Introduction
Sarcomatoid non-small cell lung cancer (NSCLC) is a rare tumor variant that comprises ~1.5% of all lung cancers.[1] Due to their rare incidence, these tumors are often called by several names such as spindle cell carcinoma, carcinosarcoma, giant cell carcinoma, or sarcomatoid carcinoma. Sarcomatoid NSCLCs are histologically heterogeneous and have varying levels of epithelial and mesenchymal features. The most common definition of sarcomatoid NSCLC consists of the tumor containing at least 10% sarcomatoid features based on immunohistochemical markers and histologic appearance. Although rare, sarcomatoid NSCLC have been reported to have more aggressive clinical phenotypes with higher recurrence rates and subsequently worse prognoses.[2] This aggressive nature is speculated in part to potentially be due to augmented angiogenesis, as prior studies note that lung metastases from sarcomas have higher microvessel density (MVD) than carcinomatous metastases.[3, 4]
Platelet-derived growth factor (PDGF) and receptor-β (PDGFR-β) have a critical role in angiogenesis and tumor cell proliferation. There have been several studies in resected lung cancer tumors evaluating PDGF and PDGFR (α, β) in tumor cells and stroma.[5–7] Although these studies have differed in opinion on the prognostic significance of PDGF/ PDGFR-β immunohistochemistry in unselected NSCLC, it is clear that the pathway has a role in regulating angiogenesis via potentiation of vascular endothelial growth factor (VEGF).[5, 7] Given the hypothesis that sarcomatous and more aggressive tumors may have higher requirements of angiogenesis and that some studies in unselected NSCLC have shown prognostic significance for PDGF-B/ PDGFR-β, we sought to characterize the presence of PDGFR-β expression and gene copy number in a large series of resected sarcomatoid NSCLC.
Material and Methods
Tumor Tissue Specimens
Archived sarcomatoid lung cancer cases were selected from a review of the University of Texas M.D. Anderson Cancer Center Thoracic Tissue Bank from 1984–2004. A total of 59 cases were identified and 43 tumor blocks had adequate tissue to perform the analyses. The diagnosis of sarcomatoid NSCLC was confirmed by an independent histopathology review and tissue markers. Comparison control cases were selected from the same Thoracic Tissue Bank and were matched to the sarcomatoid NSCLC patients by the following characteristics: age, gender, and stage. Equal numbers of adenocarcinoma and squamous cell carcinoma were chosen and will be subsequently referred to as the “control NSCLC” in the manuscript.
Immunohistochemistry
We performed standard immunohistochemical (IHC) studies of the PDGF-B ligand and PDGFR-β on 43 sarcomatoid lung cancers and 42 control NSCLCs that had been surgically resected. Sarcomatoid lung cancers were carefully selected and the IHC studies were performed on the sarcomatoid carcinoma areas. The histology sections were incubated with primary antibodies against PDGF-B (N-30, dilution 1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and PDGFR-β (P-20, dilution 1:300, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 65 min at room temperature. Tissue sections were then incubated with the secondary antibody (EnVision Dual Link+; DAKO, Carpinteria, CA) for 30 min, after which diaminobenzidine chromogen was applied for 5 min. PDGF-B and PDGFR-β protein expressions were measured from 3 different areas of the tumor specimen and given a labeling score (intensity×% tumor involvement). The average of the 3 scores was assigned to each tumor specimen, and the averages were categorized as: low (0–1), intermediate (>1–2), or high (>2–3). These data were correlated to clinical information, and a comparison between the two populations was completed. IHC expression was evaluated in the tumor cells, vasculature, and stroma.
Fluorescence in situ hybridization (FISH) for analysis of gene copy number
In addition, PDGFRB gene copy number was evaluated in tumor tissue. We analyzed the gene copy number per cell using a dual-color FISH assay. Sarcomatoid lung cancers were carefully selected and the FISH studies for gene copy number analyses were performed on the sarcomatoid carcinoma areas. The PDGFRB probe was prepared from the Vysis LSI CSF1R probe (5q33–34) SpectrumOrange (Abbott Molecular, Illinois). A similarly probe Vysis LSI D5S23, D5S721 SpectrumGreen Probe (Abbott Molecular, Illinois) mapping to chromosome 5p was used as an internal control. The 4 µm thick sections were incubated for two hours to overnight at 56°C, deparaffinized in Citri-Solv (Fisher, Waltham, MA) and washed in 100% ethanol. The slides were sequentially incubated in 2× Saline-sodium Citrate Buffer (SSC) at 75°C for 18 to 23 min, digested in 0.5mg/ml proteinase K/2×SSC at 45°C for 18 to 23 min, washed in 2×SSC for 5 min, and dehydrated in ethanol. DNA denaturation was performed for 15 min at 85°C and hybridization was allowed to occur at 37°C for 36–48 hours. Chromatin was counterstained with 4’,6-diamidino-2-phenyldole (0.3 µg/ml in Vectashield mounting medium, Vector Laboratories). Gene copy number analysis was done in approximately 50 nuclei per tumor in at least four areas, and the selection of the area was guided by H&E-stained section. PDGFRB gene copy number was evaluated by three ways: a) gene amplification, defined as presence of loose or tight gene cluster or PDGFRB gene to centromeric probe 5 radio ≥2; b) copy number gain, defined as ≥4 copies in >40% cells or presence of gene amplification; and, c) the gene copy ratio between tumor and normal cells. The definition of gene copy number gain (≥ 4 copies in > 40% of cells) was established in a prior publication by Varella-Garcia et al.[8]
Statistical Analysis
Biomarker expression was correlated to patient demographics, tumor characteristics, and overall survival. Control NSCLC tumor specimens, which consisted of equal numbers of adenocarcinoma and squamous cell carcinoma, were age, gender and stage matched to the archived sarcomatoid specimens. PDGFRB gene copy number was evaluated by three ways: presence of amplification, FISH positivity, and the gene copy ratio between tumor and normal tissue. Demographic and IHC outcomes were summarized using mean, standard deviation, range if continuous, or using frequency tables and percentages if categorical. Comparisons of raw IHC outcomes between patient groups were performed using the Wilcoxon rank sum test. Comparisons of categorized IHC outcomes between patient groups were carried out using the Fisher’s exact test. Overall survival was estimated using the Kaplan-Meier curves. Comparisons of overall survival between patient groups were made using the log-rank test. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9 (SAS Institute, Cary, NC).
Results
Patient Demographics
The median age was 57, and women comprised 43% of each group. There was no significant difference in T and N stage between the two groups, but three sarcomatoid patients had M1 disease. See Table 1.
Table 1.
Patient Demographics.
| Patients n=95 |
Sarcomatoid NSCLC N=43 |
NSCLC N=42 |
|---|---|---|
| Female gender | 43% | 43% |
| Median age | 57 | 57 |
| Histology Adenocarcinoma Squamous cell carcinoma |
N/A N/A |
21 21 |
| Stage I II III IV |
10 18 12 3 |
5 18 19 0 |
| T1 T2 T3 T4 |
4 14 19 6 |
5 18 19 0 |
| N0 N1 N2 N3 |
30 5 8 0 |
30 8 3 1 |
| M0 M1 |
40 3 |
42 0 |
PDGF-B and PDGFR-β Immunohistochemical (IHC) Results
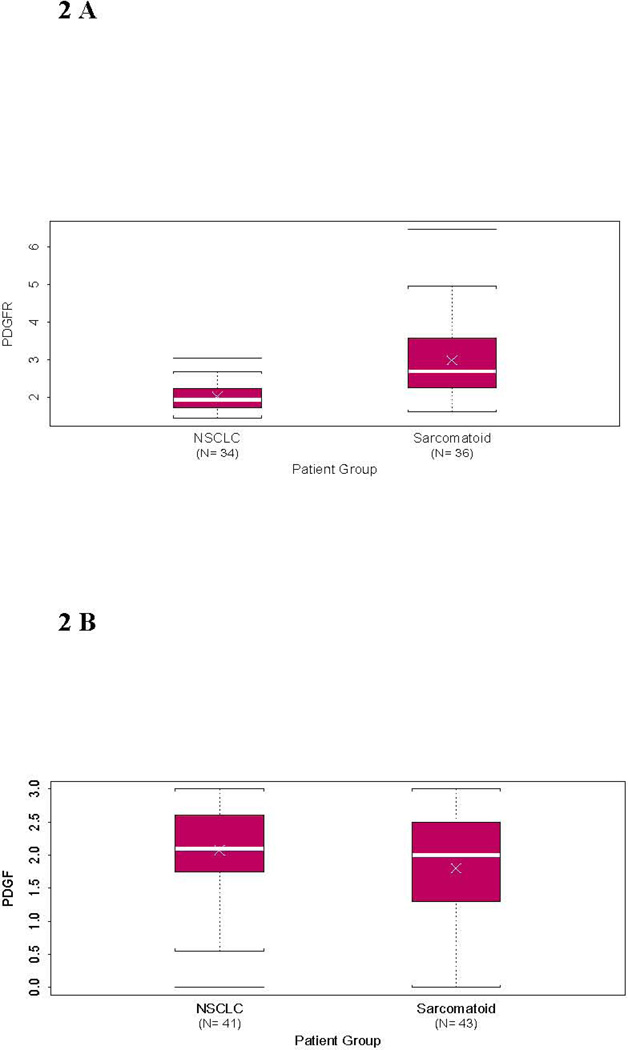
PDGF-B/PDGFR-β IHC expression was evaluated in tumor cells, vasculature, and in the stroma. There was no significant difference in IHC expression of PDGF-B or PDGFR-β between sarcomatoid or the control NSCLC in the vasculature or stroma. However, sarcomatoid lung carcinoma tumor cells had higher expression of PDGFR-β than NSCLC tumors (median score 2.69 vs. 1.93; p <0.0001), with 91% of sarcomatoid lung tumors had intermediate or high PDGFR-β staining compared to 24% of NSCLCs (p<0.0001). This analysis was conducted with Wilcoxon rank sum test and treated the IHC results as a continuous variable and by Fisher’s exact test where the IHC scores were grouped into low (0 – <1), intermediate (≥1 – <2), and high (≥ 2 – 3) scores. See Figures 1 and 2. There was no difference in IHC expression of ligand PDGF-B between the two tumor groups when evaluating the scores as a continuous variable (2 versus 2.1, p=0.16). Also, there was no difference in PDGFR-β IHC expression between the various histologic patterns of sarcomatoid lung tumors (i.e. giant cell, pleomorphic, spindle cell).
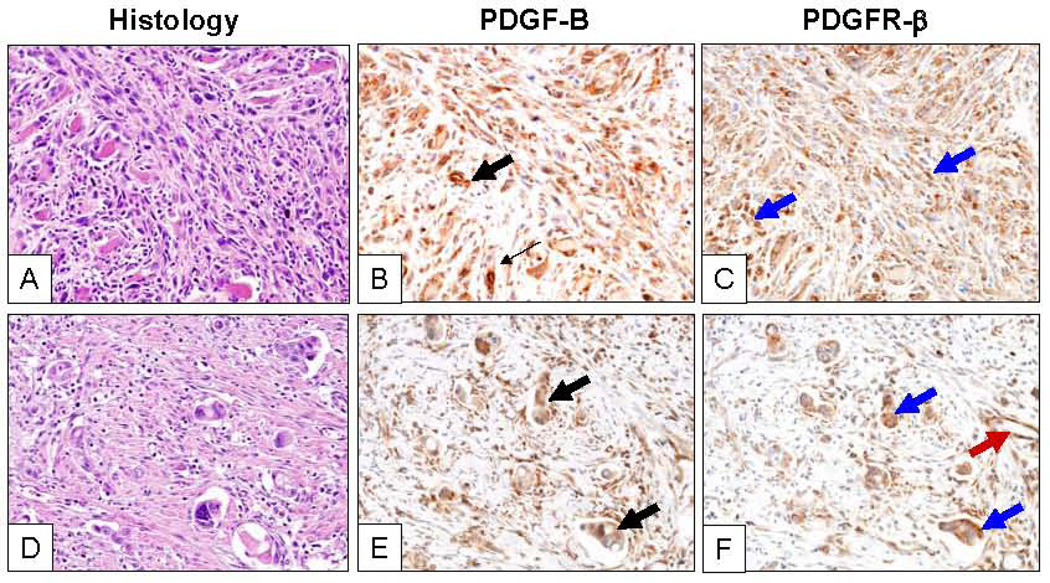
Figure 1.
Representative microphotographs of two sarcomatoid lung carcinomas (A, pleomorphic subtype, and D, giant cell, hematoxilyn-eosin, H&E) with strong cytoplasmic expression of PDGF-B (B and E; black arrows) and PDGFR-β (C and F; black arrows) in highly atypical malignant cells. Tumor stromal cells and blood vessel endothelial cells show PDGFR-β IHC expression (red arrow). Magnification of ×200.
Figure 2.
Box plots demonstrating the distribution of biomarkers by histologic subtype NSCLC versus sarcomatoid lung cancer. The white cross demonstrating the mean immunohistochemical (IHC) score and the white bar indicating the median IHC score by (A) a statistically significant difference by Wilcoxon rank sum test in PDGFRβ IHC (p<0.0001) and no significant difference in (B) PDGF-B IHC (p=0.16).
Neither PDGF-B nor PDGFR-β IHC tumor levels correlated with gender, age, TNM status, or overall pathologic stage of tumor in either the sarcomatoid or control populations. After a median follow-up time of 1.4 years, PDGF-B and PDGFR-β IHC levels in the tumor cells did not significantly impact overall or progression-free survival in either population; however, a numerical trend was seen between low PDGFR-β IHC expression and improved median overall and progression-free survival.(Table 2) When comparing overall survival between the two populations, patients with stage 2 sarcomatoid lung tumors had a trend toward worse survival than stage 2 NSCLC patients (p = 0.066).
Table 2.
Overall and Progression-Free Survival results by PDGFR-β IHC biomarker results.
| Patient Population | PDGFRβ IHC Level |
N | Median OS (mos) |
2-year OS rate |
5-year OS rate |
p-value | Median PFS (mos) |
2-year PFS rate |
5-year PFS rate |
p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | Low | 35 | 33.6 | 59% | 43% | 0.36 | 18.0 | 41% | 41% | 0.29 |
| Intermediate | 27 | 25.9 | 51% | 30% | 9.6 | 35% | 18% | |||
| High | 21 | 10.1 | 41% | 41% | 4.9 | 40% | 40% | |||
| Sarcomatoid | Low | 4 | 61.8 | 59% | 43% | 0.48 | 59.7 | 41% | 41% | 0.73 |
| Intermediate | 20 | 35.3 | 51% | 30% | 8.1 | 35% | 18% | |||
| High | 19 | 9.7 | 41% | 41% | 4.9 | 40% | 40% | |||
| Control NSCLC | Low | 31 | 33.6 | 97% | 88% | 0.39 | 18.2 | 91% | 85% | 0.22 |
| Intermediate | 7 | 13.3 | 100% | 88% | 9.6 | 96% | 78% | |||
| High | 2 | n/a* | 81% | 76% | n/a* | 80% | 50% |
no patients have died yet and a median survival results cannot be obtained
PDGFRB gene copy number
PDGFRB gene copy number was evaluated by three ways using FISH: 1) presence of amplification, 2) gene copy number gain, and 3) the gene copy ratio between tumor and normal tissue (see Figure 3). Gene copy number gain was defined as ≥4 gene copies in >40% cells or presence of gene amplification.[8] We identified PDGFRB gene amplification in six patients with sarcomatoid histology and only two in the control NSCLC group (p=0.26). Six additional patients with sarcomatoid NSCLC histology had ≥ 4 gene copies in over 40% of cells compared to none in the control NSCLC group. These patients were more likely to have advanced nodal N2 disease (p=0.0006). Thus, there were 12 patients with sarcomatoid histology that had PDGFRB gene copy number gain compared to 2 patients in the NSCLC control group (p=0.006).
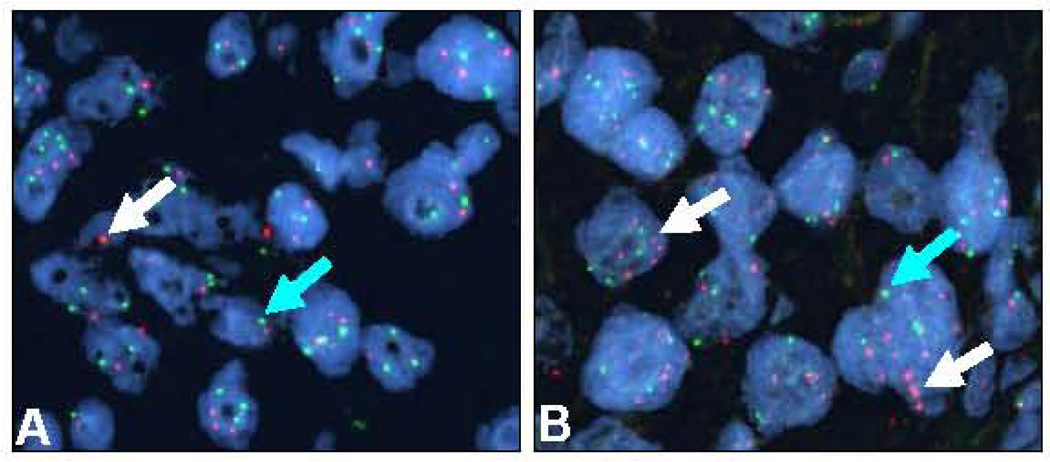
Figure 3.
Representative microphotographs of two sarcomatoid lung carcinomas examined by FISH showing normal PDGFRB copies (A) and gene amplification (B) in malignant cells. Magnification ×1,000. Red signals (white arrows) represent PDGFRB gene copies and green signals (green arrows) represent the chromosome 5 centromeric probe. Cell nuclei stained with 4’,6-diamidino-2-phenyldole.
PDGFRB gene copy number gain was associated with sarcomatoid histology (p=0.006), lower clinical and pathologic T stage (p=0.07, p=0.048), and higher pathologic N stage (p=0.013). Sarcomatoid NSCLC patients had higher gene copy ratios (above 1.83) compared to the control NSCLC patients (25 patients versus 9 patients, p=0.0004). Female patients also had higher gene copy ratios compared to men (p=0.03). There was no significant association between PDGFRB gene copy number gain and other histologic subtypes of NSCLCs (i.e. adenocarcinoma, squamous cell carcinoma). There was no correlation between gene copy number gain and overall or progression-free survival outcomes.(Table 3)
Table 3.
Overall and progression-free survival results by PDGFR copy number gain biomarker results.
| Patient Population | PDGFR copy number gain |
N | Median OS (mos) |
2-year OS rate |
5-year OS rate |
p-value | Median PFS (mos) |
2-year PFS rate |
5-year PFS rate |
p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | Positive | 14 | 39.1 | 55% | n/a* | 0.82 | 10.1 | 46% | n/a* | 0.77 |
| Negative | 55 | 25.5 | 50% | 40% | 11.5 | 38% | 33% | |||
| Sarcomatoid | Positive | 12 | 14.3 | 55% | n/a* | 0.66 | 7.2 | 46% | n/a* | 0.58 |
| Negative | 24 | 25.9 | 50% | 40% | 9.7 | 38% | 33% | |||
| Control NSCLC | Positive | 2 | n/a* | 86% | 79% | 0.31 | n/a* | 77% | 69% | 0.3 |
| Negative | 31 | 22.4 | 96% | 85% | 12.1 | 93% | 76% |
no patients have died yet and a median survival results cannot be obtained
High PDGFR-β IHC protein expression in tumor cells was associated with gene copy number gain (p=0.021) and higher gene copy ratio (tumor versus normal) status (p=0.005). This association was identified when evaluating the IHC scores as a continuous variable and also with the low, intermediate, and high cut-offs. There was also a trend towards higher gene copy ratio correlating to a higher IHC expression of PDGFR-β in the stroma (p=0.048).
Discussion
Sarcomatoid NSCLC is a rare histologic variant yet it appears to have a more aggressive phenotype[9–17], with one prior study reporting a worse 5-year overall survival rate (24.5% versus 46.3%, p=0.01) and shorter time to recurrence compared to stage-matched NSCLC.[2] Due to the rarity of sarcomatoid NSCLC, there have been few tissue-based studies evaluating tumor biomarkers and controversial results regarding the natural history of this disease.[3, 5, 18] However, this variant clearly has a unique biology and as treatment options are limited in the metastatic setting, identifying targets for systemic therapy is essential.
There have been some retrospective studies evaluating PDGF (various isoforms)/PDGFR (α, β) in NSCLC tumor tissue and cell lines[5, 6, 19] which have suggested that NSCLC tumor cells which express PDGF had a worse prognosis. However, most prior studies report that NSCLC tumor cells do not express PDGFR (α, β) and that the receptors’ expression is reserved in the surrounding vasculature and stroma.[6] Donnem et al. was an exception that reported PDGFR-α expression in NSCLC had a negative prognostic effect for disease-specific survival.[5] None of the prior studies have evaluated the sarcomatoid histologic variant of NSCLC and reported on this unique population. We are the first to report in this present study that PDGFR-β expression is highly upregulated in sarcomatoid NSCLC tumors and associated with high PDGFRB gene copy number when compared to control NSCLC. This is consistent with a prior hypothesis that sarcomatoid variants of epithelial tumors may require enhanced angiogenesis to promote their aggressive phenotype.[3, 4]
The implications of this finding suggest that anti-angiogenic therapy, specifically PDGFR inhibitors, may be of significance in the treatment of sarcomatoid NSCLC. McDermott et al. recently identified a lung and sarcoma cell lines that were highly sensitive to a PDGFR kinase inhibitor had focal gene amplification of PDGFRA gene and the PDGFC gene.[20] There appeared to be co-dependency of PDGFC activation of PDGFRA and RNA interference of either protein prevented cell proliferation. As there may be a direct anti-tumor effect with PDGFR inhibition for sarcomatoid tumors, PDGFR inhibition has also been hypothesized to decrease tumor interstitial fluid pressure and potentially enable increased chemotherapy uptake.[21]
The lack of correlation of higher PDGFR-β IHC and copy number to overall survival outcome found in our study does not necessarily argue against developing PDGF/PDGFR inhibitors in this subtype of lung cancer or in evaluating these biomarkers for prognostic/predictive value to systemic therapy. Any definitive conclusions regarding this are limited by the retrospective nature of this analysis and that our study’s tumor specimens were collected from patients whose subsequent adjuvant therapy varied significantly. There were also 3 sarcomatoid patients who were subsequently found to have M1 disease shortly after resection, which in a small study as this, could impact the results by diminishing the overall survival outcomes. When matching and comparing the stage 2 control NSCLC and sarcomatoid patients, the sarcomatoid lung cancer patients had a trend towards worse overall survival. All of these variables factor into the analyses of the overall survival data and potentially prevent a rigorous assessment of survival outcomes and the PDGFR-β IHC/gene copy number biomarkers. A prospective clinical trial that is designed to target the angiogenesis and PDGF/PDGFR pathway, would be the optimal setting to determine whether PDGFR-β IHC or gene copy number has potential as a predictive or prognostic biomarker.
Conclusion
This is the first study to demonstrate high PDGFR-β IHC expression and elevated gene copy number in sarcomatoid NSCLC tumors. Further studies are warranted to determine whether PDGFR-β is a feasible therapeutic target in sarcomatoid lung cancers that overexpress PDGFR-β by IHC or have high PDGFRB gene copy number. In addition, assessment of these biomarkers for prognostic or predictive value in a prospective trial for sarcomatoid NSCLC would be reasonable.
Acknowledgments
Supported by: National Institute of Health 5 K12 CA088084 05(PP-9)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brambilla E, et al. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 2.Martin LW, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973–980. doi: 10.1016/j.athoracsur.2007.03.099. [DOI] [PubMed] [Google Scholar]
- 3.Pelosi G, et al. Pleomorphic carcinomas of the lung show a selective distribution of gene products involved in cell differentiation, cell cycle control, tumor growth, and tumor cell motility: a clinicopathologic and immunohistochemical study of 31 cases. Am J Surg Pathol. 2003;27(9):1203–1215. doi: 10.1097/00000478-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi G, et al. Fluoro-deoxi-glucose uptake and angiogenesis are independent biological features in lung metastases. Br J Cancer. 2002;86(9):1391–1395. doi: 10.1038/sj.bjc.6600262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnem T, et al. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008;3(9):963–970. doi: 10.1097/JTO.0b013e3181834f52. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Hiroi S, Torikata C. Expression in lung carcinomas of platelet-derived growth factor and its receptors. Lab Invest. 1997;77(5):431–436. [PubMed] [Google Scholar]
- 7.Shikada Y, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65(16):7241–7248. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 8.Varella-Garcia M, et al. EGFR fluorescence in situ hybridisation assay: guidelines for application to non-small-cell lung cancer. J Clin Pathol. 2009;62(11):970–977. doi: 10.1136/jcp.2009.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venissac N, et al. Sarcomatoid lung cancer (spindle/giant cells): an aggressive disease? J Thorac Cardiovasc Surg. 2007;134(3):619–623. doi: 10.1016/j.jtcvs.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Fishback NF, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73(12):2936–2945. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Koss MN, Hochholzer L, Frommelt RA. Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am J Surg Pathol. 1999;23(12):1514–1526. doi: 10.1097/00000478-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ro JY, et al. Sarcomatoid carcinoma of the lung. Immunohistochemical and ultrastructural studies of 14 cases. Cancer. 1992;69(2):376–386. doi: 10.1002/1097-0142(19920115)69:2<376::aid-cncr2820690218>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Terzi A, et al. Biphasic sarcomatoid carcinoma of the lung: report of 5 cases and review of the literature. Eur J Surg Oncol. 1997;23(5):457. doi: 10.1016/s0748-7983(97)93733-1. [DOI] [PubMed] [Google Scholar]
- 14.Wick MR, Ritter JH, Humphrey PA. Sarcomatoid carcinomas of the lung: a clinicopathologic review. Am J Clin Pathol. 1997;108(1):40–53. [PubMed] [Google Scholar]
- 15.Raveglia F, et al. Personal experience in surgical management of pulmonary pleomorphic carcinoma. Ann Thorac Surg. 2004;78(5):1742–1747. doi: 10.1016/j.athoracsur.2004.04.084. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki T, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol. 2008;32(11):1727–1735. doi: 10.1097/PAS.0b013e3181804302. [DOI] [PubMed] [Google Scholar]
- 17.Rossi G, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003;27(3):311–324. doi: 10.1097/00000478-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima M, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer. 1999;86(4):608–616. [PubMed] [Google Scholar]
- 19.Antoniades HN, et al. Malignant epithelial cells in primary human lung carcinomas coexpress in vivo platelet-derived growth factor (PDGF) and PDGF receptor mRNAs and their protein products. Proc Natl Acad Sci U S A. 1992;89(9):3942–3946. doi: 10.1073/pnas.89.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott U, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69(9):3937–3946. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietras K, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–5484. [PubMed] [Google Scholar]