Abstract
Objective
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system associated with pathogenic autoantibodies against the astrocyte water channel protein aquaporin-4 (AQP4). The presence of neutrophils is a characteristic feature in NMO lesions in humans. Neutrophils are not generally found in multiple sclerosis lesions. We evaluated the role of neutrophils in a mouse NMO model.
Methods
NMO lesions were produced in mice by intracerebral injection of immunoglobulin G (IgG) isolated from NMO patient serum and human complement. We previously reported that this mouse model produces the characteristic histological features of NMO, including perivascular complement activation, inflammatory cell infiltration, and loss of myelin, AQP4, and glial fibrillary acidic protein. Lesions are absent when AQP4 null mice are used or when IgG from non-NMO patients is injected.
Results
We found remarkably reduced neuroinflammation, myelin loss, and AQP4 loss in brains of neutropenic mice at 24 hours and 7 days, and increased severity of NMO lesions in mice made neutrophilic by granulocyte colony stimulating factor. NMO lesions were greatly reduced by intracerebral administration of the neutrophil protease inhibitors Sivelestat and cathepsin G inhibitor I or by intraperitoneal injection of Sivelestat alone. Immunostaining of human NMO lesions for neutrophil elastase revealed many degranulating perivascular neutrophils, with no equivalent perivascular neutrophils in human multiple sclerosis lesions.
Interpretation
Our data implicate a central role of neutrophils in the pathogenesis of early NMO lesions and suggest the potential utility of neutrophil protease inhibitors such as Sivelestat in NMO therapy.
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system (CNS).1,2 The presence of circulating autoantibodies, termed NMO-IgG, directed against extracellular epitopes of the astrocytic water channel protein aquaporin-4 (AQP4), is a characteristic feature of NMO3 that distinguishes it from other inflammatory demyelinating diseases.1,4,5 There is clinical, histological, animal, and cell culture evidence that NMO–immunoglobulin G (IgG) is pathogenic. Clinically, NMO-IgG is specific to NMO1,4 and often correlates with disease activity.6,7 Reducing serum NMO-IgG levels by plasmapheresis or B-cell depletion reduces clinical disease severity.8,9 Human NMO lesions show perivascular deposition of IgG and activated complement, loss of AQP4 and glial fibrillary acidic protein (GFAP) expression, and myelin loss.10–12 It is thought that NMO-IgG binding to AQP4 on astrocytes activates complement deposition, 11–14 leading to astrocyte damage15 and an inflammatory reaction with leukocyte infiltration13 and cytokine release.16 In mice, intracerebral injection of IgG from NMO patients (IgGNMO) with human complement (hC) produces each of the characteristic histological features seen in human NMO lesions, which are absent with control (non-NMO) IgG (IgGCON) or in AQP4 null mice.13
A key histological feature of NMO is perivascular inflammatory cell infiltration.10–12 The types of leukocytes seen within NMO lesions are different from those typically found in lesions of multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), and progressive multifocal leukoencephalopathy (PML). NMO lesions contain neutrophils, eosinophils, and macrophages, with relatively few T-lymphocytes.10–12 Granulocytes are also abundant in the cerebrospinal fluid of NMO patients.17,18 Here, we utilize our mouse model of NMO lesion pathogenesis to test the involvement of neutrophils in the evolution of early and late NMO lesions. We show major effects of neutropenia and neutrophilia on the severity of NMO lesions, and remarkable protection by a small-molecule inhibitor of neutrophil elastase (NE).
Subjects and Methods
Mice
Experiments were done at St. George’s, University of London, and the University of California at San Francisco using sex- and weight-matched wild-type and AQP4 null19 CD1 mice (30–35g), 8 to 12 weeks old. Protocols were approved by the British Home Office and the University of California at San Francisco Committee on Animal Research, as appropriate. Investigators were unaware of mouse genotype or whether IgG from NMO patients or non-NMO subjects was used during the experiments.
Human IgG and hC
Serum from 5 patients with NMO and strong AQP4 autoantibody serum positivity (details in Supplementary Table i), from 3 patients with anti-NMDA receptor encephalitis and from 3 healthy volunteers was processed to obtain the IgG fraction, termed IgGNMO, IgGNMDAR, and IgGCON, respectively. The IgG concentration was 6 to 38mg/ml. hC was collected from healthy volunteers. Details are given elsewhere.13,20,21
Brain Injections
Injections were done as described.13,21 We microinfused (1μl/min) in the right hemisphere 16.8μl IgGNMO + 11.2μl hC, 16.8μl IgGCON + 11.2μl hC, 16.8μl IgGNMDAR + 11.2μl hC, or 16.8μl IgGNMO + 11.2μl hC + 5μg cathepsin G inhibitor I + 5μg Sivelestat (ONO-5046; Tocris Bioscience, Bristol, UK). In 1 experiment mice were administered intraperitoneal (i.p.) boluses of 0.2μg/g Sivelestat or 25μg/g methylprednisolone just before and 12 hours after the brain infusion. In some experiments we infused NE (5μl, 0.4mg/ml) and cathepsin G (5μl, 0.4mg/ml) from Sigma (Poole, UK). Rectal temperature was kept at 37 to 38°C. The effect of intracerebral infusion was studied at 24 hours. In 1 set of experiments the intracerebral infusion was repeated at 3 days and 5 days, and mice were killed at 7 days.
Neutropenia and Neutrophilia
To induce neutropenia mice received 2mg/kg i.p. 1A8 (rat anti-neutrophil) or isotype-matched control IgG (eBioscience, Hatfield, UK) at 24 hours before each intracerebral injection of IgGNMO and hC. 1A8 binds Ly6G, a major antigen on mature neutrophils, and selectively depletes neutrophils for 2 to 3 days after each injection.22 To induce neutrophilia mouse granulocyte colony stimulating factor (G-CSF; eBioscience, Hatfield, UK) or equal volume phosphate buffered saline (PBS) was injected daily subcutaneously (s.c.) at 200μg/g body weight for a week before intracerebral IgGNMO and hC injection.
Peripheral Leukocyte Count
We used tail vein blood. Total leukocyte count was determined in a hemacytometer and differential counts from blood smears after Wright’s stain.13,21
Mouse Brain Histology
Mice were killed, the brains were removed, fixed in 4% formaldehyde, dehydrated, immersed in Histoclear (Fisher Scientific, Loughborough, UK), and processed into paraffin.13,21 Seven adjacent coronal tissue sections 7μm thick were cut starting at 1.6mm from the frontal pole. Sections were stained with hematoxylin/eosin, Luxol Fast Blue, or immunostained as described (Table). Processed slides were mounted in VectaMount (Vector Labs, Peterborough, UK), coverslipped, and visualized using an Olympus BX-51 microscope.
Myelin Loss
Brain sections were stained using the myelin-specific dye Luxol Fast Blue (LFB) as described.13,21 Loss of myelin was quantified as the area without LFB staining divided by the ipsilateral hemispheric area.
Quantification of Loss of AQP4
After immunostaining, loss of AQP4 was expressed as the ratio of the AQP4 immunonegative area/ipsilateral hemispheric area.
Quantification of Brain Inflammation
At 24 hours we observed predominantly neutrophils adherent to vascular endothelium and perivascularly. To quantify this pattern of inflammation, we graded each vessel as: Nil (no neutrophils), Lumen (luminal neutrophils adherent to or migrating through the endothelium), or perivascular (“Periv,” neutrophils within 20μm around the vessel). We counted the number of Nil, Lumen, and Periv vessels/mm2 in a region 1.5×0.5mm, lateral to the injection site in coronal sections at 1.6mm from the frontal pole. The numbers of neutrophils adherent to, migrating through or around each vessel were also counted. We quantified leukocyte infiltration at 7 days as the ratio of the CD45 immunopositive area/ipsilateral hemispheric area.
Human Brain and Spinal Cord Analysis
We used postmortem tissue from 3 patients with NMO and 3 with MS, diagnosed according to clinical criteria. Tissue sections (7μm thick) were stained with hematoxylin/eosin, LFB, or immunostained as described (see Table). Vessels were termed inflamed if they had inflammatory cells within 50μm around them. To quantify brain neutrophil inflammation we examined 20 inflamed vessels per patient and calculated the percentage of inflamed vessels with surrounding neutrophils. Neutrophils were classed as “inactive” vs “active,” based on their morphology. Round-shaped neutrophils with NE only visible intracellularly were termed “inactive.” Degranulated neutrophils (with NE adjacent to the neutrophil) or elongated neutrophils (indicating cell migration, a NE-dependent process) were termed “active.” The investigators were unaware of the clinical diagnosis when they examined the samples.
Peripheral Leukocyte Count in NMO Patients
We identified 12 NMO patients positive for anti-AQP4 antibody, by searching through the database of St. George’s Hospital, and used data from 5 patients. For each of these 5 patients we included a pair of blood results, 1 taken during an acute exacerbation and 1 during remission. For each patient, blood results were only included if the medication used for NMO treatment was the same when the 2 blood samples were taken and there was no associated infection.
Statistics
Data are presented as mean ± standard error. Statistical comparisons were made using the Student t test or analysis of variance (ANOVA) with Student Newman-Keuls post hoc analysis. Significance is *p < 0.05, **p < 0.01, #p < 0.005, and ##p < 0.0005.
Results
Neutropenia Reduces Brain Lesions at 24 Hours
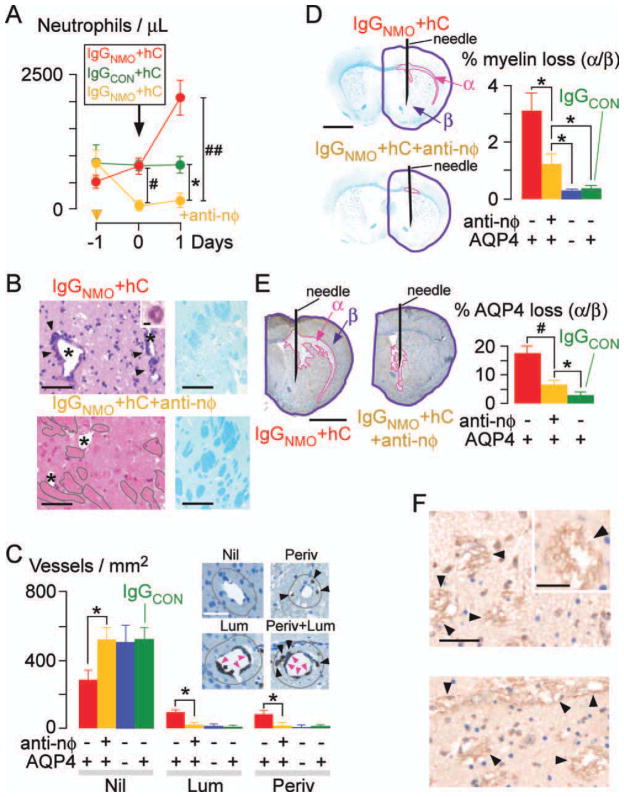
Injection of anti-neutrophil IgG caused neutropenia (Fig 1A) without significantly altering the peripheral lymphocyte, monocyte, and eosinophil counts (Supplementary Fig. i). In non-neutropenic mice intracerebral injection of IgGNMO + hC increased the peripheral neutrophil count. This is part of the NMO disease process, rather than a stress response to anesthesia and surgery or a non-specific reaction to a CNS autoantibody, because intracerebral injection of IgGCON + hC (see Fig 1A) or IgGNMDAR + hC (Supplementary Fig ii) did not increase the neutrophil count. Many neutrophils were seen in the injected hemisphere of non-neutropenic mice adherent to the vascular endothelium and the injected hemisphere appeared damaged with extensive myelin loss (see Fig 1B, upper). Neutrophils were absent in brains of neutropenic mice and their injected hemisphere had mostly intact myelinated tracts (see Fig 1B, lower). To prove that neutrophil infiltration is detrimental, we quantified cerebrovascular inflammation, as well as loss of myelin and AQP4. Non-neutropenic mice had many inflamed vessels, with neutrophils adherent to their endothelium on the luminal side (Lum), and/or perivascularly (Periv) (see Fig 1C, Supplementary Fig. iii). Compared with the non-neutropenic mice, the neutropenic mice had reduced loss of myelin (see Fig 1D) and AQP4 (see Fig 1E). Neutropenia thus abolishes vascular inflammation and reduces the loss of myelin and AQP4 at least for 24 hours.
FIGURE 1.
Neutropenia reduces brain damage at 24 hours after IgGNMO+ hC injection. (A) Peripheral neutrophil count. Antineutrophil (yellow) or isotype control (red and green) IgG was injected at day −1. Mice received IgGNMO+hC (red, yellow) or IgGCON+hC (green) at day 0 and were killed after 24 hours. (B) (Left) H&E: 0.5mm lateral to needle, non-neutropenic vs neutropenic (+anti-nφ) brain. (Inset) Perivascular neutrophil. * = lumen, arrowheads = perivascular inflammation, grey outline = intact white matter. (Right) LFB. (C) Neutrophil immunostain: Nil = no neutrophils, Lum = luminal neutrophils adherent to endothelium (pink arrowheads), Periv = perivascular neutrophils (black arrowheads) within 20μm radius (brown line), or Lum and Periv. Number of Nil, Lum, and Periv vessels/mm2. (D) (Left) LFB: Non-neutropenic vs neutropenic (+anti-nφ) brain. (Right) % loss of myelin (area-α/area-β). (E) (Left) AQP4 immunostain: non-neutropenic vs neutropenic (+anti-nφ) brain. (Right) % loss of AQP4 (area-α/area-β). (F) C5b-9 immunostain: 0.5mm lateral to needle, non-neutropenic vs neutropenic (+anti-nφ) brain. * = lumen, arrowheads = C5b-9. (Inset) C5b-9 (magnified). We injected 8 non-neutropenic (red), 8 neutropenic (yellow), and 5 non-neutropenic AQP4 null (blue) mice with IgGNMO+hC; 5 non-neutropenic mice received IgGCON+hC (green). Mean ± standard error. *p < 0.05, #p < 0.005, ##p < 0.0005 (vs IgGNMO+hC+anti-nφ). Bars = 2μm (B inset), 50μm (C inset, F inset), 100μm (B, F), 2mm (D, E).
In control experiments no NMO lesion was produced after intracerebral injection of IgGCON + hC (see Fig 1), IgGNMO + hC + complement inhibitor (C1inh),13 IgGNMO + hC in AQP4 null mice (see Fig 1), IgGNMDAR + hC (Supplementary Fig ii), or IgGNMO + hC after depleting the anti-AQP4 IgG (Supplementary Fig iv). Injecting recombinant monoclonal anti-AQP4 IgG1 (rAb-53)23 + hC produced inflammation, myelin, and AQP4 loss (Supplementary Fig v). We conclude that the AQP4 protein, the anti-AQP4 IgG within IgGNMO, and the hC are necessary and sufficient to produce the NMO lesion.
Because infiltration of neutrophils in brain occurs after NMO-IgG-induced hC activation, we hypothesized that neutropenia would have little effect on hC activation. Qualitatively, there was comparable perivascular deposition of the membrane attack complex (C5b-9) in the injected hemisphere of 3 non-neutropenic vs 3 neutropenic mice that had received IgGNMO + hC (see Fig 1F). There was no C5b-9 immunoreactivity in 2 mice injected with IgGCON + hC or 2 AQP4 null mice injected with IgGNMO + hC (not shown).
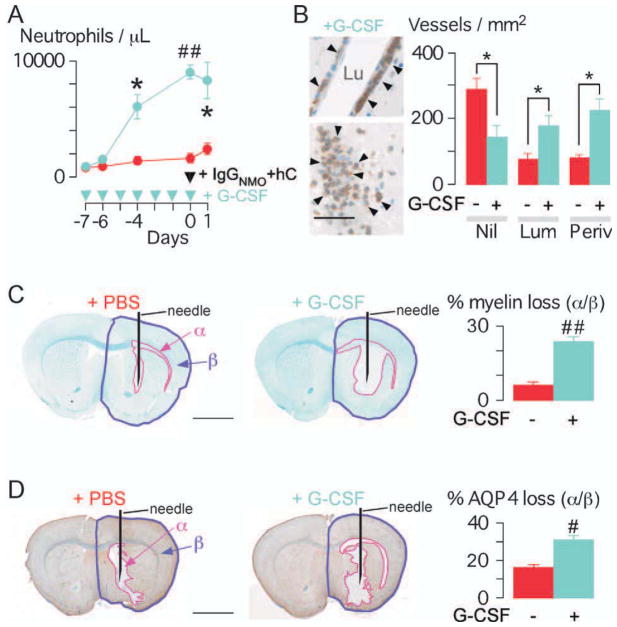
Neutrophilia Exacerbates Brain Lesions at 24 Hours
NMO lesions were compared in control mice vs mice pretreated with G-CSF, which increased peripheral neutrophil count fivefold at the time of IgGNMO + hC injection (Fig 2A). Neutrophilia remarkably increased the number of inflamed cerebral vessels at 24 hours after injecting IgGNMO + hC (see Fig 2B, Supplementary Fig iii). The inflammation around each vessel appeared more marked in neutrophilic vs. non-neutrophilic mice (see Fig 2B, left vs Fig 1D, inset). Neutrophilic mice had significantly larger areas of myelin loss (see Fig 2C) and loss of AQP4 (see Fig 2D). Neutrophilia thus increases the severity of NMO lesions after IgGNMO + hC injection.
FIGURE 2.
Neutrophilia increases brain damage at 24 hours after IgGNMO + hC injection. (A) Peripheral neutrophil count. GCSF (blue) or PBS (red) was injected daily. All mice received IgGNMO+hC at day 0 and were killed after 24 hours. (B) Neutrophil immunostain (arrowheads): vessels from +G-CSF brain. Lu = lumen. Bottom picture: neutrophils occlude lumen. Number of Nil, Lum, and Periv vessels/mm2 in +PBS (red) vs +G-CSF (blue) mice. (C) (Left) LFB: +PBS vs +G-CSF brain. (Right) % loss of myelin (area-α/area-β). (D) (Left) AQP4 immunostain: +PBS vs +G-CSF brain. (Right) Loss of AQP4 (area-α/area-β). We used 4 +PBS vs 4 +G-CSF mice. Mean ± standard error. *p < 0.05, #p < 0.005, ##p < 0.0005 for +PBS vs +G-CSF. Bar = 50μm (B); 2mm (C, D).
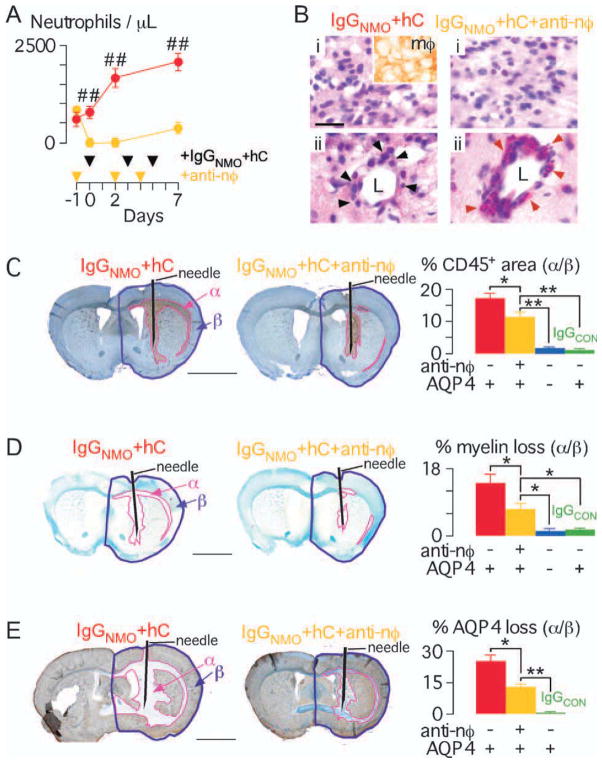
Neutropenia Reduces NMO Lesions at 7 Days
We compared NMO-IgG-induced brain injury at 7 days in non-neutropenic vs neutropenic mice. Injecting antineutrophil IgG produced neutropenia (Fig 3A) without affecting the peripheral lymphocyte, monocyte, or eosinophil counts (Supplementary Fig i).
FIGURE 3.
Neutropenia reduces brain damage at 7 days after IgGNMO+ hC injection. (A) (Left) Peripheral neutrophil count. Antineutrophil (yellow) or isotype control (red) IgG was injected at days −1, 2, and 4. All mice received IgGNMO+hC at days 0, 3, and 5 and were killed on day 7. (B) H&E: non-neutropenic vs neutropenic (+anti-nφ) brain. (i) Brain parenchyma, inset = macrophage immunostain. (ii) Perivascular leukocytes, black arrowheads = mononuclear cells, red arrowheads = eosinophils, L = lumen. (C) (Left) CD45 immunostain: non-neutropenic vs neutropenic (+anti-nφ) brain. (Right) % inflammation (area-α/area-β). (D) (Left) LFB: non-neutropenic vs neutropenic (+anti-nφ) brain. (Right) % loss of myelin (area-α/area-β). (E) (Left) AQP4 immunostain: non-neutropenic vs neutropenic (+anti-nφ) brain. (Right) % loss of AQP4 (area-α/area-β). We injected 6 non-neutropenic (red), 6 neutropenic (yellow), and 5 non-neutropenic AQP4 null (blue) mice with IgGNMO+hC; 5 non-neutropenic mice received IgGCON+hC (green). Mean ± standard error. *p < 0.05, **p < 0.01, ##p < 0.0005 vs IgGNMO+hC+anti-nφ. Bar = 20 μm (B), 2 mm (C–E).
The injected hemisphere was infiltrated with inflammatory cells in non-neutropenic and neutropenic mice (see Fig 3B). Inflammatory cells, primarily macrophages, were abundant within the brain parenchyma. Inflammatory cells were also seen around blood vessels. In the non-neutropenic mice the perivascular cells were thinly scattered macrophages with some neutrophils and eosinophils. Interestingly, in the neutropenic mice, the perivascular cells were densely packed eosinophils with relatively few macrophages. There was a significantly smaller CD45+ area in the neutropenic vs non-neutropenic mice (see Fig 3C). Compared with non-neutropenic mice, the neutropenic mice had markedly reduced area of loss of myelin (see Fig 3D) and AQP4 (see Fig 3E). Therefore, the protective effect of neutropenia persists for at least 7 days after NMO-IgG-induced hC activation in the brain.
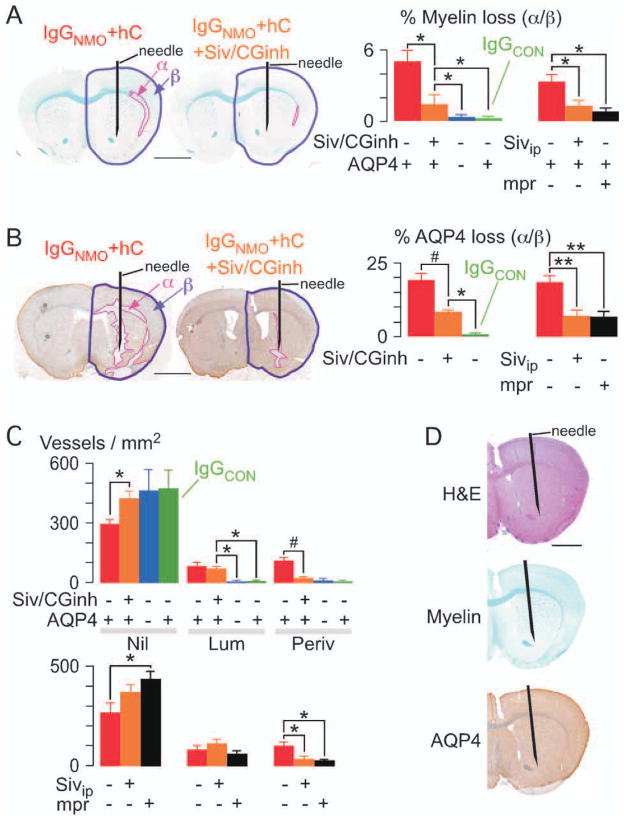
Neutrophil Protease Inhibition Reduces NMO Lesions at 24 Hours
We determined whether selective inhibition of the major neutrophil proteases, NE (with Sivelestat) and cathepsin G (with cathepsin G inhibitor I), protects mice from NMO-IgG-initiated brain injury. Compared with the mice that received IgGNMO + hC, the mice that also received Sivelestat + cathepsin G inhibitor I intracerebrally had remarkably less loss of myelin (Fig 4A) and AQP4 (see Fig 4B). Intraperitoneal (i.p.) administration of Sivelestat (without cathepsin G inhibitor I) or methylprednisolone also reduced NMO-IgG-initiated brain injury (see Figs 4A, B, far right). These findings suggest that the detrimental effect of neutrophils in NMO lesions is NE-dependent and can be treated systemically.
FIGURE 4.
Neutrophil protease inhibitors reduce brain damage at 24 hours after IgGNMO+hC injection. (A) LFB, and (B) AQP4 immunostain. (Left) Brain injected with IgGNMO+hC alone (IgGNMO+hC) or with Sivelestat and cathepsin G inhibitor I (IgGNMO+hC+Siv/CGinh). (Right) % area-α/area-β (A) loss of myelin and (B) loss of AQP4 after IgGNMO+hC (6 mice, red), IgGNMO+hC+Siv/CGinh (6 mice, orange), IgGNMO+hC (5 AQP4 null mice, blue), or IgGCON+hC (5 mice, green). (Far Right) % area-α/area-β (A) loss of myelin and (B) loss of AQP4 after injecting IgGNMO+hC without (6 mice, red) vs with i.p. Sivelestat (6 mice, orange) vs with i.p. methylprednisolone (5 mice, black). (C) Number of Nil, Lum, and Periv vessels/mm2. (Top) IgGNMO+hC (6 mice, red), IgGNMO+hC+Siv/CGinh (6 mice, orange), IgGNMO+hC (5 AQP4 null mice, blue), or IgGCON+hC (5 mice, green). (Bottom) IgGNMO+hC without (6 mice, red) vs with i.p. Sivelestat (6 mice, orange) vs. with i.p. methylprednisolone (5 mice, black). (D) Brain at 24 hours after intracerebral injection of NE and Cath G: H&E (top), LFB (middle), AQP4 immunostain (bottom). Mean ± standard error. *p < 0.05, **p < 0.01, #p < 0.005 vs IgGNMO+hC+Siv/CGinh or +Sivip. Bar = 2mm (A, B, D).
Possible mechanisms for the protection by NE inhibition include reduced neutrophil protease-dependent brain injury or reduced neutrophil entry into the brain. In Figure 4C, intracerebral Sivelestat and cathepsin G inhibitor I (top) or i.p. Sivelestat (bottom) markedly reduced neutrophil entry into the brain, as evidenced by fewer vessels with perivascular neutrophils (Periv) in the proteasetreated mice. Neutrophil protease inhibition did not inhibit neutrophil adhesion to the endothelium (ie, number of “Lum” vessels) after IgGNMO + hC injection. Direct intracerebral injection of NE and cathepsin G did not produce myelin or AQP4 loss at 24 hours in 3 mice (see Fig 4D) or 4 days in 3 other mice (not shown). Our data suggest that NE facilitates neutrophil entry into the NMO lesion.
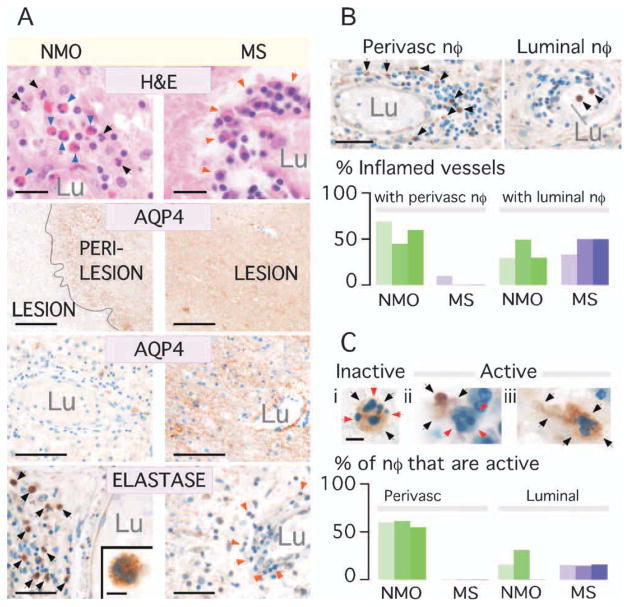
Neutrophil Elastase Is Abundant Within Human NMO But Not MS Lesions
Hematoxylin-eosin staining (Fig 5A, top row) revealed perivascular inflammation in all NMO and MS samples. In NMO, the inflammatory cells were neutrophils, eosinophils, and mononuclear cells, but the MS tissues only had mononuclear cells. There was loss of AQP4 immunoreactivity in NMO, with strong AQP4 expression in MS (see Fig 5A second and third rows). About 40% to 60% of the inflamed vessels in NMO were surrounded by NE+ cells, compared with 0% to 15% in MS (see Fig 5A bottom row and B). The percentage of inflamed vessels with NE+ cells within their lumen was comparable (40–50%) in NMO vs MS, suggesting neutrophil transmigration in NMO but not MS. We classed neutrophils as “inactive” or “active” based on their morphology (see Fig 5C, top). Round neutrophils with intracellular NE were termed ‘inactive’ (see Fig 5C, i). “Active” neutrophils were degranulated, ie, with extracellular NE (see Fig 5C, ii), or elongated, indicating cell migration, a NE-dependent process (see Fig 5C, iii). About one-half of the perivascular neutrophils in NMO were “active,” with only 15% “active” neutrophils observed intraluminally in NMO and MS (see Fig 5C, bottom). These results support the conclusion that neutrophil activation is unique to NMO lesions. Further histological characterization is provided in Supplementary Table ii.
FIGURE 5.
Abundant neutrophil elastase within human NMO but not MS lesions. (A) NMO vs MS brain lesions. H&E: Arrows = neutrophils (black), eosinophils (blue), mononuclear cells (orange). AQP4 immunostain: (Top) Lesion and perilesion, (Bottom) Vessel in lesion. Lu = lumen. NE immunostain: Perivascular region. Arrows = NE+ (black) and NE− (orange) leukocytes. (Inset) NE+ cell. (B) (Top) Vessel with perivascular (no intraluminal) and 1 with intraluminal (no perivascular) neutrophils. (Bottom) % inflamed vessels with perivascular NE+ cells and % with circulating (luminal) NE+ cells in 3 NMO (green) vs 3 MS (purple) samples. (C) Top (i) Inert and (ii,iii) active neutrophils. (i) NE (black arrows) is intracellular (red arrows). (ii) Extracellular NE. (iii) Elongated neutrophil (black arrows). (Bottom) Number of perivascular and luminal active neutrophils in 3 NMO (green) and 3 MS (purple) samples. Bar = 20μm (A: H&E), 0.5mm (A: AQP4 top), 100μm (A: AQP4 bottom), 50μm (A: Elastase, B), 5μm (A: Elastase inset, C).
Peripheral Leukocyte Counts in NMO Patients
The cell counts (×109/liter, mean ± standard error of the mean [SEM]) for an acute exacerbation vs baseline for the 5 NMO patients were as follows: 8.9 ± 0.6 vs 4.6 ± 0.8, p = 0.04 (neutrophils); 0.1 ± 0.1 vs 0.1 ± 0.1, p = 1.00 (eosinophils); 0.7 ± 0.1 vs 0.4 ± 0.1, p = 0.07 (monocytes); and 1.8 ± 0.3 vs 2.2 ± 0.5, p = 0.41 (lymphocytes). These data support a rise in the peripheral neutrophil count during acute NMO exacerbations.
Discussion
Our data support a major role for neutrophils in the pathogenesis of NMO lesions. hC activation by NMO-IgG leads to a significant increase in the number of circulating neutrophils in mice and humans. The mechanism may involve the release of factors by injured astrocytes into the bloodstream that stimulate neutrophil production by the bone marrow. Injured or activated rodent astrocytes secrete G-CSF and GM-CSF in vitro24,25 and reactive human astrocytes immunostain strongly for these factors in vivo.24,26 Within hours, circulating neutrophils enter the CNS and exacerbate the injury initiated by hC activation. Because neutrophils produce CNS damage largely through a NE-dependent mechanism, we propose, based on the experimental data here, that selective NE inhibitors such as Sivelestat may be useful in treating the acute phase of NMO.
Human NMO lesions contain neutrophils, macrophages, and eosinophils, with relatively few lymphocytes. 10,11 The mouse model here allowed us to determine the order of leukocyte entry into the CNS and the contribution of different types of leukocytes in NMO pathogenesis. Within 12 hours there is perivascular complement activation, with loss of AQP4 and early myelin loss.13,21 By 24 hours neutrophils enter the lesion and accumulate around small vessels,13,21 where AQP4 is highly expressed. Here, we showed that these neutrophils exacerbate the CNS damage. By 7 days few neutrophils remain in the lesions, whereas macrophages predominate and infiltrate extensively into the white matter.13,21 Perivascular eosinophils are also seen at 7 days and become more abundant in neutropenia. The role of macrophages and eosinophils in NMO lesions is unclear. The few scattered T cells found within the lesions do not play a major role in our mouse model, since T-cell ablation did not reduce the inflammation, myelin breakdown, or AQP4 loss at 24 hours or 5 days.21
The human data support our finding in mice that neutrophils are detrimental in NMO. NE immunostaining revealed perivascular neutrophil infiltration in NMO with evidence of NE-dependent neutrophil activity in the lesions including degranulation and cell migration.27,28 The associated morphological changes in perivascular neutrophils—elastase release and cell elongation—are part of the NMO disease process, not a postmortem artifact, because they were not seen in circulating neutrophils.
The detrimental effect of neutrophil recruitment and activation, as shown here in NMO, is seen in several other diseases. Neutrophils infiltrate into mouse CNS in the preclinical (acute) phase of experimental autoimmune encephalomyelitis29,30 and in vivo depletion of neutrophils prevents acute experimental autoimmune encephalomyelitis. 31 Neutrophils exacerbate brain damage during brain ischemia-reperfusion in mice,32 lung damage in acute respiratory distress syndrome,33 cartilage damage in inflammatory arthritis,34 as well as liver damage during ischemia, alcoholism, and cholestasis.35 Neutrophils damage tissues by releasing proteases (including elastase and cathepsin G) and free radicals (including superoxide) that may injure cells directly and degrade the extracellular matrix.
In NMO the astrocytes and reactive astrocytes are specifically targeted by hC and neutrophils. It is well established that reactive astrocytes are essential for preserving the integrity of oligodendrocytes. Eliminating reactive astrocytes using genetic manipulation caused profound myelin loss after brain36 or spinal cord37 injury. We propose that by destroying the reactive astrocytes, IgGNMO + hC secondarily produces extensive demyelination. In other CNS conditions associated with neutrophil infiltration the reactive astrocytes are preserved and therefore extensive demyelination does not occur.
A clinically important finding is the remarkable attenuation in NMO lesion generation with Sivelestat. Sivelestat is a potent, substrate-competitive, and highly specific inhibitor of neutrophil elastase with one-half maximal inhibitory concentration (IC50) of 19 to 49nM for rat, rabbit, hamster, human, and mouse NE.38 Sivelestat is cell-permeable38,39 and should thus inhibit NE intracellularly in circulating neutrophils before their entry into the CNS. Sivelestat is used in Japan to treat acute respiratory distress syndrome and is well tolerated with no major side effects.33 Although neutrophils secrete several proteolytic enzymes, selective NE inhibition impairs neutrophil migration and phagocytosis and abolishes the formation of neutrophil extracellular traps.27,28,40 We showed that by inhibiting NE, Sivelestat inhibits the entry of neutrophils into the NMO lesion. The half-life of activated human41 and mouse42 neutrophils is short (~8 hours); therefore, inhibition of NE may be therapeutic even after some neutrophils have entered the CNS, by preventing further neutrophil entry. Sivelestat might be useful as a corticosteroid-sparing agent because its therapeutic benefit is comparable to that of methylprednisolone.
We conclude that neutrophil entry into the CNS is an early event in the evolution of NMO lesions and that the development of the early lesion is NE-dependent. Our results provide proof of concept for the use of the NE inhibitor Sivelestat for NMO treatment to reduce CNS damage in acute exacerbations.
Supplementary Material
TABLE 1.
TABLE Primary Antibodies Used for Immunohistochemistry
| Antibody | Target | Concentration | Source |
|---|---|---|---|
| Mouse tissue | |||
| Rat 1A8 anti-Ly6G | Neutrophils | 1:100 | BD Biosciences, Oxford, UK |
| Rabbit anti-AQP4 | AQP4 | 1:100 | Millipore, Livingston, UK |
| Rat anti-macrophage | Macrophages | 1:10 | eBioscience, Hatfield, UK |
| Mouse anti-CD45 | Leukocytes | 1:10 | BD Biosciences, Oxford, UK |
| Rabbit anti-C5b-9 | Fixed complement | 1:100 | Abcam, Cambridge, UK |
| Human tissue | |||
| Rabbit anti-AQP4 | AQP4 | 1:100 | Millipore, Livingston, UK |
| Rabbit anti-NE | Neutrophil elastase | 1:2,000 | Abcam, Cambridge, UK |
| Mouse anti-GFAP | GFAP | 1:200 | Millipore, Livingston, UK |
Tissue was unmasked in citric acid and incubated with the primary antibody for 1 hour at room temperature, followed by appropriate species secondary antibody (1:500, 1 hour, room temperature, Vector Laboratories, Peterborough, UK). Immunostaining was visualized brown using the Vectastain HRP kit (Vector Laboratories, Peterborough, UK) followed by DAB/H2O2. Nuclei were counterstained blue with hematoxylin.
Acknowledgments
This research was supported by a grant from the Guthy- Jackson Charitable Foundation (to A.S.V. and M.C.P. and the Oxford NIHR Biomedical Research Centre (PW and AV). A.S.V. is funded by grants EY13574 (National Eye Institute) and DK35124 (National Institute for Diabetes and Digestive and Kidney Diseases) from the National Institutes of Health).
We thank Prof. J.L. Bennett of the University of Colorado at Denver for providing the recombinant monoclonal anti-AQP4 antibody. Human CNS tissue samples were supplied by The Thomas Willis Oxford Brain Collection, and the UK Multiple Sclerosis Tissue Bank funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland (registered charity 207495).
Footnotes
Potential Conflicts of Interest
A.S.V.: grants: EY13574 and DK35124 from the National Institutes of Health, and Guthy-Jackson Charitable Foundation. A.V.: grant: Oxford NIHR Biomedical Research Centre. M.C.P.: grant: Guthy-Jackson Charitable Foundation. P.W.: grant: Oxford NIHR Biomedical Research Centre; employment: University of Oxford; patents: patents for diagnostic assays for Lgi1 and Caspar2.
Additional Supporting Information can be found in the online version of this article.
References
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: aquaporin- 4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6:383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 6.Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock-Guttman B, Miller C, Yeh E, et al. Neuromyelitis optica immunoglobulins as a marker of disease activity and response to therapy in patients with neuromyelitis optica. Mult Scler. 2008;14:1061–1067. doi: 10.1177/1352458508092811. [DOI] [PubMed] [Google Scholar]
- 8.Cree BA, Lamb S, Morgan K, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto K, Kusunoki S. Intermittent plasmapheresis prevents recurrence in neuromyelitis optica. Ther Apher Dial. 2009;13:505–508. doi: 10.1111/j.1744-9987.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 10.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 11.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 13.Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita M, Nakatsuji Y, Moriya M, et al. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport. 2009;20:508–512. doi: 10.1097/wnr.0b013e32832776f4. [DOI] [PubMed] [Google Scholar]
- 16.Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16:1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 17.O’Riordan JI, Gallagher HL, Thompson AJ, et al. Clinical, CSF, and MRI findings in Devic’s neuromyelitis optica. J Neurol Neurosurg Psychiatry. 1996;60:382–387. doi: 10.1136/jnnp.60.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarius S, Franciotta D, Paul F, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 2011;7:82–90. doi: 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma T, Yang B, Gillespie A, et al. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters P, Jarius S, Littleton E, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–919. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 21.Saadoun S, Waters P, MacDonald C, et al. T cell deficiency does not reduce neuromyelitis optica lesions in mice following intracerebral injection of NMO-IgG and complement. J Neuroimmunol. 2011;235:27–32. doi: 10.1016/j.jneuroim.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Daley JM, Thomay AA, Connolly MD, et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic antiaquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stan AC, Walter GF, Welte K, Pietsch T. Immunolocalization of granulocyte-colony-stimulating factor in human glial and primitive neuroectodermal tumors. Int J Cancer. 1994;59:306–312. doi: 10.1002/ijc.2910570303. [DOI] [PubMed] [Google Scholar]
- 25.Ohno K, Suzumura A, Sawada M, Marunouchi T. Production of granulocyte/macrophage colony-stimulating factor by cultured astrocytes. Biochem Biophys Res Commun. 1990;169:719–724. doi: 10.1016/0006-291x(90)90390-9. [DOI] [PubMed] [Google Scholar]
- 26.Malipiero UV, Frei K, Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol. 1990;144:3816–3821. [PubMed] [Google Scholar]
- 27.Kaynar AM, Houghton AM, Lum EH, et al. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2008;39:53–60. doi: 10.1165/rcmb.2007-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young RE, Thompson RD, Larbi KY, et al. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004;172:4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 29.Wu F, Cao W, Yang Y, Liu A. Extensive infiltration of neutrophils in the acute phase of experimental autoimmune encephalomyelitis in C57BL/6 mice. Histochem Cell Biol. 2010;133:313–322. doi: 10.1007/s00418-009-0673-2. [DOI] [PubMed] [Google Scholar]
- 30.Nygardas PT, Maatta JA, Hinkkanen AE. Chemokine expression by central nervous system resident cells and infiltrating neutrophils during experimental autoimmune encephalomyelitis in the BALB/c mouse. Eur J Immunol. 2000;30:1911–1918. doi: 10.1002/1521-4141(200007)30:7<1911::AID-IMMU1911>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.McColl SR, Staykova MA, Wozniak A, et al. Treatment with antigranulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- 32.Prestigiacomo CJ, Kim SC, Connolly ES, Jr, et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 33.Iwata K, Doi A, Ohji G, et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med. 2010;49:2423–2432. doi: 10.2169/internalmedicine.49.4010. [DOI] [PubMed] [Google Scholar]
- 34.Eyles JL, Hickey MJ, Norman MU, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 35.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 36.Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 37.Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabata K, Suzuki M, Sugitani M, et al. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun. 1991;177:814–820. doi: 10.1016/0006-291x(91)91862-7. [DOI] [PubMed] [Google Scholar]
- 39.Nakatani K, Takeshita S, Tsujimoto H, et al. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001;69:241–247. [PubMed] [Google Scholar]
- 40.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinski A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of GCSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.