Hz), 129.1, 128.6, 127.5, 127.5, 127.4, 126.7, 126.7, 126.0, 123.5, 123.4, 114.5 (d, 2JCF = 28 Hz), 114.0, 111.9, 106.1, 69.0; DART MS m/z calculated for C26H17FO4 (M+) 412.11; found 412.14. Anal. Calcd for C26H17FO4: C, 75.72; H, 4.15. Found: C, 75.72; H, 4.17.
5.4.22. 5-[(3-Iodobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B22)
Mp 165–168 °C, yield 86%; IR (cm–1): 1674, 1597, 1551, 1458, 1265, 1188, 1095, 1018, 856, 779, 748; 1H NMR (300 MHz, DMSO-d6): δ 8.69 (s, 1H, Ar-H), 8.26 (d, J = 9 Hz, 1H, Ar-H), 8.05–7.95 (m, 3H, Ar-H), 7.88 (s, 1H, Ar-H), 7.71 (d, J = 8 Hz, 1H, Ar-H), 7.65–7.57 (m, 4H, Ar-H), 7.53 (d, J = 8 Hz, 1H, Ar-H), 7.25–7.19 (m, 1H, Ar-H), 7.06 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.15 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 165.4, 154.8, 148.3, 139.9, 136.4, 136.0, 133.0, 132.3, 130.5, 129.1, 128.5, 128.1, 127.5, 126.9, 126.5, 125.9, 114.2, 111.5, 106.3, 94.7, 68.8; DART MS m/z calculated for C26H17IO4 (M+) 520.02; found 520.04. Anal. Calcd for C26H17IO4: C, 60.02; H, 3.29. Found: C, 60.00; H, 3.26.
5.4.23. 5-[(4-Bromobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B23)
Mp 222–224 °C, yield 89%; IR (cm–1): 1674, 1605, 1558, 1458, 1273, 1188, 1088, 1011, 849, 802, 748; 1H NMR (300 MHz, DMSO-d6): δ 8.65 (s, 1H, Ar-H), 8.18 (dd, J = 2, 9 Hz, 1H, Ar-H), 8.05–7.96 (m, 3H, Ar-H), 7.64–7.55 (m, 5H, Ar-H), 7.49–7.38 (m, 3H, Ar-H), 7.09 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.19 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6):165.1, 154.9, 148.3, 139.8, 133.1, 133.0, 132.3, 130.2, 128.6, 128.6, 128.5, 127.6, 127.5, 127.3, 127.2, 127.1, 126.5, 126.1, 125.9, 114.4, 111.6, 106.2, 68.9; DART MS m/z calculated for C26H17BrO4 (M+) 472.03; found 472.04. Anal. Calcd for C26H17BrO4: C, 65.98; H, 3.62. Found: C, 66.02; H, 3.61.
5.4.24. 5-[(3-Chlorobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B24)
Mp 140–142 °C, yield 88%; IR (cm–1): 1674, 1605, 1551, 1458, 1273, 1188, 1095, 779, 748; 1H NMR (300 MHz, DMSO-d6): δ 8.61 (s, 1H), 8.11 (dd, J = 1, 9 Hz, 1H, Ar-H), 8.06–7.97 (m, 3H, Ar-H), 7.65–7.56 (m, 6H, Ar-H), 7.46 (d, J = 8 Hz, 2H, Ar-H), 7.09 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.16 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 164.7, 159.2, 155.1, 148.3, 136.6, 133.2, 132.2, 131.3, 129.7, 128.9, 128.6, 128.2, 127.5, 127.4, 126.8, 126.6, 126.0, 120.8, 114.5, 111.8, 106.2, 69.2; DART MS m/z calculated for C26H17ClO4 (M+) 428.08; found 428.09. Anal. Calcd for C26H17ClO4: C, 72.82; H, 4.00. Found: C, 72.83; H, 3.97.
5.4.25. 5-[(2,6-Difluorobenzyl)oxy]-2-(2-naphthyl)benzofuran-3-carboxylic acid (B25)
Mp 222–225 °C, yield 91%; IR (cm–1): 1674, 1628, 1556, 1466, 1281, 1188, 787; 1H NMR (300 MHz, DMSO-d6): δ 13.16 (br s, 1H, COOH), 8.59 (s, 1H, Ar-H), 8.09–7.98 (m, 4H, Ar-H), 7.67–7.60 (m, 4H, Ar-H), 7.58–7.48 (m, 1H, Ar-H), 7.11 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.21 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 164.5, 161.2 (d, 1JCF = 249 Hz), 161.1 (d, 1JCF = 249 Hz), 160.2, 155.2, 148.6, 133.4, 132.2, 131.6 (t, 3JCF = 11 Hz), 129.3, 128.7, 127.9, 127.5, 127.5, 126.7, 126.6, 126.0, 114.8, 112.2 (d, 2JCF = 22 Hz), 111.8, 111.7 (d, 2JCF = 25 Hz), 111.6, 109.8, 58.5; DART MS m/z calculated for C26H16F2O4 (M+) 430.10; found 430.12. Anal. Calcd for C26H16F2O4: C, 72.56; H, 3.75. Found: C, 72.58; H, 3.75.
5.4.26. 2-(2-Naphthyl)5-{[3-(trifluoromethyl)benzyl]oxy}-1-benzofuran-3-carboxylic acid (B26)
Mp 168–170 °C, yield 83%; IR (cm–1): 1682, 1551, 1474, 1327, 1273, 1180, 1119, 795; 1H NMR (300 MHz, DMSO-d6): δ 13.26 (br s, 1H, COOH), 8.60 (s, 1H, Ar-H), 8.11–7.98 (m, 4H, Ar-H), 7.88 (s, 1H, Ar-H), 7.84 (d, J = 7 Hz, 1H, Ar-H), 7.77–7.57 (m, 6H, Ar-H), 7.14 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.30 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 164.6, 159.6, 155.2, 148.4, 138.7, 133.3, 132.2, 131.6, 129.5, 129.3, 129.1, 128.9, 128.6, 128.1, 127.5, 127.5, 127.4, 126.7, 126.7, 126.0, 124.4 (q, 3JCF = 4 Hz), 124.0 (q, 3JCF = 4 Hz), 114.7, 111.9, 110.4, 106.1, 69.0; DART MS m/z calculated for C27H17F3O4 (M+) 462.11; found 462.12. Anal. Calcd for C27H17F3O4: C, 70.13; H, 3.71. Found: C, 70.15; H, 3.74.
5.4.27. 5-[(3,4-Difluorobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B27)
Mp 186–190 °C, yield 87%; IR (cm–1): 1682, 1612, 1551, 1520, 1458, 1281, 1196, 1095, 810, 779; 1H NMR (300 MHz, DMSO-d6): δ 8.64 (s, 1H, Ar-H), 8.16 (d, J = 9 Hz, 1H, Ar-H), 8.03 (d, J = 9 Hz, 2H, Ar-H), 7.97 (d, J = 9 Hz, 1H, Ar-H), 7.64–7.54 (m, 5H, Ar-H), 7.50–7.37 (m, 2H, Ar-H), 7.09 (dd, J = 2, 9 Hz, Ar-H), 5.16 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 165.1, 158.3, 154.9, 149.2 (dd, 1JCF = 244 Hz, 2JCF = 30 Hz,) 149.0 (dd, 1JCF = 244 Hz, 2JCF = 30 Hz), 148.4, 135.0 (dd, 3JCF = 5 Hz, 4JCF = 4 Hz), 124.4 (dd, 3JCF = 7 Hz, 4JCF = 3 Hz), 133.2, 132.3, 128.7, 128.6, 128.5, 127.5, 127.4, 127.3, 127.0, 126.6, 125.9, 117.4 (d, 2JCF = 17 Hz), 116.7 (d, 2JCF = 17 Hz), 114.5, 111.7, 106.2, 68.6; DART MS m/z calculated for C26H16F2O4 (M+) 430.10; found 430.13. Anal. Calcd for C26H16F2O4: C, 72.56; H, 3.75. Found: C, 72.59; H, 3.73.
5.4.28. 5-[(4-Fluorobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B28)
Mp 225–228 °C, yield 86%; IR (cm–1): 1674, 1605, 1558, 1512, 1466, 1273, 1227, 1188, 1095, 1018, 825, 771; 1H NMR (300 MHz, DMSO-d6): δ 13.12 (s, 1H, COOH), 8.58 (s, 1H, Ar-H), 8.07–8.05 (m, 3H, Ar-H), 7.99 (dd, J = 2, 7 Hz, 1H, Ar-H), 7.67–7.53 (m, 6H, Ar-H), 7.23 (t, J = 9 Hz, 1H, Ar-H), 7.11 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.16 (s, 2H, OCH2); 13C NMR (75 MHz, DMSO-d6): 164.5, 161.7 (d, 1JCF = 242 Hz), 155.4, 148.4, 133.3, 133.3, 132.3, 132.2, 129.9 (d, 3JCF = 8 Hz), 129.2, 128.7, 127.8, 127.6, 127.5, 126.7, 126.6, 126.0, 115.1 (d, 2JCF = 21 Hz), 114.7, 111.9, 109.7, 106.0, 69.2; DART MS m/z calculated for C26H17FO4 (M+) 412.11; found 412.12. Anal. Calcd for C26H17FO4: C, 75.72; H, 4.15. Found: C, 75.73; H, 4.11.
5.4.29. 5-[(2-Bromobenzyl)oxy]-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B29)
Mp 212–215 °C, yield 84%; IR (cm–1): 1682, 1605, 1535, 1474, 1265, 1211, 1095, 1026, 849, 802, 741; 1H NMR (300 MHz, DMSO-d6): δ 13.12 (s, 1H, COOH), 8.59 (s, 1H, Ar-H), 8.07–8.03 (m, 3H, Ar-H), 8.00 (dd, J = 2, 9 Hz, 1H, Ar-H), 7.71–7.58 (m, 6H, Ar-H), 7.47–7.43 (m, 1H, Ar-H), 7.35–7.30 (dt, J = 2, 8 Hz, 1H, Ar-H), 7.14 (dd, J = 3, 9 Hz, 1H, Ar-H), 5.21 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 164.5, 160.2, 155.3, 148.5, 135.9, 133.3, 132.6, 132.1, 130.1, 129.2, 128.6, 127.5, 127.5, 126.7, 126.6, 126.0, 122.7, 114.7, 112.0, 106.0, 69.7; DART MS m/z calculated for C26H17BrO4 (M+) 472.03; found 472.05. Anal. Calcd for C26H17BrO4: C, 65.98; H, 3.62. Found: C, 65.95; H, 3.61.
5.4.30. 5-([1,1’-Biphenyl]-3-ylmethoxy)-2-(2-naphthyl)-1-benzofuran-3-carboxylic acid (B30)
Mp 110–112 °C, yield 82%; IR (cm–1): 1674, 1597, 1543, 1458, 1265, 1203, 1095, 1011, 833, 748; 1H NMR (300 MHz, DMSO-d6): δ 8.70 (s, 1H, Ar-H), 8.28 (d, J = 9 Hz, 1H, Ar-H), 8.03–7.93 (m, 3H, Ar-H), 7.79 (s, 1H, Ar-H), 7.71–7.67 (m, 3H, Ar-H), 7.62–7.50 (m, 8H, Ar-H), 7.39–7.34 (m, 1H, Ar-H), 7.07 (dd, J = 2, 9 Hz, 1H, Ar-H), 5.23 (s, 2H, OCH2); 13C NMR (75.5 MHz, DMSO-d6): 165.7, 156.2, 155.0, 148.2, 140.3, 139.9, 137.9, 132.9, 132.4, 129.3, 129.0, 128.9, 128.5, 127.9, 127.6, 127.5, 127.2, 127.0, 126.7, 126.4, 126.1, 126.0, 125.9, 114.2, 111.4, 106.4, 69.8; DART MS m/z calculated for C32H22O4 (M+) 470.15; found 470.18. Anal. Calcd for C32H22O4: C, 81.69; H, 4.71. Found: C, 81.72; H, 4.68.
6. Bioassay
6.1. Cell lines and culture
FRT cells were stably transfected with human TMEM16A (TMEM16A (abc), cDNA provided by Dr. Luis Galietta, Gaslini Institute, Genoa, Italy) and the halide sensor YFP-F46L/H148Q/I152L. Cells were incubated in coon's modified F12 medium supplemented with 5% fetal calf serum, 2 mM l-glutamine 100 U/ml penicillin and 100 μg/ml streptomycin. Primary cultures of normal human bronchial epithelial (CF-HBE) cells were obtained and grown as described earlier.33 For the iodide quenching experiment, the FRT cells were plated in 96-well black-walled microplates (Corning, NY, USA) at a density of 20,000 cells/well and then incubated for 2 days.
6.2. Iodide quenching
TMEM16A-mediated I– influx was measured by quenching of the intracellular fluorescence generated by the halide sensor YFPF46L/H148Q/I152L, as described previously.29 Each well of a 96-well plate was washed 3 times in phosphate buffered saline (PBS) (200 μL/wash), leaving 100 μL PBS. Test compounds (1 μL) were added to each well at different concentrations. After 10 min, 96-well plates were transferred to a plate reader for fluorescence assay. Each well was assayed individually for TMEM16A-mediated I– influx by recording fluorescence continuously (400 ms/point) for 2 s (baseline), then 100 μL of 140 mM I– and ATP (200 μM) containing solution was added at 2 s to activate TMEM16A through the ATP-induced intracellular calcium increase. The initial rate of I– influx following each of the solution additions was compared from fluorescence data by nonlinear regression.
6.3. Short circuit current
Snap well inserts containing TMEM16A-expressing FRT cells and primary cultured human bronchial epithelial cells were mounted in Ussing chambers (physiological Instruments San Diego, CA, USA). Symmetrical HCO3– -buffered solutions were used for human bronchial epithelial cells. For FRT cells, the hemi chambers were filled with 5 ml of half-Cl solution (apical), and HCO3–-buffered solution (basolateral). The newly synthesized benzofuran derivatives were added to the apical solution, and equal volume of the vehicle was added simultaneously to the basolateral solution. The HCO3–-buffered solution contained 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM D-glucose, 5 mM HEPES, and 25 mM NaHCO3. In the half-Cl– solution, 65 mM NaCl in the HCO3–-buffered solution was replaced with Na-gluconate. In human bronchial epithelial cells, ENaC (epithelial sodium channel) was blocked by pretreatment of 10 μM amiloride and then CFTR was activated by 10 μM forskolin, which activates CFTR via raised cAMP. Cells were bathed for a 10-min stabilization period and aerated with 95% O2/5% CO2 at room temperature and then short circuit current was measured using an EVC 4000 Multi-channel V/I clamp (World Precision Instruments Sarasota, FL) and recorded using PowerLab/8sp (AD Instruments, Castle Hill, Australia).
Acknowledgements
One of the authors (S.K.) is grateful to University Grants Commission (UGC), New Delhi, India for financial support and award of Senior Research Fellowship. The authors are thankful to Mr. Jared W. Rigoli, Mr. Joe D. Rigoli and Ms. Ali Heckman for their help in purification of compounds.
Footnotes
This research is dedicated in remembrance of our former collaborator Late Professor Aaron D. Mills (University of Idaho, USA)
References and notes
- 1.Eggermont J. Proc. Am. Thorac. Soc. 2004;1:22. doi: 10.1513/pats.2306010. [DOI] [PubMed] [Google Scholar]
- 2.Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. J. Biol. Chem. 2009;284:14875. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner JA, Cozens AL, Schulman H, Gruenert DC, Stryer L, Gardner P. Nature. 1991;349:793. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- 4.Tarran R, Button B, Boucher RC. Annu. Rev. Physiol. 2006;68:543. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 5.Ahluwalia J. Biochem. Biophys. Res. Commun. 2008;365:328. doi: 10.1016/j.bbrc.2007.10.176. [DOI] [PubMed] [Google Scholar]
- 6.Sardinia A, Ameya JS, Weylandth KH, Noblesc M, Valverded MA, Higgins CF. Biochim. Biophys. Acta. 2003;1618:153. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Hartzell C, Putzier I, Arreola J. Annu. Rev. Physiol. 2005;67:719. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 8.Frings S, Reuter D, Kleene SJ. Prog. Neurobiol. 2000;60:247. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 9.Badar CR, Bertrand D, Schwartz EA. J. Physiol. 1982;331:253. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotlikoff MI, Wang YX. Am. J. Respir. Crit. Care Med. 1998;158:S109. doi: 10.1164/ajrccm.158.supplement_2.13tac600. [DOI] [PubMed] [Google Scholar]
- 11.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. Nature. 2008;455:1210. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Zhang X, Liu X, Chen T, Zhao M. Exp. Eye Res. 2010;90:771. doi: 10.1016/j.exer.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwanyanya A, Macianskiene R, Bito V, Sipido KR, Vereecke J, Mubagwa K. Biochem. Biophys. Res. Commun. 2010;402:531. doi: 10.1016/j.bbrc.2010.10.069. [DOI] [PubMed] [Google Scholar]
- 14.Verkman AS, Galietta LJ. Nat. Rev. Drug Disc. 2009;8:153. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oriowo MA. Eur. J. Pharm. 2004;506:157. doi: 10.1016/j.ejphar.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. Science. 2008;322:590. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder BC, Cheng T, Jan YN, Jan LY. Cell. 2008;134:1019. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, Rock JR, Harfeb BD, Chenga T, Huangc X, Jana YN, Jana LY. PNAS. 2009;106(50):21413. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper AS, Greenwood IA, Large WA. J. Physiol. 2002;539:119. doi: 10.1113/jphysiol.2001.013270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruickshank SF, Baxter LM, Drummond RM. Br. J. Pharmacol. 2003;140:1442. doi: 10.1038/sj.bjp.0705571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liantonio A, Giannuzzi V, Picollo A, Babini E, Pusch M, Camerino DC. Br. J. Pharmacol. 2007;150:235. doi: 10.1038/sj.bjp.0706954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Chen S, Lu X, Cheng H, Oua Y, Cheng H, Zhou G. Bioorg. Med. Chem. 2009;19:1851. doi: 10.1016/j.bmcl.2009.02.082. [DOI] [PubMed] [Google Scholar]
- 23.Dawood KM, Abdel-Gawad H, Rageb EA, Ellithey M, Mohamed HA. Bioorg. Med. Chem. 2006;14:3672. doi: 10.1016/j.bmc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Safiye E, Tamer K, Hüseyin A. Bioorg. J. Med. Chem. 2011;19:1179. [Google Scholar]
- 25.Vangveravong S, Taylor M, Xu J, Cui J, Calvin W, Babic S, Luedtke RR, Mach RH. Bioorg. Med. Chem. 2010;18:5291. doi: 10.1016/j.bmc.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixit M, Tripathi BK, Tamrakar AK, Srivastava AK, Kumar B, Goel A. Bioorg. Med. Chem. 2007;15:727. doi: 10.1016/j.bmc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Namkung W, Phuan PW, Verkman AS. J. Biol. Chem. 2011;286:2365. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holub JM, O'Toole-Colin K, Getzel A, Argenti A, Evans MA, Smith DC, Dalglish GA, Rifat S, Wilson DL, Taylor BM, Miott U, Glersaye J, Lam KS, McCranor BJ, Berkowitz JD, Miller RB, Lukens JR, Krumpe K, Gupton JT, Burnham BS. Molecule. 2004;9:135. doi: 10.3390/90300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. FASEB J. 2010;24(11):4178. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmar VS, Jain R, Singh S. Bull. Chem. Soc. Jpn. 1988;61:2277. [Google Scholar]
- 31.Nakano J, Katagiri N, Kato T. Chem. Pharm. Bull. 1982;30(7):2590. [Google Scholar]
- 32.Zotova SA, Gololobova TM, Stolyarchuk AA, Stepanyuk GI, Mostovaya NI. Pharm. Chem. J. 1988:26. [Google Scholar]
- 33.Namkung W, Finkbeiner WE, Verkman AS. Mol. Biol. Cell. 2010;21:2639. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]