Abstract
This report describes a patient with metastatic epithelioid hemangioendothelioma treated with bevacizumab and nanoparticle albumin-bound paclitaxel. The treatment was well tolerated and led to the stabilization of an aggressive variant of the disease. This case report is the first one that describes the activity of the combination of chemotherapy and bevacizumab in epithelioid hemangioendothelioma. Literature describing the activity of bevacizumab and other agents (thalidomide, lenalidomide, and interferon) believed to possess anti-angiogenic activities is also reviewed.
KEY WORDS: hemangioendothelioma, angiogenesis inhibitors, nab-paclitaxel, bevacizumab
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor with an incidence of less than one in a million. Considering the rarity of this disease, there is no published large trial to guide therapy. Surgical resection is generally performed for localized disease. The management of metastatic disease is difficult because it is generally resistant to most chemotherapeutic agents. Histologically, the tumor shows proliferation of epithelioid cells, which expresses vascular markers such as CD31, CD34, and Factor VIII. Consequently, interest in employing anti-angiogenic therapy for this disease is increasing. Bevacizumab, a humanized monoclonal antibody directed against vascular endothelial growth factor, shows activity in numerous solid malignancies. This case report describes a patient with metastatic thoracic EHE who was treated with bevacizumab and nanoparticle albumin-bound (nab) paclitaxel. Literature describing the activity of bevacizumab and other anti-angiogenic agents in EHE is also reviewed.
Case Report
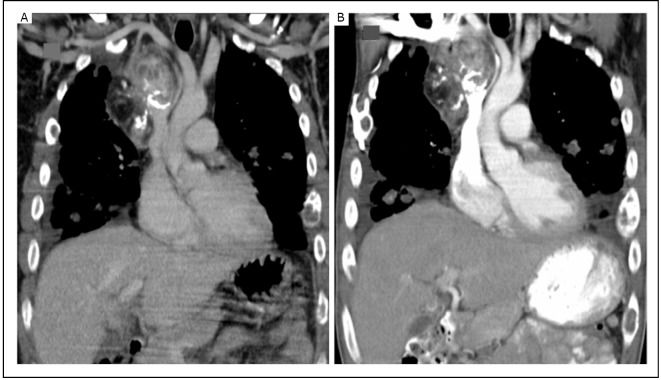
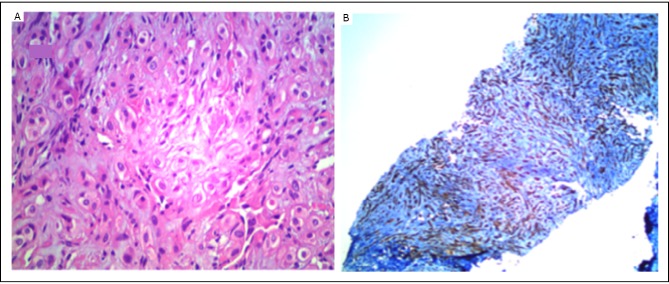
A 35-year old Hispanic male presented with a 12-month history of cough, shortness of breath, and pain involving the chest wall, lower back, and both legs. He lost 10 kg of his body weight over this time period. He did not smoke and had no other significant medical history. Physical examination was remarkable for 88% oxygen saturation in room air. He had crackles in both lung fields. Computed tomography (CT) of the thorax (Figure 1A) showed a heterogeneous mass in the superior and anterior mediastinum that involved the superior vena cava and right brachiocephalic vein. Innumerable 1-2 cm pulmonary nodules were present in both lung fields. A bone scan showed extensive metastatic disease in the calvarium, sternum, ribs, pelvis, spine, and femur. Magnetic resonance imaging of the head did not show brain involvement. CT-guided core biopsy of the mediastinal mass showed nests of rounded and slightly spindled epithelioid cells embedded in a myxoid matrix. Immunostains were positive for CD31 and CD34, consistent with EHE (Figure 2). The alpha-fetoprotein and beta-human chorionic gonadotropin levels were normal.
Figure 1.
A: CT scan (coronal view) showing a mass in the anterior and superior mediastinum containing a solid-enhancing component, coarse calcification, and fat. The mass involves the superior vena cava and right brachiocephalic vein. B: CT scan after 6 months showing stable appearance.
Figure 2.
A: Nests of rounded and slightly spindled epithelioid cells embedded in a myxoid matrix H&E (× 400). B: CD34 immunostaining (× 100). The tumor cells stained for CD34 are consistent with a vascular origin.
He received palliative radiotherapy on the sites of painful metastases in his legs and pelvis. Subsequently, he was treated with nab-paclitaxel at a dose of 100 mg/m2 on a weekly schedule and bevacizumab at a dose of 15 mg/kg every 3 weeks. In view of extensive bony metastases, he also received zoledronic acid infusions. Treatment was well tolerated and led to improvement in his pain and exercise tolerance. Interim CT scans after 3 and 6 months of therapy indicated stable disease with no new metastatic lesion (Figure 1B). Nab-paclitaxel was discontinued after 6 months to avoid cumulative neurotoxicity, but bevacizumab was continued.
Discussion
The World Health Organization categorizes EHE as a malignant vascular neoplasm [1]. However, the clinical spectrum of this disease is variable and a more accepted concept is that its malignant potential varies between those of an indolent hemangioma and an aggressive angiosarcoma[2]. Pro-angiogenic factors are believed to promote the growth of these vascular tumors [3, 4]. Given that chemotherapy is generally ineffective in EHE, angiogenesis inhibition is a reasonable approach to manage patients with metastatic EHE.
A PubMed search using the keywords “hemangioen-dothelioma” and “bevacizumab” identified a total of 6 case reports (Table 1). Five cases involved patients with thoracic primaries [5-8], and one patient had a skeletal EHE[9]. One had partial response for 13 months, 1 had stable disease, and 4 had progressive disease. Bevacizumab was believed to cause cerebral infarction and death in one patient [7]. Carboplatin and paclitaxel were the most commonly used chemotherapeutic agents with bevacizumab. We treated our patient with both nab-paclitaxel and bevacizumab. This formulation of paclitaxel has many theoretical advantages over the solvent-based formulation. It enters the tumor endothelium by glycoprotein 60-caveolin-1-mediated endocytosis. This targeting of the endothelium can lead to anti-angiogenic activity that can be further potentiated by bevacizumab [10]. The leaky endothelial junctions of the tumor allow for greater permeation of nab-paclitaxel than solvent mixed paclitaxel [11]. The drug can also be retained longer in the tumor by binding to secreted protein acid rich cysteine, which is present in tumor stroma [12]. The treatment was well tolerated and led to disease stabilization without significant toxicity.
Table 1. Bevacizumab in epithelioid hemangioendothelioma.
| Author | Reference | Age (years) |
Gender (M/F) |
Dose and schedule | Chemotherapy | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| Belmont et al. | 5 | 41 | M | 15 mg/kg, every 21 days | Carboplatin, paclitaxel, docetaxel | Partial response | 13 months |
| Kim YH et al. | 6 | 44 | F | 15 mg/kg, every 21 days | Carboplatin,paclitaxel | Progression | |
| Mizota et al. | 7 | 59 | F | 15 mg/kg, every 21 days | Carboplatin, paclitaxel | Progression | 3 months |
| Lazarus et al. | 8 | 42 | M | ? | Paclitaxel | Progression | 1-2 months |
| Lazarus et al. | 8 | 42 | M | ? | Carboplatin, etoposide | Progression | 2-3 months |
| Trautmann et al. | 9 | 19 | F | 7.5 mg/m2, every 21 days | None | Stable disease | 16 months |
Although the mechanism of action of thalidomide and its analog, lenalidomide, are not fully understood, they are believed to have immunomodulatory and anti-angiogenic properties. A PubMed search using “thalidomide” and “hemangioenothelioma” identified 5 case reports, whereas “lenalidomide” and “hemangioendothelioma” resulted in 1 case report (Table 2). Among them, 6 had hepatic primary and 1 had multifocal skin and central nervous system involvement [13-18]. None had thoracic primary involvement. Two cases had partial responses, which lasted up to 9 years in 1 case. Another two patients had stable disease lasting up to 7 years. The dose of thalidomide varied from 300 mg daily to 400 mg twice a day. At these doses, thalidomide can induce prohibitive toxicity and cannot be recommended for all patients. Lenalidomide is a better tolerated alternative that warrants further exploration. The use of immunomodulation in pulmonary EHE is also worthy of further study.
Table 2. Thalidomide and lenalidomide (IMID’s) in epithelioid hemangioendothelioma.
| Author | Reference | Age (years) | Gender (M/F) | IMID | Dose and schedule | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| Salech F el al. | 13 | 40 | F | Thalidomide | 300mg daily | Partial response | 109 months |
| Raphael el al. | 14 | 53 | F | Thalidomide | 400 mg daily | Stable disease | 84 months |
| Kassam el al. | 15 | 13 | F | Thalidomide | 400 mg twice a day | Progressive disease | _ |
| Bolke et al. | 16 | 47 | M | Thalidomide | ? | Progressive disease/death | -- |
| Mascarenhas el al. | 17 | 52 | M | Thalidomide | ? | Partial response | -- |
| Sumrall et al. | 18 | 31 | F | Lenalidomide | 25 mg daily for 21/28 days |
Stable disease | 48 months |
Interferon alpha, which has anti-angiogenic properties, is also used in EHE. Table 3 summarizes the literature on the use of interferon over the last 10 years [19-25]. We excluded case reports describing adjuvant use of interferon after surgical resection because the efficacy of the drug in these case reports cannot be evaluated. Remarkably, 1 patient with EHE of breast metastasis to the lungs had a complete clinical response to interferon. She was treated with bilateral mastectomies and subsequently given interferon. The authors described a complete clinical response that lasted for 7 years[23]. Reduced metastatic lesion sizes after primary tumor removal has been described in multiple malignancies. In patients with metastatic EHE who have an easily resectable primary tumor, this approach may be reasonable.
Table 3. Interferon in epithelioid hemangioendothelioma.
| Author | Reference | Primary site | Interferon | Dose and schedule | Outcome | Follow-up |
|---|---|---|---|---|---|---|
| Radzikowaska et al. | 19 | Thoracic | Alpha-2a | 3 million units, 3 times a week |
Stable disease | 3 months |
| Saleiro et al. | 20 | Thoracic | Alpha 2b | - | Progressive disease | 9 months |
| Calabro et al. | 21 | Thoracic | Alpha 2a | - | Stable disease | - |
| Hassan et al. | 22 | Thyroid | Alpha | 3 million units, 5 times a week |
Progressive disease | 2 months |
| Marsh et al. | 23 | Breast | Alpha | 3 millon units, 5 days a week for 1 year |
Complete response | 84 months |
| Al-Sharim et al. | 24 | Thoracic | Alpha | 7 million units, 3 times a week. |
Progressive disease | 2 months |
| Kayler et al. | 25 | Hepatic | Alpha-2b | 3 million units daily | Partial response | 4 months |
A better understanding of the pathogenesis of these neoplasms and large clinical trials are needed to develop enhanced therapeutic approaches. Recently, chromosomal translocation involving t(1;3)(p36;q25) leading to a fusion gene WWTR1/CAMTA1 was described in virtually all patients with EHE [26]. Nevertheless, anti-angiogenesis therapy presently remains a valid approach for managing EHE.
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.Fletcher C, Unni K, Mertens F. World health organization classification of tumors. Pathology and Genetics. Tumors of soft tissue and bone. Lyon; IARC press; 2002. [Google Scholar]
- 2.Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: a study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epitheliod hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol 2003; 34: 680-689 [DOI] [PubMed] [Google Scholar]
- 3.Koch M, Nielsen GP, Yoon SS. Malignant tumors of blood vessels: Angiosarcomas, hemangioendotheliomas, and hemangiopericytomas. J Surg Oncol 2008; 97: 450-456 [DOI] [PubMed] [Google Scholar]
- 4.Emamaullee JA, Edgar R, Toso C, et al. Vascular endothelial growth factor expression in hepatic epithelioid hemangioendothelioma: Implications for treatment and surgical management. Liver Transpl 2010; 16: 191-197 [DOI] [PubMed] [Google Scholar]
- 5.Belmont L, Zemoura L, Couderc LJ. Pulmonary epithelioid haemangioendothelioma and bevacizumab. J Thorac Oncol 2008; 3: 557-558 [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Mishima M, Miyagawa-Hayashino A. Treatment of pulmonary epithelioid hemangioendothelioma with bevacizumab. J Thorac Oncol 2010; 5: 1107-1108 [DOI] [PubMed] [Google Scholar]
- 7.Mizota A, Shitara K, Fukui T.Bevacizumab chemotherapy for pulmonary epithelioid hemangioendothelioma with severe dyspnea. J Thorac Oncol 2011; 6: 651-652 [DOI] [PubMed] [Google Scholar]
- 8.Trautmann K, Bethke A, Ehninger G, et al. Bevacizumab for recurrent hemangioendothelioma. Acta Oncol 2011; 50:153-154 [DOI] [PubMed] [Google Scholar]
- 9.Lazarus A, Fuhrer G, Malekiani C, et al. Primary pleural epithelioid hemangioendothelioma (EHE)--two cases and review of the literature. Clin Respir J 2011; 5: e1-5 [DOI] [PubMed] [Google Scholar]
- 10.Volk LD, Flister MJ, Bivens CM. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor therapy. Neoplasia 2008; 10: 613-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin cancer res 2006; 12:1317–1324 [DOI] [PubMed] [Google Scholar]
- 12.Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment- where there’s SPARC, there’s fire. J Cell Biochem 2008; 104: 721-732 [DOI] [PubMed] [Google Scholar]
- 13.Salech F, Valderrama S, Nervi B, et al. Thalidomide for the treatment of metastatic hepatic epithelioid hemangioendothelioma: a case report with a long term follow-up. Ann Hepatol 2011; 10: 99-102 [PubMed] [Google Scholar]
- 14.Raphael C, Hudson E, Williams L, et al. Successful treatment of metastatic hepatic epithelioid hemangioendothelioma with thalidomide: a case report. J Med Case Rep 2010; 4: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassam A, Mandel K.Metastatic hepatic epithelioid hemangioendothelioma in a teenage girl. J Pediatr Hematol Oncol 2008; 30: 550-552 [DOI] [PubMed] [Google Scholar]
- 16.Bölke E, Gripp S, Peiper M, et al. Multifocal epithelioid hemangioendothelioma: case report of a clinical chamaeleon. Eur J Med Res 2006; 11: 462-466 [PubMed] [Google Scholar]
- 17.Mascarenhas RC, Sanghvi AN, Friedlander L, et al. Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology 2004; 67: 471-475 [DOI] [PubMed] [Google Scholar]
- 18.Sumrall A, Fredericks R, Berthold A, et al. Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. J Neurooncol 2010; 97: 275-277 [DOI] [PubMed] [Google Scholar]
- 19.Radzikowska E, Szczepulska-Wójcik E, Chabowski M, et al. Pulmonary epithelioid haemangioendothelioma--interferon 2-lpha treatment--case report. Pneumonol Alergol Pol. 2008; 76: 281-285 [PubMed] [Google Scholar]
- 20.Saleiro S, Barbosa M, Souto Moura C, Almeida J, Ferreira S.Epithelioid hemangioendothelioma - a rare pulmonary tumor. Rev Port Pneumol. 2008; 14: 421-425 [PubMed] [Google Scholar]
- 21.Calabrò L, Di Giacomo AM, Altomonte M, et al. Primary hepatic epithelioid hemangioendothelioma progressively responsive to interferon-alpha: is there room for novel anti-angiogenetic treatments? J Exp Clin Cancer Res. 2007; 26: 145-150 [PubMed] [Google Scholar]
- 22.Hassan I, Barth P, Celik I, et al. An authentic malignant epithelioid hemangioendothelioma of the thyroid: a case report and review of the literature. Thyroid 2005; 15: 1377-1381 [DOI] [PubMed] [Google Scholar]
- 23.Marsh RW, Walker MH, Jacob G, et al. Breast implants as a possible etiology of epithelioid hemangioendothelioma and successful therapy with interferon-alpha2. Breast J 2005; 11: 257-261 [DOI] [PubMed] [Google Scholar]
- 24.Al-Shraim M, Mahboub B, Neligan PC, et al. Primary pleural epithelioid haemangioendothelioma with metastases to the skin. A case report and literature review. J Clin Pathol 2005; 58:107-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayler LK, Merion RM, Arenas JD, et al. Epithelioid hemangioendothelioma of the liver disseminated to the peritoneum treated with liver transplantation and interferon alpha-2B. Transplantation 2002; 74: 128-130 [DOI] [PubMed] [Google Scholar]
- 26.Tanas MR, Sboner A, Oliveira AM, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med 2011; 3: 98ra82. [DOI] [PubMed] [Google Scholar]