Introduction
Radiographically, malignant pleural mesothelioma (MPM) is difficult to measure given the non-radial and inconsistent pattern of growth and response to treatment. Clinical trials that utilize primary endpoints dependent on radiographic imaging, such as progression-free survival and response rate, may be compromised by inaccuracy of tumor measurements, inconsistency in measurement technique between clinicians, and a lack of experience in measuring pleural tumors, given the relative rarity of MPM. Currently, the measurement methods for MPM are either the RECIST1,2 or the newer modified RECIST criteria3. Modified RECIST was developed based upon speculation that RECIST did not address the unique shape of mesothelioma tumors with the pleural rind and thus led to inaccurate measurements.4–6
The summarized definitions of the two methods are below:
RECIST1,2
A measurable target lesion must be 2 cm or more when using cross-sectional imaging with a non-spiral CT scan or MRI. If using a spiral CT scan, the measurements must be greater than 1 cm. Measure the long axis of the tumor to achieve the longest uni-dimensional measurement. With the updated RECIST criteria (version 1.1)2, the number of target lesions required to assess clinical response has been reduced to a maximum of five measurements. Each involved organ site that has a measurable target lesion should be represented in this measurement. However, only 2 maximum measurements per organ are allowed – therefore, only two pleural tumor uni-dimensional measurements for mesothelioma are required.
Tip for RECIST v1.1 reporting on pleural rind-like masses
Measure the longest diameter of the tumor along the chest wall.
Modified RECIST3
Uni-dimensional measurements of tumor thickness perpendicular to the chest wall or mediastinum should be performed, measured in 2 sites at 3 different levels on CT scan. Transverse cuts used for measurement must be at least 1 cm apart, and related to anatomical landmarks in the thorax, preferably above the level of division of the main bronchi. At reassessment, pleural thickness must be measured at the same position and level. Nodal, subcutaneous, and other bi-dimensionally measurable lesions are measured uni-dimensionally as per the RECIST2 criteria. Uni-dimensional measurements are added to produce the total tumor measurement.
The sum of 6 pleural thickness measurements = one univariate diameter
Although both methods define distinct rules of MPM measurements, these measurement techniques have been applied in a highly variable fashion by medical oncologists and radiologists, and obtaining accurate tumor measurements remains a dilemma for mesothelioma multi-center clinical trials. Ultimately, the future of mesothelioma clinical research may be dependent on volumetric measurements or FDG-PET CT scans; these systems provide numerical values which should minimize inter-observer variability and simplify the technique. To date, these volumetric methods are not validated and require further study before their implementation in large scale clinical trials.7–9 Educating clinicians in the proper application of the RECIST and modified RECIST systems and standardization of the manner in which the measurements are made is essential. There is no practical guideline to assist in the radiological assessment of pleural mesothelioma. We will attempt to provide detailed practical instructions on how to measure MPM by the updated RECIST version 1.1 and modified RECIST guidelines detailed above.
How to make measurements of malignant pleural mesothelioma by modified RECIST
Tips:
Larger lesions are easier and more accurate to measure. Choose the larger or thickest of the pleural disease to measure. Magnifying the image may enable an easier measurement.
The short-axis diameter is defined as the shortest pathway from the tip of the tumor to the chest wall (see Figure 1).
Ensure that differentiation of normal chest wall from tumor is easy at the point of measurement (see Figure 2).
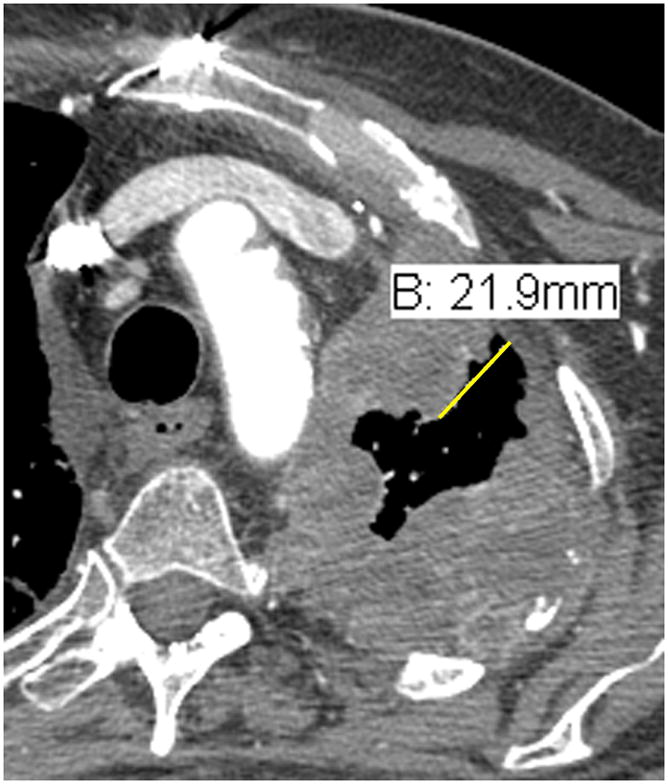
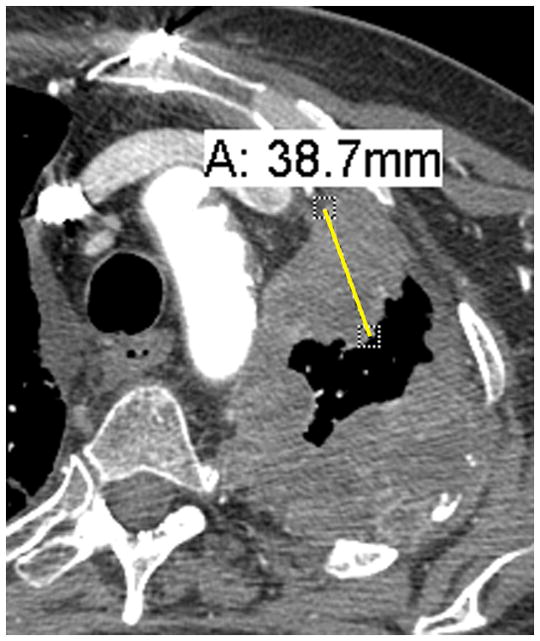
Figure 1. Example of well formulated and erroneous measurements using modified RECIST.
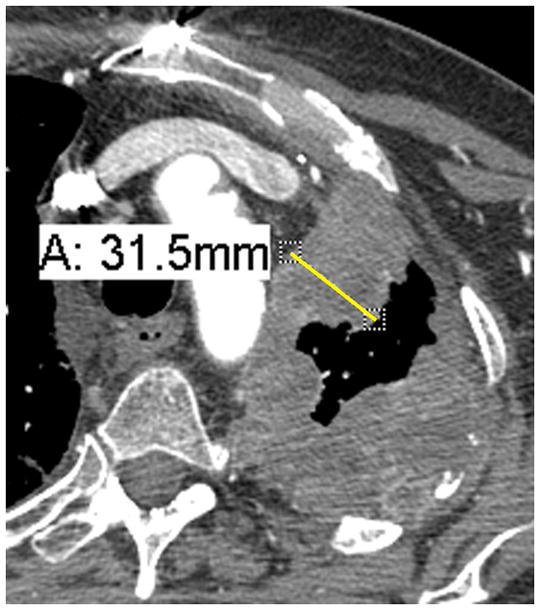
A. Good measurement: The pleural disease has a short-axis measurement of 3.2 cm. The short-axis is always measured perpendicular, or 90 degrees, to the chest wall or mediastinum and is the measurement between the point the pleural disease touches the chest wall/mediastinum and the point where the pleural disease touches normal lung.
B. Malpositioned caliper: Although this measurement takes a short path to the chest wall, perpendicular to the chest wall, it does not pass entirely through the tumor before meeting the chest wall and is therefore an erroneous measurement.
C. Malpositioned caliper: Although the measurement goes through tumor tissue, the path is not the shortest going to the chest wall, i.e. perpendicular. It forms an acute angle to the point where the pleural disease interfaces with the mediastinum and is therefore incorrect.
Figure 2. Examples of pleural disease interfaces with the chest wall or mediastinum.
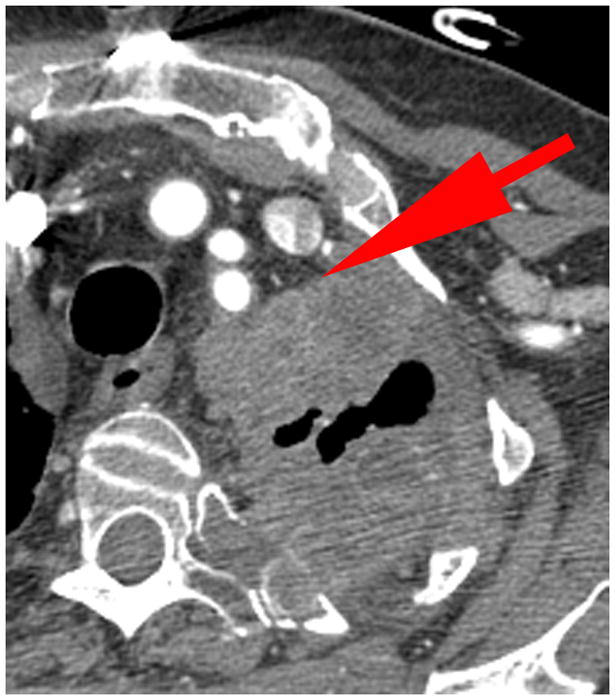
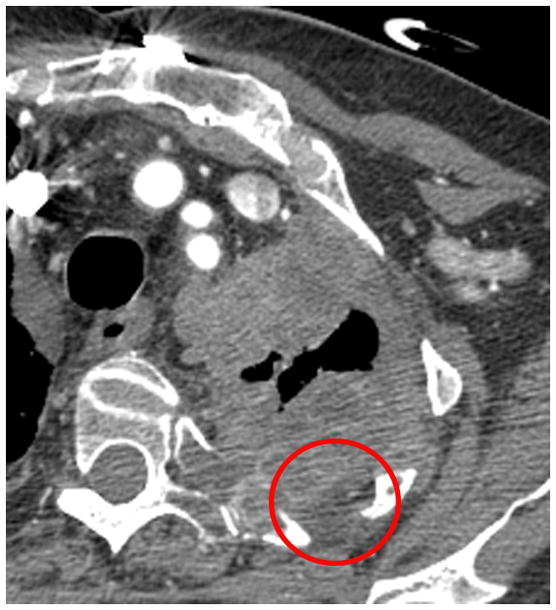
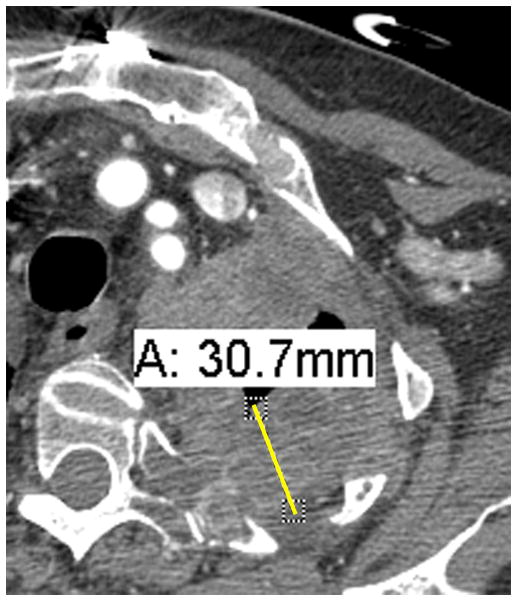
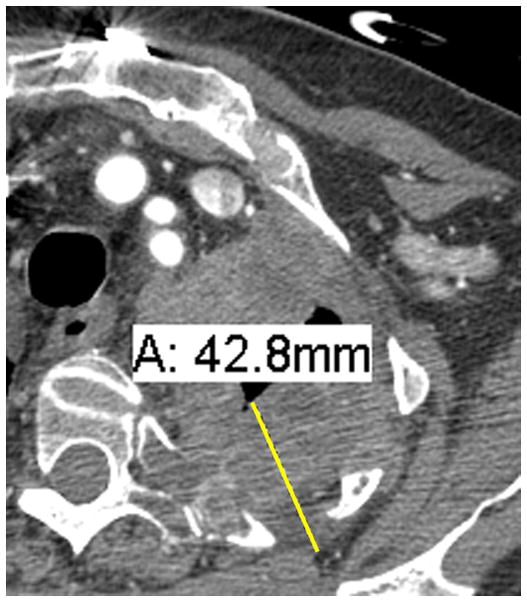
A. Example of a good interface to measure. There is a clear, well marginated differentiation between the pleural tumor and the normal mediastinal fat (arrow).
B. Example of a poor interface to measure. The border of the pleural tumor compared to normal tissues is not clear or distinct (circle).
C. If choosing to measure the interface seen in Figure 2B, measurements may erroneously underestimate tumor burden.
D. If choosing to measure the interface seen in Figure 2B, measurements may erroneously overestimate tumor burden.
1) To ensure accuracy, all measurements must be performed electronically in the same window setting on consecutive studies. We recommend using the “soft tissue” setting (not lung windows) as it enables accurate pleural mass measurements with the electronic calipers and avoids the incorporation of chest wall or mediastinal fat within the calculations, which are not as easily identified when using lung windows.
2) CT scan slices up to 5 mm can be used, but for greater accuracy, thinner slices such as 2.5 mm are preferable. Ideally, all subsequent CT scans should utilize the same slice thickness.
3) To limit inter-observer variability in measuring tumor response, it is preferred that the same clinician measure the tumors at baseline and on all subsequent CT scans.
4) The pleural disease to be measured should have a short-axis diameter of at least 1 cm, as lesions < 1 cm are considered non-measurable.
5) To obtain the short-axis of the disease, with electronic calipers, measure the distance between the point where the tumor abuts the chest or the mediastinal border and the point where the pleural disease touches normal lung (see Figure 1A). The shortest route possible is the one perpendicular to the chest wall/mediastinum.
6) Avoid measuring regions where tumor infiltration obscures the interface of tumor to normal tissue. A good interface for measurement is usually one where the tumor abuts fat or an intact rib/vertebral body, as the density of the pleural tumor differs significantly from bone or fat and the difference can be easily observed. Avoid choosing an interface of tumor with muscle as muscle and tumor have similar CT densities and are often difficult to distinguish when they abut one another (see Figure 1).
7) Following the instructions in #5, choose three different axial slices, preferably above the level of the main bronchi and record two measurements per slice, thus resulting in 6 separate measurements (see Figure 3).
Figure 3.
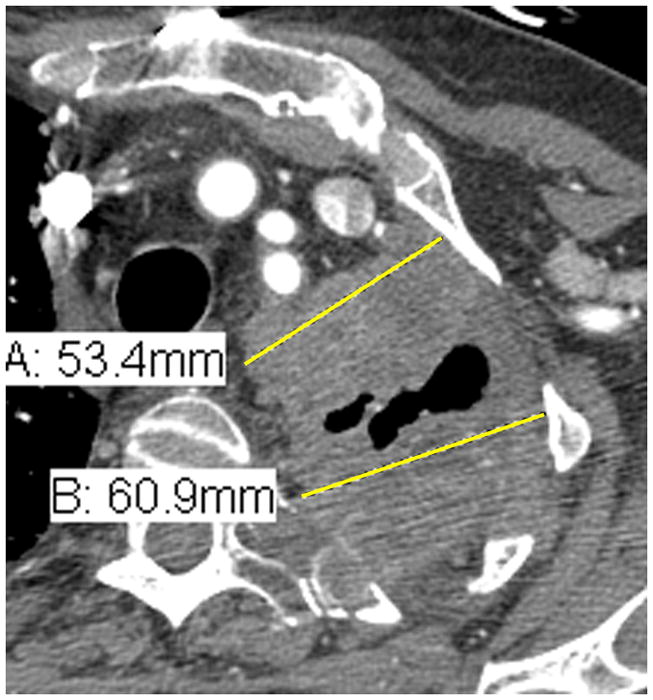
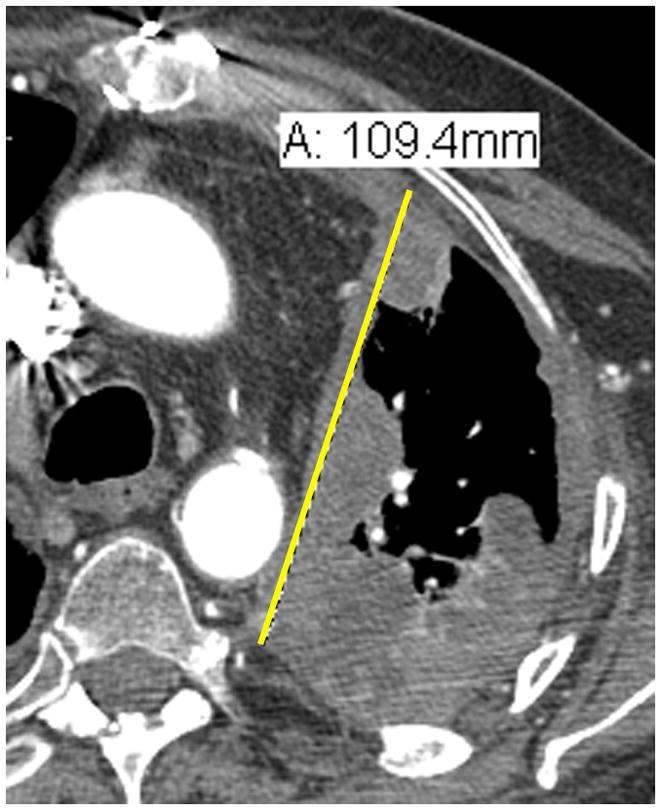
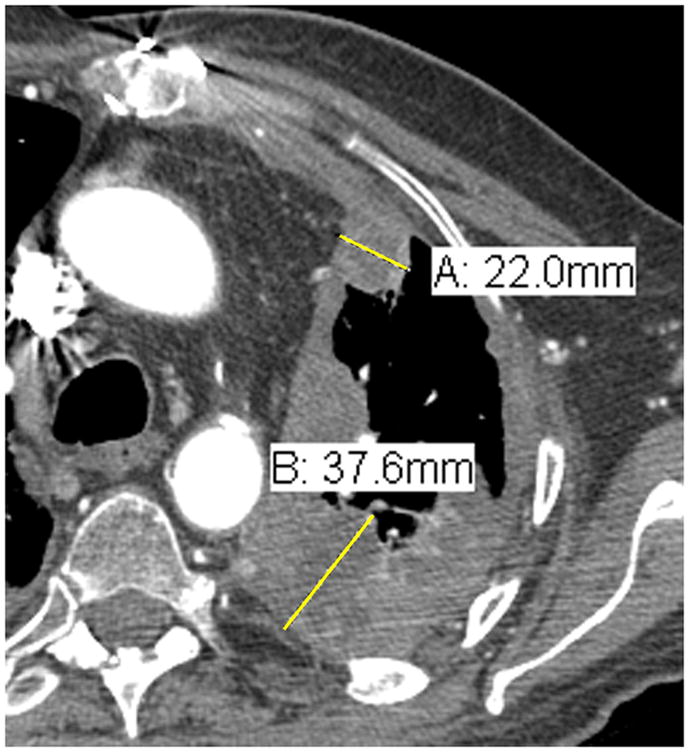
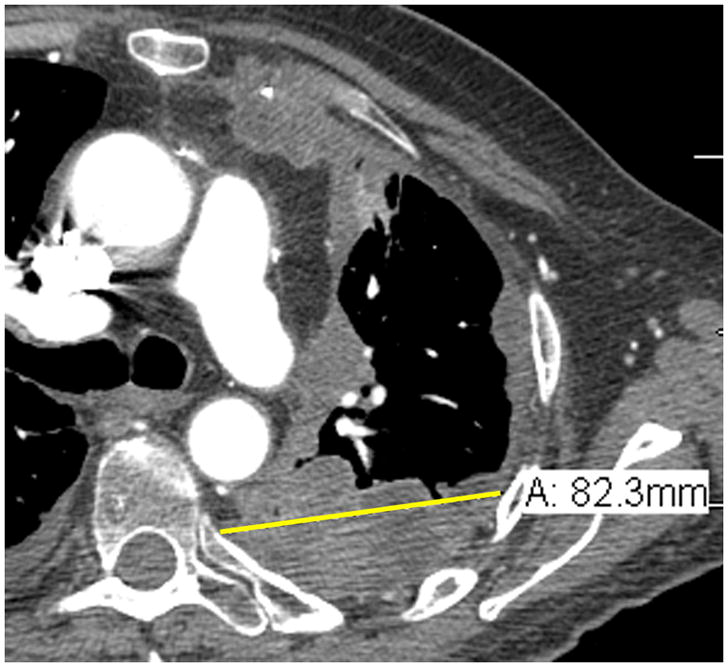
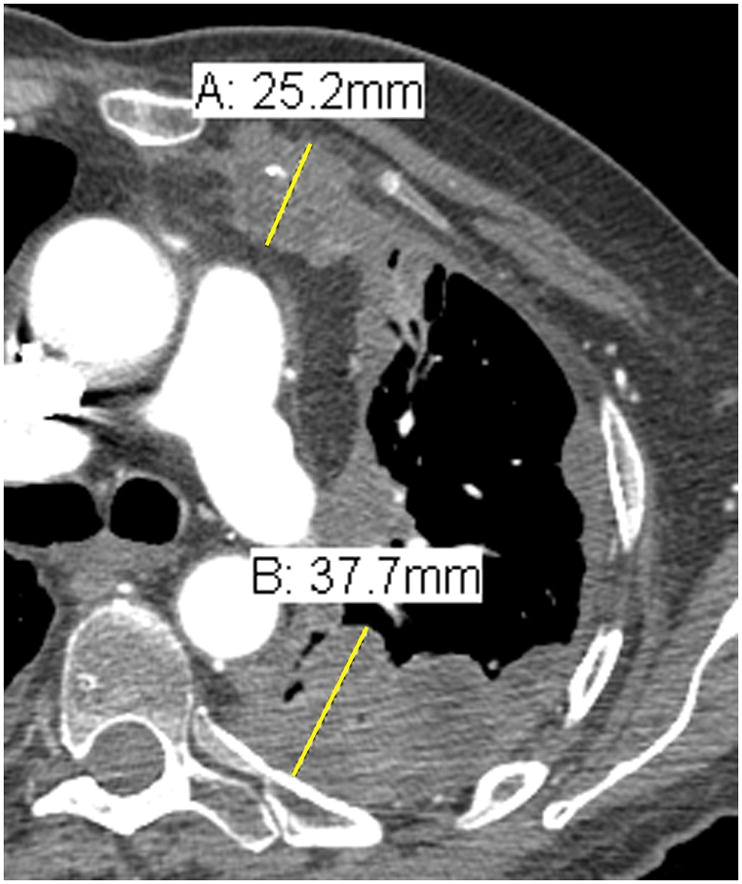
Comparison of tumor measurements using the updated RECIST criteria version 1.1 (i, iii, v) and the modified RECIST criteria (ii, iv, vi).
| RECIST v1.1 | modified RECIST |
|---|---|
| i. | ii. |
| A: 5.3 cm | A: 3.2 cm |
| B: 6.1 cm | B: 3.3 cm |
| iii. | iv. |
| A: 10.9 cm | A: 2.2 cm |
| B: 3.8 cm | |
| v. | vi. |
| A: 8.2 cm | A: 2.5 cm |
| B: 3.8 cm |
RECIST method*: Total measurement of the two longest diameters in the pleura = 10.9 + 8.2 = 19.1 cm
Modified RECIST: Total measurement = 3.2 + 3.3 + 2.2 + 3.8 + 2.5 + 3.8 = 18.8 cm
*Although additional attempted measurements (Figure 3i.) were made of the pleural tumor in the patient example for RECIST measurements, by RECIST version 1.1, only the 2 longest measurements of the pleural tumor are used in calculating the total RECIST measurement.
8) If any additional nodal, subcutaneous or other bi-dimensionally measureable lesions are available, they should undergo uni-dimensional measurements by RECIST version 1.1 and be added to the total obtained from #7.
Tips on lymph node measurements
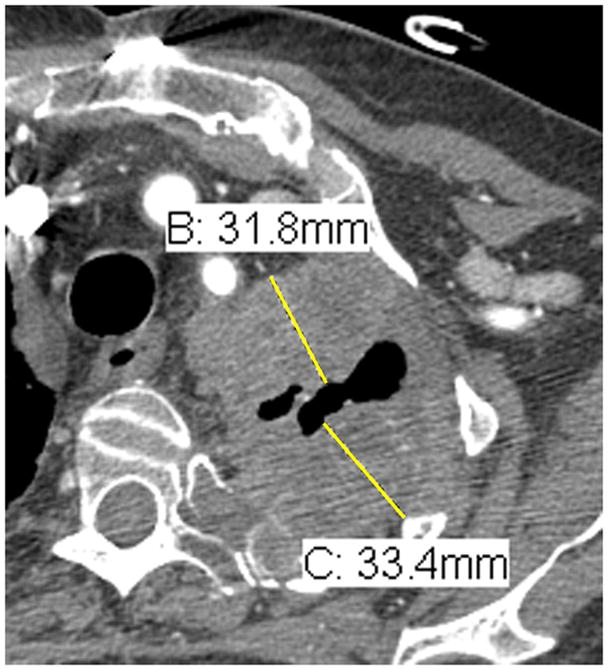
Lymph nodes are considered a separate organ to measure and up to 2 lymph nodes can be measured per patient by RECIST version 1.1.2 For uni-dimensional lymph node measurements10, use the short-axis of the lymph node at baseline and then at every follow-up scan (see Figure 4). To be considered as pathologically enlarged and measurable at baseline, the lymph node short-axis diameter should be ≥ 15 mm.
Figure 4.
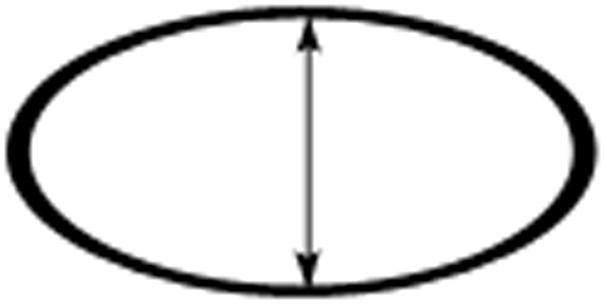
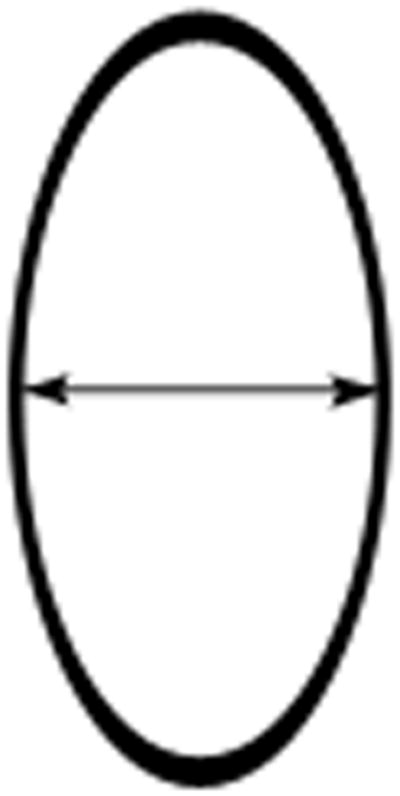
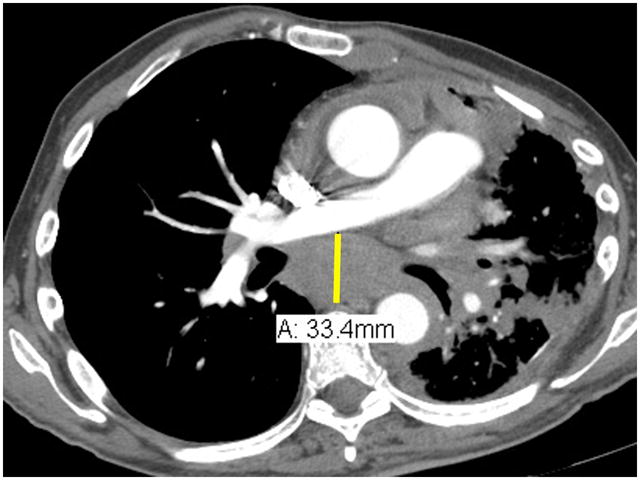
Lymph node measurements. Schematic representation of a horizontally oriented (A) or vertically oriented (B) lymph node as well as an enlarged subcarinal lymph node on contrast enhanced chest CT at the level of the right pulmonary artery (C). Arrow denotes the short-axis of the lymph nodes in figures 4A and 4B, and in the caliper measurements of the short-axis of the 33.4 mm subcarinal lymph node seen in 4C. As an example, the measurements of the lymph node in 4C would be added to the pleural measurements obtained in Figure 3.
RECIST method v1.1:
Total measurement = Pleura (10.9 + 8.2 = 19.1 cm) + lymph node (3.3 cm) = 22.4 cm
Modified RECIST:
Total measurement = Pleura (3.2 + 3.3 + 2.2 + 3.8 + 2.5 + 3.8 = 18.8 cm) + lymph node (3.3 cm) = 22.1 cm
Acknowledgments
This Southwest Oncology Group investigation supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA105409, CA13612, CA46368, CA46441
References
- 1.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–60. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 4.van Klaveren RJ, Aerts JG, de Bruin H, et al. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;43:63–9. doi: 10.1016/s0169-5002(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 5.Nowak AK. CT, RECIST, and malignant pleural mesothelioma. Lung Cancer. 2005;49 (Suppl 1):S37–40. doi: 10.1016/j.lungcan.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol. 2008;18:1635–43. doi: 10.1007/s00330-008-0918-9. [DOI] [PubMed] [Google Scholar]
- 7.Ak G, Metintas M, Metintas S, et al. Three-dimensional evaluation of chemotherapy response in malignant pleural mesothelioma. Eur J Radiol. 74:130–5. doi: 10.1016/j.ejrad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Ceresoli GL, Chiti A, Zucali PA, et al. Assessment of tumor response in malignant pleural mesothelioma. Cancer Treat Rev. 2007;33:533–41. doi: 10.1016/j.ctrv.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Zhao B, Krug LM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol. 5:879–84. doi: 10.1097/JTO.0b013e3181dd0ef1. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]


