Abstract
Biotargeted nanomedicines have captured the attention of academic and industrial scientists who have been motivated by the theoretical possibilities of the ‘magic bullet’ that was first conceptualized by Paul Ehrlich at the beginning of the 20th century. The Biotargeting Working Group, consisting of more than 50 pharmaceutical scientists, engineers, biologists and clinicians, has been formed as part of the National Cancer Institute’s Alliance for Nanotechnology in Cancer to harness collective wisdom in order to tackle conceptual and practical challenges in developing biotargeted nanomedicines for cancer. In modern science and medicine, it is impossible for any individual to be an expert in every aspect of biology, chemistry, materials science, pharmaceutics, toxicology, chemical engineering, imaging, physiology, oncology and regulatory affairs. Drawing on the expertise of leaders from each of these disciplines, this commentary highlights six tenets of biotargeted cancer nanomedicines in order to enable the translation of basic science into clinical practice.
Keywords: cost–effectiveness analysis, good manufacturing practice, ligand, nanoparticle, receptor targeted
Nanotechnology has been applied to cancer to enhance the utility of US FDA-approved chemo-therapeutics. Practical benefits of nanoparticle-based chemotherapeutics include increased drug solubility, reduced toxicity to healthy organs, increased tumor accumulation, and protection of the payload from premature metabolism and degradation. Nanoparticles can further be engineered to target specific tumor cells that express particular cell surface molecules. The selection of appropriate targets, component materials, formulation strategies and characterization methods are critical to achieving successful outcomes. Manufacturing and quality control requirements that are mandated by regulatory bodies are also important factors to clinical translation. Finally, market acceptance, pricing and reimbursement issues must be considered. As one embarks on the creation and development of biotargeted nanomedicines for cancer, six important tenets should guide the process.
Tenet 1: sights on the target
Biotargeted nanomedicines are defined as nanoparticles containing a drug and/or imaging agent administered to the body and targeted to a specific organ [1], tissue [2], cell [3] or subcellular [4] compartment in order to treat [5] or diagnose [6,7] disease, or both (Figure 1) [8,9].
Figure 1.
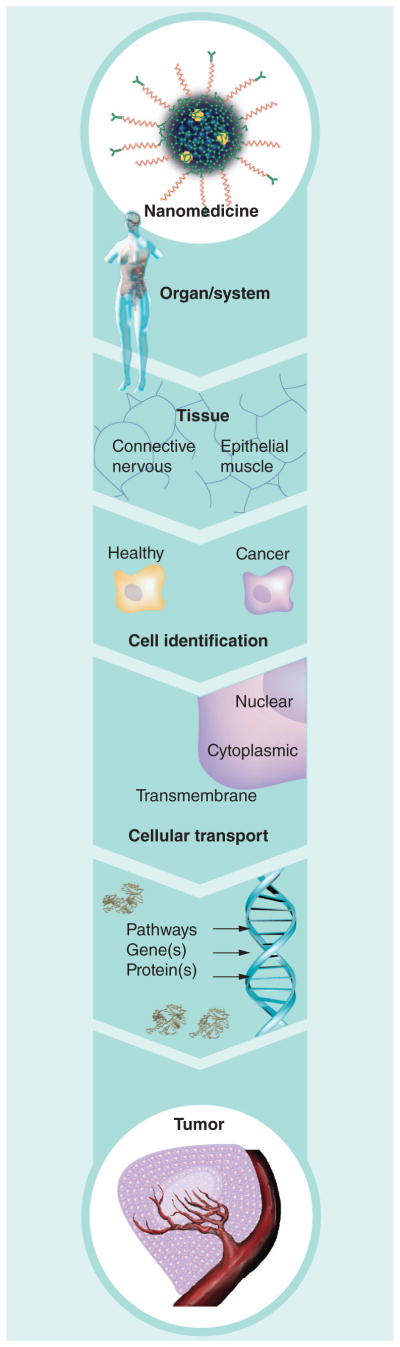
Sequential barriers and opportunities for targeting a nanomedicine to cancer.
Biotargeting is afforded by the use of targeting ligands on the surface of nanoparticles, including small molecules [10], peptides [11], antibodies [12], affibodies [13] and aptamers [14]. These particles, when loaded with drugs, biologics, nucleic acids or imaging agents, have shown tremendous utility as in vitro diagnostics and as therapies in animal models of cancer. Translating such nanoparticles into biotargeted nanomedicines requires that they confer improved efficacy or safety in patients. Challenges and possible solutions are discussed herein.
Biotargeted nanomedicines have the potential to transform the diagnosis and treatment of many human diseases; however, selection of the appropriate target is critical for successful outcomes. While some early progress has been achieved, the full realization of the impact of this approach will depend largely on the determination of suitable clinical applications. Biotargeted nanomedicines can be developed at the level of the drug (molecular targeted therapy such as Gleevec®; Novartis Pharmaceuticals Corporation, NJ, USA) or at the level of the delivery system, which is the primary focus of this article.
Biotargeted nanoparticles have the potential to substantially improve the therapeutic index of their payloads by increasing drug potency via selective delivery to target cancer cells or tumor stroma, thereby reducing their systemic toxicity and undesired off-target effects. Twenty nanomedicines have been approved by the FDA [15] and there are currently four targeted nanomedicines under clinical investigation, all in the area of cancer: BIND-014 [101], CALAA-01 [102], MBP-426 [103] and SGT-53 [104]. BIND-014 is a prostate-specific membrane antigen-targeting PLGA nanoparticle containing docetaxel. CALAA-01 is a transferrin receptor-targeting cyclodextrin-based nanoparticle containing siRNA directed against the M2 subunit of ribonucleotide reductase. MBP-426 is a transferrin receptor-targeting liposome containing oxaliplatin. SGT-53 is a transferrin receptor-targeting liposome containing plasmid DNA encoding p53.
It is noteworthy that, among the four ongoing trials, there are three different classes of therapeutics represented: small molecules, siRNA and DNA. It is also noteworthy that three of the four formulations target the transferrin receptor. This receptor is often targeted because it is one of the most abundant receptors on the cell membrane, is released from clathrin-coated pits by endosomal acidification and recycles rapidly back to the cell membrane; together, these features promote high-efficiency uptake into the cell. Both native transferrin and antibodies to the transferrin receptor can be conjugated to nanoparticles to facilitate internalization. Although multiple therapeutic modalities are being advanced through clinical trials, there clearly remains a shortage of validated receptors to target. The use of defined criteria represents a novel approach to identifying and validating targetable biomarkers [16]. Among the criteria considered were extracellular localization of the target, diffuse upregulation of the target throughout tumor tissue and upregulation of the target in most patients.
Several features are desirable for targeting, but the identification of receptors that satisfy all of these criteria remains difficult. First, exclusive presentation (e.g., clusters of differentiation markers within immune cell subpopulations) or at least marked upregulation (e.g., EGF receptor, HER2, MUC1 on cancer cells with a tumor:normal cell expression ratio of at least 10:1) of the receptor enables the nanomedicine to discriminate between the targeted cell and other cells in the body. Second, receptor function in the diseased state must be considered when designing a targeting strategy. Examples include sensitivities to known ligands, crosstalk between downstream signaling pathways, mechanisms of receptor internalization and/or turnover, and receptor saturation and/or downregulation after repeated doses [17]. Third, although releasing small molecules in close proximity to the target cell can be beneficial in the case of small-molecule therapeutics, particle uptake by receptor-mediated processes is essential for nucleic acid-based therapies. Indeed, it has been shown that the principal advantage of targeted nanoparticles is related to uptake by cancer cells rather than overall tumor localization [18,19]. Consequently, optimization of particle internalization will probably enhance the specificity and efficacy of nanomedicines.
Tenet 2: leaping biological hurdles
The validation of additional targets is important, but the complexity of tumor biology must also be recognized [20]. Recent evidence indicates that tumors are composed of highly interactive and cooperatively functioning cellular communities. These cell subpopulations modulate one another’s biological characteristics, such as growth rate, metastatic ability and sensitivity to anticancer therapy [20,21]. In this regard, tumors can be regarded as self-generated heterogeneous cell populations contained within a permissive tumor microenvironment that is a complex system of many cell types. Collectively, these diverse cells make up the complex organ system of solid tumors, which includes vasculature, stroma and tumor parenchyma, and contribute dynamically to tumor progression, metastasis and resistance to therapy [22].
One of the fundamental considerations for biotargeting is the determination of which cell type to target: endothelium, tumor cells, stroma or a combination of cell types. Our currently limited understanding of the repertoire of clinically relevant, targetable cellular receptors (or other plasma membrane proteins) and their interactions, cell surface densities, shedding status, turnover, internalization rates and dynamics hampers translation. Further fundamental biological studies are needed to understand these processes in both cancer and healthy cells, since the majority of tumor targets are expressed at some level in healthy cells. Basic science will inform applied science not only at the level of the cell surface but also at the level of the tumor–organ system.
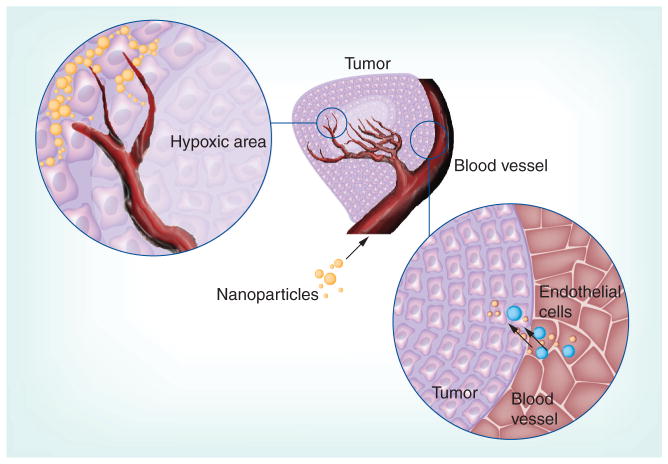
Owing to the intrinsic heterogeneity of tumors and the multiple barriers within tumors, robust biotargeting has proven to be a formidable challenge for the most common forms of solid cancer. Receptor-targeted or ligand-enabled nanomedicines and imaging agents must travel from the systemic circulation into the tumor vas-culature, penetrate into the tumor parenchyma, diffuse deeply into the hypoxic core of the tumor and discriminate their target cells from healthy neighboring cells (Figure 2).
Figure 2.
Transport of nanoparticles from the vasculature into the tumor parenchyma and hypoxic core.
Several physical barriers limit the efficacy of tumor penetration and delivery by nanomedicines directed against solid tumors, including heterogeneous circulation caused by the abnormal and irregular architecture of tumor vasculature, intratumoral vascular hyperpermeability contributing to increased interstitial pressure in the targeted tumors that substantially reduces the convective transport of nanoparticles into the mass, and impaired diffusion in the context of an unusual, highly dense extracellular matrix in the tumor microenvironment. It has been postulated that these barriers are responsible for the modest survival benefit that has been observed using FDA-approved nanotherapeutics, as delivery of insufficient quantities of drug in the tumor core can lead to resistance and/or incomplete treatment [23]. Accordingly, the development of innovative strategies aimed at abnormal tumor vasculature and matrix barriers are needed as components of a multipronged treatment strategy in which nanomedicines play a role. The use of antiangiogenic therapies and matrix-modifying enzymes can normalize the vascular network and can even be combined with cytotoxic reagents to improve efficacy [24].
Novel strategies are being pursued to engineer solutions through multistage nanoparticle delivery [25]. The inclusion of tumor micro-environment-sensitive shells allows for site-specific activation of the particles. One example of this approach combined the natural ability of approximately 100-nm particles to accumulate in tumors (owing to enhanced permeation and retention by the leaky tumor vasculature) with the ability of smaller particles (~10 nm) to penetrate more deeply into the tumor tissue by including a tumor protease-responsive peptide [26]. Related approaches have demonstrated the active biotargeting domains in the presence of such matrix metalloproteinases [27] or in response to decreased pH in the hypoxic tumor core [28]. Engineering efforts will surely benefit from and hopefully synergize with studies that enhance our understanding of tumor biology.
Tenet 3: these are not tablets
Even if the biology were simple, the ability to control the material properties of nanomedicines remains a major obstacle to the realization of the potential of medical nanotechnology. Many additional parameters, such as physicochemical properties, are critical to the translation of nanomedicine candidates for specific clinical applications. Key properties influencing the biodistribution and pharmacokinetics of nanomedicines include particle composition, size, shape, modulus, charge and surface characteristics [29]. These properties also affect clearance routes and particle–target cell interaction, the latter of which is crucial for internalization.
Surface modification can be used both to alter the particles’ physicochemical properties and also to incorporate targeting moieties. Due to their high surface-to-volume ratio, nanoparticles are particularly well suited to displaying targeting ligands on their surfaces in a dense manner. Unlike current conventional imaging agents that cannot recognize more than one biomarker, nanoparticles can be functionalized with multiple ligands to detect several biomarkers simultaneously [30,31] and at much lower concentrations [32,33], thus allowing for multiplexed anatomic and functional imaging [34].
Important considerations for biotargeted nanoparticles include the type of ligand, the preferred ligand architecture and optimal stoichiometry/density per particle. Furthermore, since there are many potential sites of attachment, the inherent heterogeneity of ligand distribution on the surface of the nanoparticles must be recognized and addressed. Controlled, reproducible surface modification remains a difficult task. Additionally, the characterization of ligands, as well as the aforementioned parameters, is crucial. Table 1 highlights some common roadblocks and possible solutions in characterizing clinical and preclinical nanoparticle formulations. Formulation often relies on self-assembly, and the resultant particles can exhibit high poly-dispersity. Extrusion can be used to narrow the size distribution [35], and templated manufacturing [29] and microfluidic-based flow focusing are emerging strategies [36].
Table 1.
Common roadblocks to the realization of biotargeted nanomedicines
| Characterization parameters | Common roadblock | Possible solutions | Ref. |
|---|---|---|---|
| Biological activity/ potency | Difficulties characterizing a number of ligands on the surface of the nanoparticles, including orientation and activity. Changes in the manufacturing process can readily alter these parameters | Characterization is key. Multiple orthogonal methods should be applied to evaluate batch-to-batch consistency. ζ-potential and microscopy methods can be evaluated to probe nanoparticle surface. Whenever possible, nanomedicines should be evaluated using functional bioassays | [46,47] |
| Polydispersity/ heterogeneity | Individual molecules of a nanoparticle can contain hundreds or even thousands of atoms. Therefore, many possible permutations in the arrangement and chemical attachment leads to an inherent structural heterogeneity, even in a ‘pure’ nanomedicine | Nanomedicine properties must be defined by an acceptable range that affords the necessary safety and efficacy profile for the nanoparticle formulation rather than by an absolute standard | [48,49] |
| Biocompatibility | Non-API components of the nanoparticles cause adverse effects (e.g., surfactants used in synthesis may be cytotoxic or linkers used to attach targeting ligands may be immunotoxic) | The inclusion of appropriate controls in cytotoxicity tests, including those that distinguish and compare the toxicity of various components of a nanomedicine (i.e., buffer, supernatant/ filtrate, platform and linker molecules), may illuminate toxicity due to non-API components | [50–52] |
| In vivo stability | The drug and nanoparticle do not stay associated in vivo, and/or the rate of drug release may be rapid | Dual radiolabeling of drug and nanoparticle as well as in vitro release assays that predict in vivo fate (i.e., release assays in whole blood or 100% plasma) can be highly informative | [52–54] |
| Biodistribution | Rapid uptake from the systemic circulation by cells of the immune system (MPS) | Coating with hydrophilic polymers such as PEG can reduce interactions with plasma proteins and uptake by MPS cells | [55,56] |
| Immune reactions | Nanoparticles may cause platelet aggregation, hemolysis, coagulation, activation of the complement system or innate and/or adaptive responses | Robust in vitro assays for hematological interactions and immunological responses should be used to screen for such effects early in preclinical development | [57–59] |
API: Active pharmaceutical ingredient; MPS: Mononuclear phagocytic system; PEG: Polyethylene glycol.
Critical issues to address in the formulation of drugs entrapped in nanoparticles include entrapment efficiency, particle stability and drug release rate. Release kinetics represents a particularly salient feature of particle-based formulations, which often demonstrate rapid release of drug (‘burst release’) or very slow release. Mathematical models are being used to predict drug release from bulk- and surface-eroding systems [37], and combinatorial library screening followed by optimization can reveal pharmacokinetic profiles that are desirable [38]. Novel drug-delivery systems have been designed to require an external stimulus to trigger release [39,40], adding an extra level of control. Such advanced platforms can combine spatial targeting with functional targeting.
Nanoparticles should be well characterized prior to application since batch-to-batch variability continues to plague preclinical studies even in the most conscientious laboratories. The National Cancer Institute’s (NCI) Nanotechnology Characterization Laboratory (NCL) has been and continues to be a central player in developing reliable assays to measure in vitro characteristics of complex nanoparticles, as well as pharmacokinetic and toxicological profiles using animal models. The NCL has developed reliable screens for full nanoparticle characterization, blood contact properties, in vitro immunology and toxicity, as well as cell-based assays to assess inflammation, oxidative stress and apoptosis/necrosis. Detailed protocols can be found on their website [105]. Following in vitro characterization, nanoparticles should be evaluated for pharmacokinetics, safety and efficacy in vivo. Moving forward, improved understanding of the underlying mechanisms that dictate nanoparticle interactions with biological systems is critical.
Tenet 4: from one to millions
Large-scale manufacturing remains a costly and challenging aspect in the clinical translation of biotargeted nanomedicines. Current clinically used nanomedicines have undergone long development processes through contract research organizations to address potential issues with scale-up. While making milligram quantities to test whether the technology is feasible in the academic laboratory setting, scale-up manufacturing en route to obtaining high-quality clinical-grade material can be intimidating for small firms and large companies alike.
While more challenges exist in the development of nanomedicines as compared with traditional small-molecule drugs, it is possible to develop a robust approach that incorporates current good manufacturing practices (GMP) with standardized and validated synthetic, as well as analytical methods for the Chemistry, Manufacturing and Controls section of an Investigational New Drug filing with the FDA. A thorough preclinical investigation for biotargeted nanomedicines must include appropriate analytical, bioanalytical and biochemical methods that identify and monitor nanomedicine components that have the potential to trigger significant differences in biological end points, safety and efficacy. Size distribution (in the dry state and in physiologically relevant solution), shape, targeting ligand activity, coating uniformity and density, homogeneity of components, surface charge/ζ-potential, therapeutic payload incorporation (free vs bound/encapsulated) and release kinetics under biologically relevant conditions (Table 1) must all be analyzed using standard operating procedures and validated methods.
Another important parameter for consideration in the manufacturing and scale-up of clinical materials is preparation of the nanomedicine under sterile conditions and within the allowable limits of endotoxin contamination. Initial preparation of the material under sterile and endotoxin-free conditions is generally a better practice than post-manufacturing purification, as this approach minimizes potential problems relating to nanoparticle compatibility with sterilization techniques. Nanoparticle analysis pre- and post-sterilization should include assays that verify consistency, functional integrity and reproducible bioactivity or biological outcome.
For clinical production and pharmaceutical quality, a uniform product that is standardized and can be manufactured with insignificant batch-to-batch variation is desired. This is often difficult with nanoparticles, as an extremely controlled manufacturing environment is needed, and the scale of production dictates the intricacies of the manufacturing process. A recent FDA publication offers a chronology for nanotherapeutic development, and manufacturing characterization is highlighted [41]. However, it is prudent not to wait until the final GMP phase to consider how such a product can be made in order to meet current GMP requirements.
Tenet 5: technology meets reality
Drug and imaging products that are developed for use in humans in the USA are subject to regulatory review and approval by the FDA. The agency has formed a Nanotechnology Task Force both to address regulatory and scientific issues and to draft guidance for researchers and manufacturers [106]. Although the FDA has guidelines for what it considers to be a nanoparticle [107], it has not yet articulated specific review criteria for nanoparticle-based products. The FDA is evaluating products on a case-by-case basis, using the quality of science as a key barometer. Issues relating to synthesis, characterization, pharmacology and toxicology, as well as the manufacturing facility, are of utmost importance for regulatory approval to be granted.
Notably, materials previously approved for clinical use may have different properties from the parent material when formulated at the nanoscale or when incorporated into a nanoscale delivery platform. A well-characterized component, such as a small-molecule drug, needs to be re-assessed when incorporated into a nanoparticle, since its known pharmacokinetic profile no longer applies. Two fundamentally different components exist: the therapeutic drug, often a small molecule and a well-defined compound from a structural perspective; and the nanoparticle carrier, often comprising multiple components that may not be homogeneous. The fate of each component must be tracked and assessed separately. Moreover, the individual components may interact with one another or affect characterization [42].
Each cancer type varies in how well animal models mimic the human disease, and different species can exhibit differing absorption, distribution, metabolism, excretion and toxicological profiles. Additionally, there are lingering questions as to what effects extremely stable entities, such as quantum dots, might have on the body over extended time periods. To date, the FDA has only issued specific guidance for liposomal drug entities [108]; however, this guidance document is referenced for other nanomedicine products. With respect to nanoparticle-based products, the guidance pertains to physicochemical properties, manufacturing process, excipients, drug product specifications, stability, (bio)analytical methods, pharmacokinetics and bioavailability, and labeling. While such GMP-related issues are critical, the most important criterion for approval remains unchanged: the new nanomedicine entity must demonstrate acceptable safety, purity and efficacy, especially compared with the standard-of-care.
Tenet 6: at what cost?
Drug improvements and innovations in cancer medicines have traditionally been assessed and analyzed with respect to safety and efficacy. An often overlooked factor is cost, which is especially important in the face of ever increasing healthcare expenses. There are a number of FDA-registered nanoparticle-based products on the market that can be compared directly with their non-nanoparticle-based standard-of-care counterparts. These nanoparticle-based formulations may not be more efficacious than their counterparts; however, the nanoparticle-based formulation could reduce dose-limiting toxicities. Cost–effectiveness analysis (CEA) addresses the cost-to-benefit ratio of a new therapy versus the standard-of-care therapy and should not be confused with the risk/safety-to-benefit ratio analysis commonly performed by the FDA.
CEA is currently used as a post-marketing measure for physicians and insurers in therapy decision-making. CEA should also be used to determine whether to proceed with a particular nanoparticle technology, including analysis of raw materials, manufacturing, and therapeutic and safety outcomes. This may allow one to predict the financial implications of the nanomedicine compared with standard therapies.
At present, there are few cost–benefit studies available for nanomedicine products. As an example, the cost–effectiveness of Doxil® (Janssen Biotech Inc, PA, USA; PEGylated liposomal doxorubicin) and Abraxane® (Celgene Corporation, NJ, USA; nano-albumin bound paclitaxel) is evaluated compared with their conventional standard-of-care generic alternatives, doxorubicin and paclitaxel, respectively. In 2009, the average cost per dose of Doxil was US$5594 compared with $62–162 for doxorubicin, and the average cost per dose for Abraxane was $5054 compared with $90–454 for paclitaxel [109]. It is worth noting that Doxil and Abraxane have either exclusivity or patent protection, whereas doxorubicin and paclitaxel are generics and inherently demand a lower pricing structure. The risk and benefit factors for both nanoparticle-based products versus their small-molecule counterparts have been established [43] and are continuing to be elucidated in greater detail as more clinical trials are conducted. Notable health- and cost-related benefits of Doxil and Abraxane are lower cardiac toxicity and reduced vehicle toxicity, respectively. Although neither nanomedicine products have shown an increase in overall patient survival, the reduction in toxicities and their associated cost have largely justified the higher cost. Increasing pressure to reduce healthcare costs puts an even greater burden on the nanomedicine innovator to justify the real cost-to-benefit ratio, and CEA provides a needed tool to do so. Since targeting approaches are designed to improve efficacy while reducing toxicity, targeted nanotherapeutics have the potential to reduce overall healthcare cost of an illness despite the higher cost of the therapeutic.
Many of the companies that are currently developing biotargeted cancer therapies are smaller start-ups featuring pipelines based on technologies that were originally developed in academic laboratories, rendering high development costs even more daunting. There is a movement towards collaborative efforts between large and small pharma, government, nonprofit agencies and venture capital firms to defray costs of new therapeutic development. Biotargeted nanomedicines is one area that could significantly benefit from such an approach to bridge ‘the valley of death’. Funding programs, such as the newly established Small Business Innovation Research (SBIR) Bridge program, as well as several consortia that address clinical trials costs, such as the Prostate Cancer Clinical Trials Consortium and the NIH Biomarkers Consortium, will surely benefit both biomedicine developers and patients. As an example of sharing the costs of clinical trials, the Biomarkers Consortium is conducting two studies in which nine industry partners jointly contribute a total of $6.53 million and the NCI contributes an additional $3.75 million [110]. Likewise, in the area of nanomedicine, the NCI has established a consortium, Translation of Nanotechnology in Cancer (TONIC), to foster collaborative efforts between government, industry partners and academic researchers.
An incentive for innovators of nanomedicine products is that approved biotargeted nano-medicines are less prone to competition from generic companies, as it is very difficult to demonstrate bioequivalence of a generic version of a nanomedicine owing to the complexities of the product [44]. Thus, makers of safe, efficacious nanomedicines will probably experience a steady or increasing demand for these therapeutics for many years, potentially even after patent expiry.
Conclusion & future perspective
Biotargeted nanomedicines are now actively being investigated in human clinical trials. It is likely that the first group of FDA-registered biotargeted nanomedicines will be niche products that address specific applications. As the field continues to advance, however, we foresee solutions to the complexities described herein through the application of rigorous characterization techniques and utilization of the collected data to inform subsequent iterations of nanomedicine design. By combining the principles of engineering, chemistry and medicine – particularly in the context of an improved understanding of fundamental biology – the field of nanotechnology will move closer to making the elusive ‘magic bullet’ a reality [45].
Executive summary.
Tenet 1: sights on the targe
The selection of the appropriate target for biotargeted nanomedicines is critical for successful outcomes.
Tenet 2: leaping biological hurdles
The validation of new targets is important, but the complexity of tumor biology must also be recognized.
Tenet 3: these are not tablets
The ability to control the material properties of nanomedicines remains a major obstacle to the realization of the potential of medical nanotechnology.
Tenet 4: from one to millions
A thorough investigation for biotargeted nanomedicines must include appropriate analytical, bioanalytical and biochemical methods.
Tenet 5: technology meets reality
The new nanomedicine entity must demonstrate acceptable safety, purity and efficacy, especially compared with the standard-of-care.
Tenet 6: at what cost?
The nanomedicine innovator must justify the real cost-to-benefit ratio of their new nanomedicine as compared with the standard-of-care.
Acknowledgments
The authors would like to thank J Whitley, Creative Lead for Instructional Innovation in the Educational Technology Research and Development Group at the UNC Eshelman School of Pharmacy, for his creation of Figures 1 & 2.
Footnotes
Financial & competing interests disclosure
This project has been funded in whole or in part with federal funds from the National Cancer Institute (NCI)–NIH under contract number HHSN261200800001E. All authors are part of the NCI Alliance for Nanotechnology in Cancer and hold either Center of Cancer Nanotechnology Excellence or Cancer Nanotechnology Platform Partnership grants. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Contributor Information
Michael S. Goldberg, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02215, USA
Sara S. Hook, Center for Strategic Scientific Initiatives, Office of the Director, National Cancer Institute, NIH, Bethesda, MD 20892, USA
Andrew Z. Wang, Department of Radiation Oncology, School of Medicine, University of North Carolina, Chapel Hill, NC 27514, USA
Jeff WM. Bulte, Russell H Morgan Department of Radiology & Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD21205, USA
Anil K. Patri, Nanotechnology Characterization Laboratory, National Cancer Institute, Frederick, MD 21702, USA
Fatih M. Uckun, Department of Pediatrics, Keck School of Medicine, University of Southern California, & Children’s Center for Cancer & Blood Diseases/Children’s Hospital Los Angeles, Los Angeles, CA 90033, USA
Vincent L. Cryns, Department of Medicine, University of Wisconsin School of Medicine & Public Health, Madison, WI 53706, USA
Justin Hanes, Department of Radiology, School of Medicine, Stanford University, Palo Alto, CA 94305, USA.
Demir Akin, Department of Radiology, School of Medicine, Stanford University, Palo Alto, CA 94305, USA.
Jennifer B. Hall, Nanotechnology Characterization Laboratory, National Cancer Institute, Frederick, MD 21702, USA
Nastaran Gharkholo, Center for Nanotechnology in Drug Delivery, Division of Molecular Pharmaceutics, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27514, USA.
Russell J. Mumper, Email: mumper@email.unc.edu, Center for Nanotechnology in Drug Delivery, Division of Molecular Pharmaceutics, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27514, USA, Tel.: +1 919 966 1271, Fax: +1 919 966 6919
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Nolan BW, Schermerhorn ML, Powell RJ, et al. Restenosis in gold-coated renal artery stents. J Vasc Surg. 2005;42(1):40–46. doi: 10.1016/j.jvs.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Park J-H, Von Maltzahn G, Xu MJ, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci USA. 2010;107(3):981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol Pharm. 2011;8(1):143–152. doi: 10.1021/mp100203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agemy L, Friedmann-Morvinski D, Kotamraju VR, et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc Natl Acad Sci USA. 2011;108(42):17450–17455. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon Z, Lee JA, Huang S, Prevost RJ, Hammond PT. Highly stable, ligand-clustered “patchy” micelle nanocarriers for systemic tumor targeting. Nanomedicine (Lond) 2011;7(2):201–209. doi: 10.1016/j.nano.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Mao H, Cao Z, et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology. 2009;136(5):1514.e2–1525.e2. doi: 10.1053/j.gastro.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Mao H, Wang YA, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5(2):235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6(18):2041–2049. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santra S, Kaittanis C, Santiesteban OJ, Perez JM. Cell-specific, activatable, and theranostic prodrug for dual-targeted cancer imaging and therapy. J Am Chem Soc. 2011;133(41):16680–16688. doi: 10.1021/ja207463b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Li J, Wang Y, et al. A folate receptor-targeting nanoparticle minimizes drug resistance in a human cancer model. ACS Nano. 2011;5(8):6184–6194. doi: 10.1021/nn200739q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33(2):583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama T, Tam J, Kuroda S, et al. EGFR-targeted hybrid plasmonic magnetic nanoparticles synergistically induce autophagy and apoptosis in non-small cell lung cancer cells. PLoS One. 2011;6(11):e25507. doi: 10.1371/journal.pone.0025507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexis F, Basto P, Levy-Nissenbaum E, et al. HER-2-targeted nanoparticle–affibody bioconjugates for cancer therapy. Chem Med Chem. 2008;3(12):1839–1843. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153(1):16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 15▪.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. Comprehensive review highlighting major classes of nanoparticles that have been investigated in human clinical trials. [DOI] [PubMed] [Google Scholar]
- 16.Van Oosten M, Crane LM, Bart J, Van Leeuwen FW, Van Dam GM. Selecting potential targetable biomarkers for imaging purposes in colorectal cancer using Target Selection Criteria (TASC): a novel target identification tool. Transl Oncol. 2011;4(2):71–82. doi: 10.1593/tlo.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon DJ, Liu CT, Quinlan DS, Nafisi PM, Kamei DT. Intracellular trafficking considerations in the development of natural ligand–drug molecular conjugates for cancer. Ann Biomed Eng. 2011;39(4):1235–1251. doi: 10.1007/s10439-011-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104(39):15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 20▪.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. Excellent report on the six complexities of human tumors including proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis. [DOI] [PubMed] [Google Scholar]
- 21.Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila) 2010;3(11):1361–1364. doi: 10.1158/1940-6207.CAPR-10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheel C, Eaton EN, Li SH-J, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. Critical read to understand the challenges and opportunities in delivering drugs to solid tumors using nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta S, Eavarone D, Capila I, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436(7050):568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 25.Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102(7):1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA. 2011;108(6):2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris TJ, Von Maltzahn G, Lord ME, et al. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4(9):1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5(6):4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Byrne JD, Napier ME, Desimone JM. More effective nanomedicines through particle design. Small. 2011;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy DC, Hoop KA, Tay L-L, Pezacki JP. Development of nanoparticle probes for multiplex SERS imaging of cell surface proteins. Nanoscale. 2010;2(8):1413–1416. doi: 10.1039/c0nr00122h. [DOI] [PubMed] [Google Scholar]
- 31.Ko MH, Kim S, Kang WJ, et al. In vitro derby imaging of cancer biomarkers using quantum dots. Small. 2009;5(10):1207–1212. doi: 10.1002/smll.200801580. [DOI] [PubMed] [Google Scholar]
- 32.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 33.Thaxton CS, Elghanian R, Thomas AD, et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci USA. 2009;106(44):18437–18442. doi: 10.1073/pnas.0904719106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim Biophys Acta. 2011;1810(3):317–329. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hossann M, Wang T, Wiggenhorn M, et al. Size of thermosensitive liposomes influences content release. J Control Release. 2010;147(3):436–443. doi: 10.1016/j.jconrel.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Rhee M, Valencia PM, Rodriguez MI, Langer R, Farokhzad OC, Karnik R. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv Mater. 2011;23(12):H79–H83. doi: 10.1002/adma.201004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothstein SN, Federspiel WJ, Little SR. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials. 2009;30(8):1657–1664. doi: 10.1016/j.biomaterials.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrkach J, Von Hoff D, Ali MM, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128–139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 39.Ge J, Neofytou E, Cahill TJ, Beygui RE, Zare RN. Drug release from electric-field-responsive nanoparticles. ACS Nano. 2012;6(1):227–233. doi: 10.1021/nn203430m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44(10):947–956. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Tyner K, Sadrieh N. Considerations when submitting nanotherapeutics to FDA/CDER for regulatory review. Methods Mol Biol. 2011;697:17–31. doi: 10.1007/978-1-60327-198-1_3. Informative US FDA perspective on how current FDA guidances apply to nanoparticles and nanotherapeutics. [DOI] [PubMed] [Google Scholar]
- 42.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaitanis A, Staal S. Liposomal doxorubicin and nAb-paclitaxel: nanoparticle cancer chemotherapy in current clinical use. Methods Mol Biol. 2010;624:385–392. doi: 10.1007/978-1-60761-609-2_26. [DOI] [PubMed] [Google Scholar]
- 44.Burgess P, Hutt PB, Farokhzad OC, Langer R, Minick S, Zale S. On firm ground: IP protection of therapeutic nanoparticles. Nat Biotechnol. 2010;28(12):1267–1270. doi: 10.1038/nbt.1725. [DOI] [PubMed] [Google Scholar]
- 45.Sharp PA, Langer R. Promoting convergence in biomedical science. Science. 2011;333(6042):527. doi: 10.1126/science.1205008. [DOI] [PubMed] [Google Scholar]
- 46.Dobrovolskaia MA, Patri AK, Simak J, et al. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol Pharm. 2012;9(3):382–393. doi: 10.1021/mp200463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern ST, Hall JB, Yu LL, et al. Translational considerations for cancer nanomedicine. J Control Release. 2010;146(2):164–174. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNeil SE, editor. Characterization of Nanoparticles Intended for Drug Delivery. Humana Press Inc; NY, USA: 2011. [Google Scholar]
- 49.Roebben G, Ramirez-Garcia S, Hackley VA, et al. Interlaboratory comparison of size and surface charge measurements on nanoparticles prior to biological impact assessment. J Nanopart Res. 2011;13(7):2675–2687. [Google Scholar]
- 50.Boeckler C, Frisch B, Muller S, Schuber F. Immunogenicity of new heterobifunctional cross-linking reagents used in the conjugation of synthetic peptides to liposomes. J Immunol Methods. 1996;191(1):1–10. doi: 10.1016/0022-1759(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 51.Leonov AP, Zheng J, Clogston JD, Stern ST, Patri AK, Wei A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano. 2008;2(12):2481–2488. doi: 10.1021/nn800466c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zara J, Pomato N, McCabe RP, Bredehorst R, Vogel CW. Cobra venom factor immunoconjugates: effects of carbohydrate-directed versus amino group-directed conjugation. Bioconjug Chem. 1995;6(4):367–372. doi: 10.1021/bc00034a005. [DOI] [PubMed] [Google Scholar]
- 53.Lo ST, Stern S, Clogston JD, et al. Biological assessment of triazine dendrimer: toxicological profiles, solution behavior, biodistribution, drug release and efficacy in a PEGylated, paclitaxel construct. Mol Pharm. 2010;7(4):993–1006. doi: 10.1021/mp100104x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zolnik BS, Stern ST, Kaiser JM, et al. Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug Metab Dispos. 2008;36(8):1709–1715. doi: 10.1124/dmd.107.019679. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simanek E, Patri AK. Biodistribution of nanomedicines. Mol Pharm. 2008;5(4):473. doi: 10.1021/mp800083a. [DOI] [PubMed] [Google Scholar]
- 57.Dobrovolskaia MA, Clogston JD, Neun BW, Hall JB, Patri AK, McNeil SE. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008;8(8):2180–2187. doi: 10.1021/nl0805615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009;4(7):411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 59.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.A Study of BIND-014 Given to Patients With Advanced or Metastatic Cancer. http://clinicaltrials.gov/ct2/show/NCT01300533?term=NCT01300533&rank=1.
- 102.Safety Study of CALAA-01 to Treat Solid Tumor Cancers. http://clinicaltrials.gov/ct2/show/NCT00689065?term=NCT00689065&rank=1.
- 103.Study of MBP-426 in Patients With Second Line Gastric, Gastroesophageal, or Esophageal Adenocarcinoma. http://clinicaltrials.gov/ct2/show/NCT00964080?term=NCT00964080&rank=1.
- 104.Safety Study of Infusion of SGT-53 to Treat Solid Tumors. http://clinicaltrials.gov/ct2/show/NCT00470613?term=NCT00470613&rank=1.
- 105 ▪▪.National Cancer Institute’s Nanotechnology Characterization Laboratory. http://ncl.cancer.gov/Very informative website that has excellent resources including assay cascades and standard operating procedures for physical/chemical characterization and in vitro and in vivo testing of nanoparticles.
- 106.US FDA. Nanotechnology task force report. 2007 www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/UCM2006659.htm.
- 107▪.US FDA. Guidance for industry: considering whether an FDA-regulated product involves the application of nanotechnology. 2011 www.fda.gov/regulatoryinformation/guidances/ucm257698.htmMust-read guidance that describes the FDA’s current thinking on whether FDA-regulated products contain nanomaterials or otherwise involve the application of nanotechnology.
- 108.US FDA. Guidance for industry: liposome drug products chemistry, manufacturing, and controls; human pharmacokinetics and bioavailability; and labeling documentation. 2002 www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070570.pdf.
- 109.EvaluatePharma. Worldwide sales 2007–2009. 2011 www.evaluatepharma.com.
- 110.The Biomarkers Consortium. www.biomarkersconsortium.org/