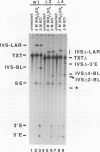
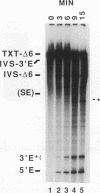
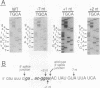
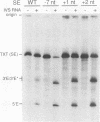
Abstract
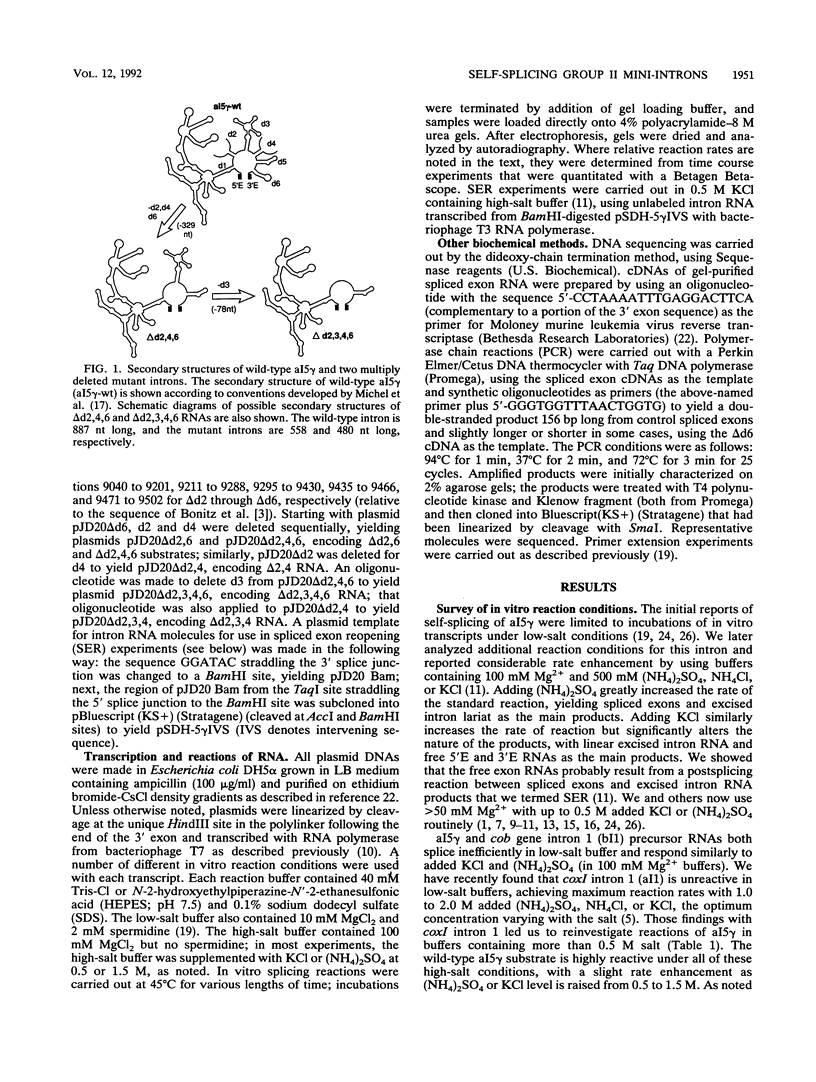
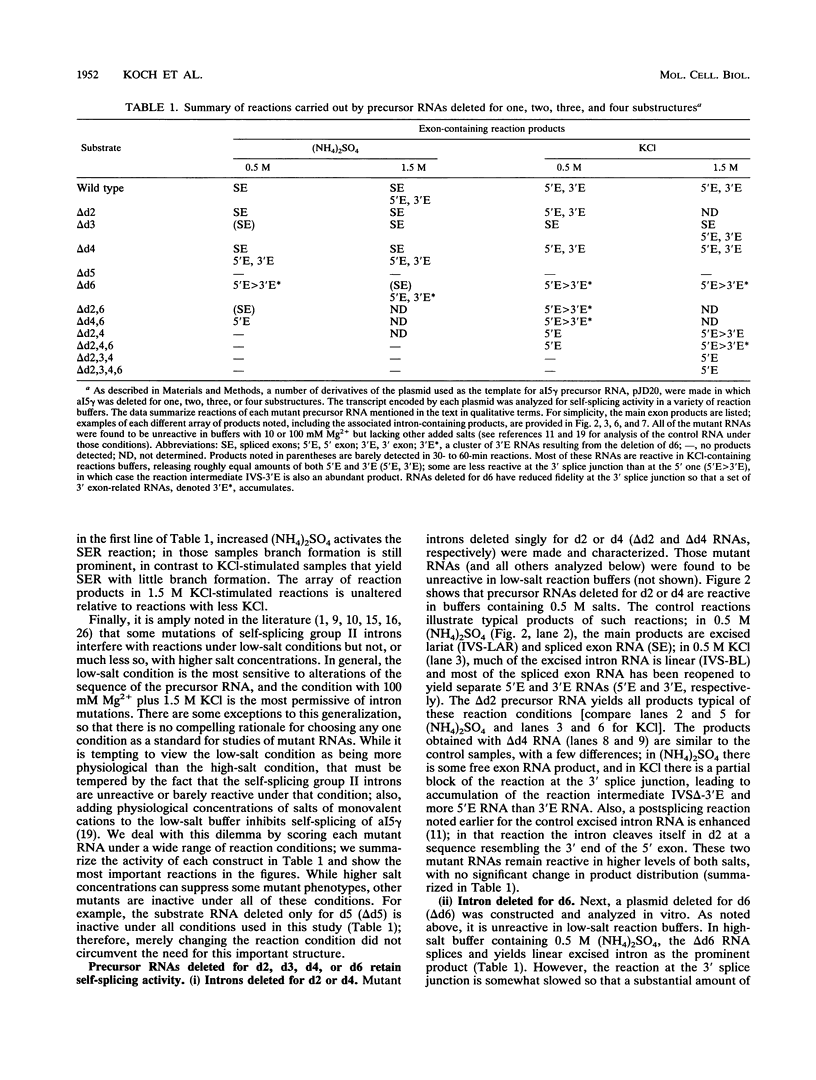
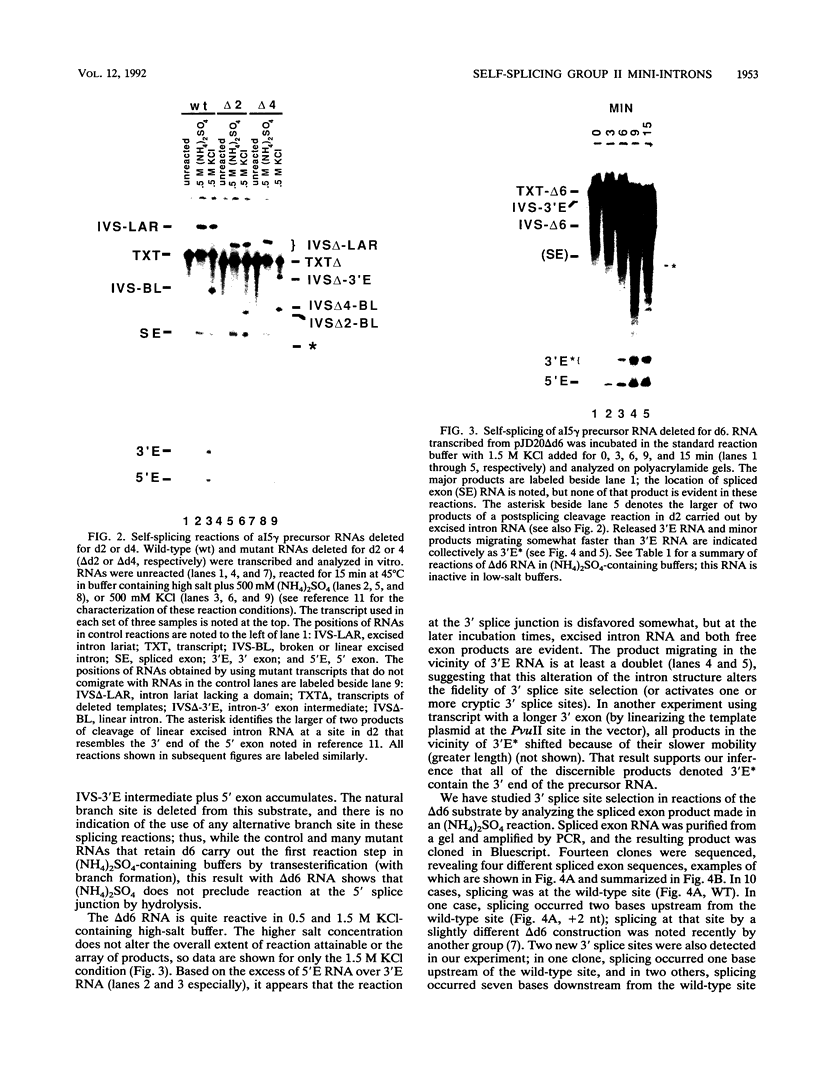
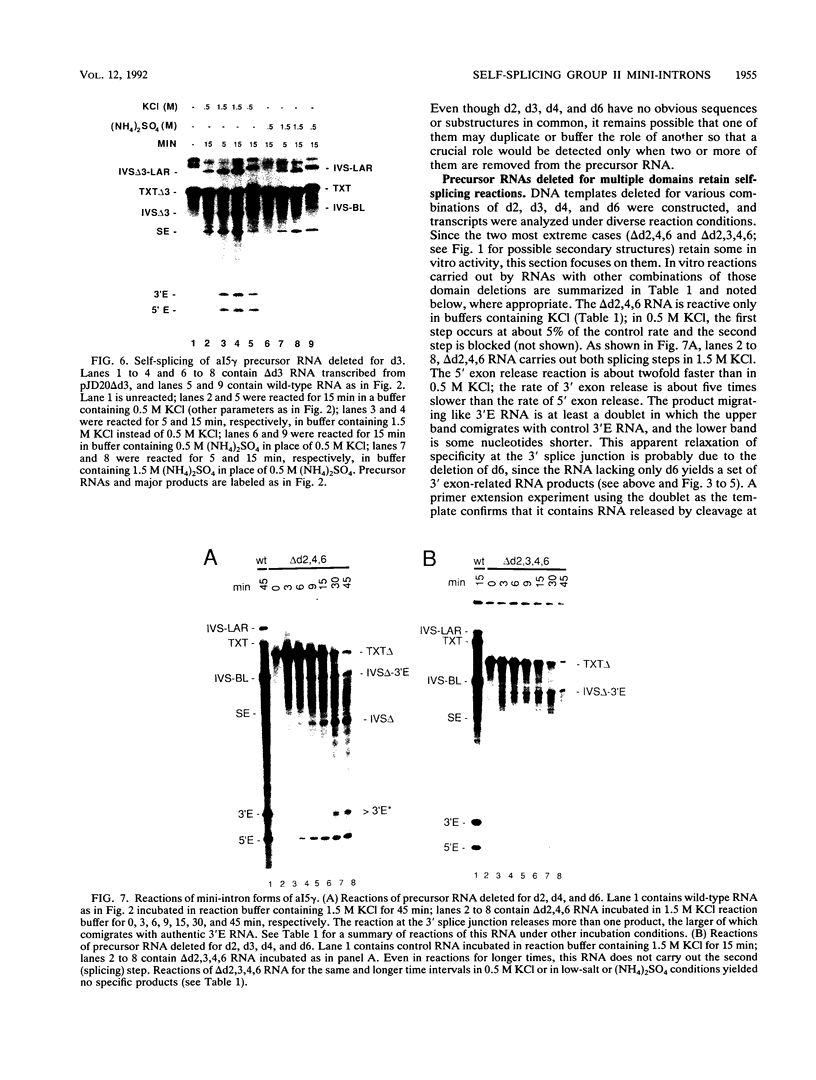
Group II introns can be folded into highly conserved secondary structures with six major substructures or domains. Domains 1 and 5 are known to play key roles in self-splicing, while the roles of domains 2, 3, 4, and 6 are less clear. A trans assay for domain 5 function has been developed which indicates that domain 5 has a binding site on the precursor RNA that is not predicted from any secondary structure element. In this study, the self-splicing group II intron 5 gamma of the coxI gene of yeast mitochondrial DNA was deleted for various intron domains, singly and in combinations. Those mutant introns were characterized for self-splicing reactions in vitro as a means of locating the domain 5 binding site. A single deletion of domain 2, 3, 4, or 6 does not block in vitro reactions at either splice junction, though the deletion of domain 6 reduces the fidelity of 3' splice site selection somewhat. Even the triple deletion lacking domains 2, 4, and 6 retains some self-splicing activity. The deletion of domains 2, 3, 4, and 6 blocks the reaction at the 3' splice junction but not at the 5' junction. From these results, we conclude that the binding site for domain 5 is within domain 1 and that the complex of 5' exon, domain 1, and domain 5 (plus short connecting sequences) constitutes the essential catalytic core of this intron.
Full text
PDF




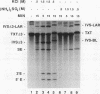
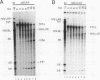
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachl J., Schmelzer C. Effect of deletions at structural domains of group II intron bI1 on self-splicing in vitro. J Mol Biol. 1990 Mar 5;212(1):113–125. doi: 10.1016/0022-2836(90)90308-9. [DOI] [PubMed] [Google Scholar]
- Beaudry A. A., Joyce G. F. Minimum secondary structure requirements for catalytic activity of a self-splicing group I intron. Biochemistry. 1990 Jul 10;29(27):6534–6539. doi: 10.1021/bi00479a027. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Doudna J. A., Szostak J. W. Miniribozymes, small derivatives of the sunY intron, are catalytically active. Mol Cell Biol. 1989 Dec;9(12):5480–5483. doi: 10.1128/mcb.9.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A., Jacquesson-Breuleux N. Splice site selection and role of the lariat in a group II intron. J Mol Biol. 1991 Jun 5;219(3):415–428. doi: 10.1016/0022-2836(91)90183-7. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Base-pairing interactions involving the 5' and 3'-terminal nucleotides of group II self-splicing introns. J Mol Biol. 1990 Jun 5;213(3):437–447. doi: 10.1016/S0022-2836(05)80206-2. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jacquier A. Self-splicing group II and nuclear pre-mRNA introns: how similar are they? Trends Biochem Sci. 1990 Sep;15(9):351–354. doi: 10.1016/0968-0004(90)90075-m. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. A., Peebles C. L., Dietrich R. C., Romiti S. L., Perlman P. S. Group II intron self-splicing. Alternative reaction conditions yield novel products. J Biol Chem. 1988 Mar 5;263(7):3432–3439. [PubMed] [Google Scholar]
- Joyce G. F., van der Horst G., Inoue T. Catalytic activity is retained in the Tetrahymena group I intron despite removal of the large extension of element P5. Nucleic Acids Res. 1989 Oct 11;17(19):7879–7889. doi: 10.1093/nar/17.19.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakman J. H., Konings D., Pel H. J., Grivell L. A. Structure-function relationships in a self-splicing group II intron: a large part of domain II of the mitochondrial intron aI5 is not essential for self-splicing. Nucleic Acids Res. 1989 Jun 12;17(11):4205–4216. doi: 10.1093/nar/17.11.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U., Godehardt I., Schmidt U. A self-splicing group II intron in the mitochondrial large subunit rRNA (LSUrRNA) gene of the eukaryotic alga Scenedesmus obliquus. Nucleic Acids Res. 1990 May 11;18(9):2691–2697. doi: 10.1093/nar/18.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Müller M. W., Schweyen R. J., Schmelzer C. Selection of cryptic 5' splice sites by group II intron RNAs in vitro. Nucleic Acids Res. 1988 Aug 11;16(15):7383–7395. doi: 10.1093/nar/16.15.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Müller M. W. Self-splicing of group II introns in vitro: lariat formation and 3' splice site selection in mutant RNAs. Cell. 1987 Dec 4;51(5):753–762. doi: 10.1016/0092-8674(87)90098-5. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Schweyen R. J. Self-splicing of group II introns in vitro: mapping of the branch point and mutational inhibition of lariat formation. Cell. 1986 Aug 15;46(4):557–565. doi: 10.1016/0092-8674(86)90881-0. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Riederer B., Mörl M., Schmelzer C., Stahl U. Self-splicing of the mobile group II intron of the filamentous fungus Podospora anserina (COI I1) in vitro. EMBO J. 1990 Jul;9(7):2289–2298. doi: 10.1002/j.1460-2075.1990.tb07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallasch C., Mörl M., Niemer I., Schmelzer C. Structural requirements for selection of 5'- and 3' splice sites of group II introns. Nucleic Acids Res. 1991 Jun 25;19(12):3307–3314. doi: 10.1093/nar/19.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh D. S., Green C. J., Pace N. R. The design and catalytic properties of a simplified ribonuclease P RNA. Science. 1989 Jun 30;244(4912):1569–1571. doi: 10.1126/science.2472671. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., Grivell L. A. Self-splicing of a group II intron in yeast mitochondria: dependence on 5' exon sequences. EMBO J. 1987 Apr;6(4):1079–1084. doi: 10.1002/j.1460-2075.1987.tb04861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]