Abstract
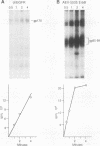
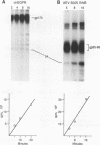
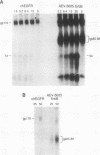
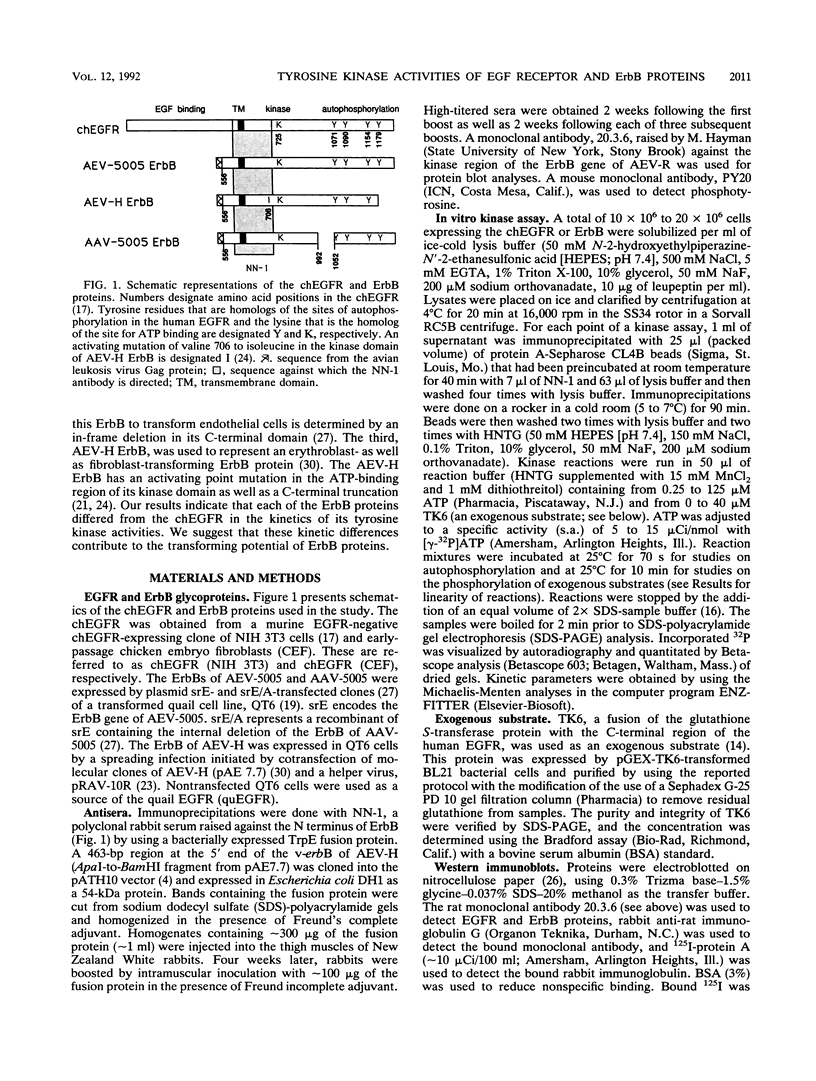
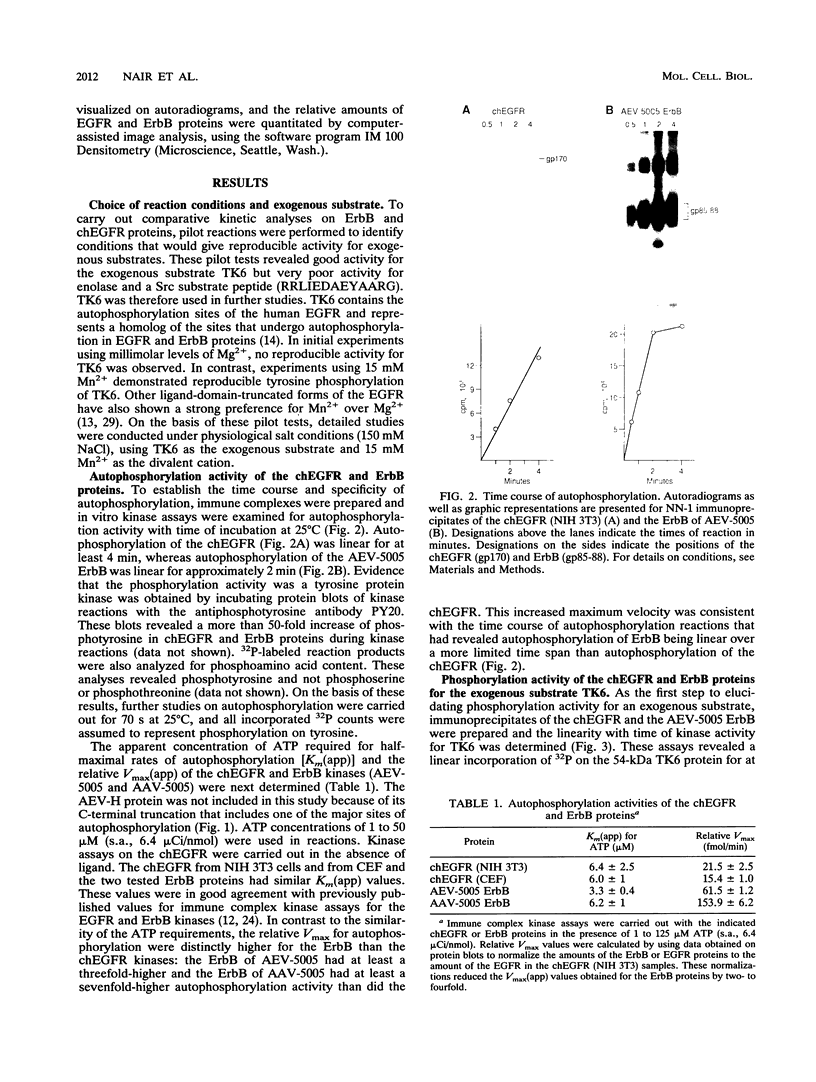
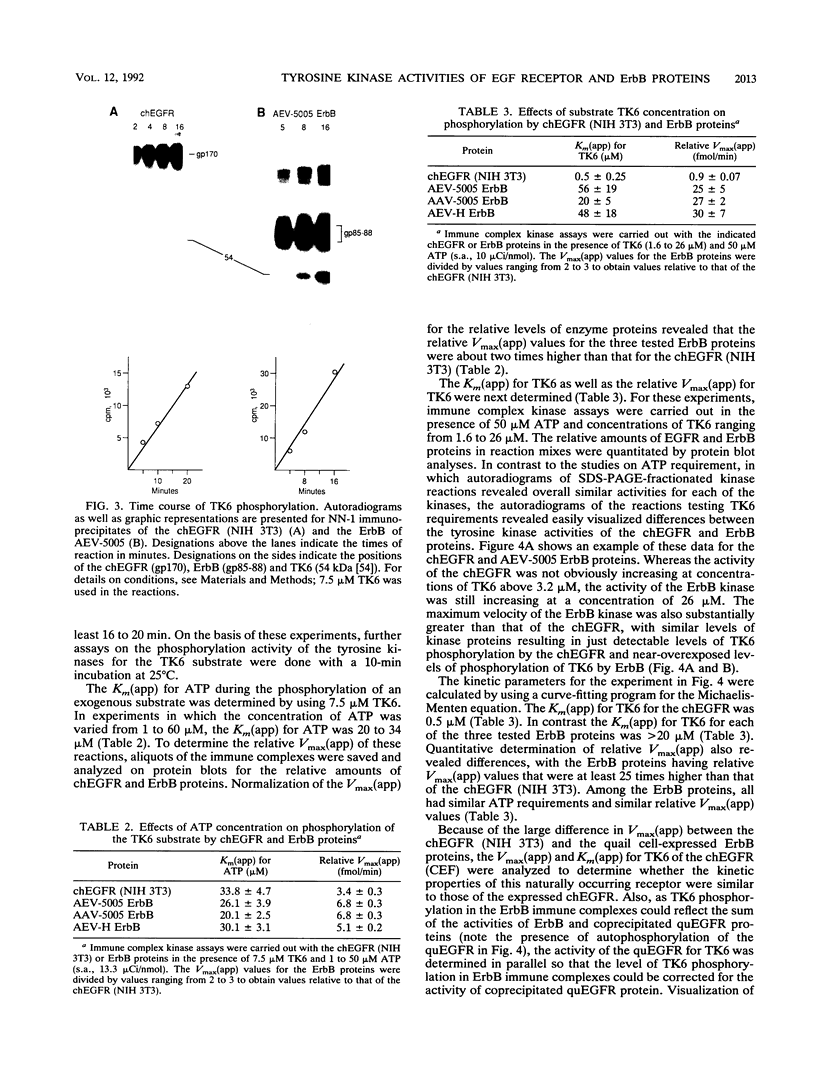
We have compared the protein tyrosine kinase activities of the chicken epidermal growth factor receptor (chEGFR) and three ErbB proteins to learn whether cancer-activating mutations affect the kinetics of kinase activity. In immune complex assays performed in the presence of 15 mM Mn2+, ErbB proteins and the chEGFR exhibited highly reproducible tyrosine kinase activity. Under these conditions, the ErbB and chEGFR proteins had similar apparent Km [Km(app)] values for ATP. The ErbB proteins appeared to be activated, as they had at least 3-fold-higher relative Vmax(app) for autophosphorylation and approximately 2-fold higher relative Vmax(app) for the phosphorylation of the exogenous substrate TK6 (a bacterially expressed fusion protein containing the C-terminal domain of the human EGFR). The ErbB kinases had both higher Km(app) and higher Vmax(app) for the phosphorylation of the exogenous substrate TK6 than did the chEGFR. The ratios of the Vmax(app) to the Km(app) for TK6 phosphorylation suggested that the ErbB proteins had lower catalytic efficiencies for the exogenous substrate than did the chEGFR. The three tested ErbB proteins had cytoplasmic domain mutations that conferred distinctive disease potentials. These mutations did not affect the kinetics for the phosphorylation of the exogenous substrate TK6. Two of the ErbB proteins contained all of the sites used for autophosphorylation. In these, a mutation that broadened oncogenic potential to endothelial cells caused an additional increase in Vmax(app) for autophosphorylation. Thus, mutations that change the EGFR into an ErbB oncogene cause multiple changes in the kinetics of protein tyrosine kinase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Raines M. B., Kung H. J., Vennström B. Rous-associated virus 1-induced erythroleukemic cells exhibit a weakly transformed phenotype in vitro and release c-erbB-containing retroviruses unable to transform fibroblasts. J Virol. 1986 Mar;57(3):1127–1138. doi: 10.1128/jvi.57.3.1127-1138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J. Phosphorylation of the erbB gene product from an avian erythroblastosis virus-transformed chick fibroblast cell line. J Biol Chem. 1985 Feb 25;260(4):2003–2006. [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Erneux C., Cohen S., Garbers D. L. The kinetics of tyrosine phosphorylation by the purified epidermal growth factor receptor kinase of A-431 cells. J Biol Chem. 1983 Apr 10;258(7):4137–4142. [PubMed] [Google Scholar]
- Gamett D. C., Tracy S. E., Robinson H. L. Differences in sequences encoding the carboxyl-terminal domain of the epidermal growth factor receptor correlate with differences in the disease potential of viral erbB genes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6053–6057. doi: 10.1073/pnas.83.16.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T., DeClue J. E., Martin G. S. Protein phosphorylation at tyrosine is induced by the v-erbB gene product in vivo and in vitro. Cell. 1985 Mar;40(3):609–618. doi: 10.1016/0092-8674(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Kitchener G., Knight J., McMahon J., Watson R., Beug H. Analysis of the autophosphorylation activity of transformation defective mutants of avian erythroblastosis virus. Virology. 1986 Apr 15;150(1):270–275. doi: 10.1016/0042-6822(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Heimann B., Beimling P., Pfaff E., Schaller H., Moelling K. Analysis of a tyrosine-specific protein kinase activity associated with the retroviral erbB oncogene product. Exp Cell Res. 1985 Nov;161(1):199–208. doi: 10.1016/0014-4827(85)90504-x. [DOI] [PubMed] [Google Scholar]
- Hihara H., Yamamoto H., Shimohira H., Arai K., Shimizu T. Avian erythroblastosis virus isolated from chick erythroblastosis induced by lymphatic leukemia virus subgroup A. J Natl Cancer Inst. 1983 May;70(5):891–897. [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Szapary D., Komoriya A., Kris R., Ullrich A., Schlessinger J. Kinetic parameters of the protein tyrosine kinase activity of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3053–3060. doi: 10.1002/j.1460-2075.1988.tb03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koland J. G., Cerione R. A. Activation of the EGF receptor tyrosine kinase by divalent metal ions: comparison of holoreceptor and isolated kinase domain properties. Biochim Biophys Acta. 1990 May 22;1052(3):489–498. doi: 10.1016/0167-4889(90)90160-f. [DOI] [PubMed] [Google Scholar]
- Koland J. G., O'Brien K. M., Cerione R. A. Expression of epidermal growth factor receptor sequences as E. coli fusion proteins: applications in the study of tyrosine kinase function. Biochem Biophys Res Commun. 1990 Jan 15;166(1):90–100. doi: 10.1016/0006-291x(90)91915-f. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lax I., Johnson A., Howk R., Sap J., Bellot F., Winkler M., Ullrich A., Vennstrom B., Schlessinger J., Givol D. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol Cell Biol. 1988 May;8(5):1970–1978. doi: 10.1128/mcb.8.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Ng M., Privalsky M. L. Structural domains of the avian erythroblastosis virus erbB protein required for fibroblast transformation: dissection by in-frame insertional mutagenesis. J Virol. 1986 May;58(2):542–553. doi: 10.1128/jvi.58.2.542-553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley R. J., Maihle N. J., Boerkoel C., Shu H. K., Carter T. H., Moscovici C., Kung H. J. Disease tropism of c-erbB: effects of carboxyl-terminal tyrosine and internal mutations on tissue-specific transformation. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7164–7168. doi: 10.1073/pnas.86.18.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Moscovici M. G., Kung H. J. Molecular characterization of three erbB transducing viruses generated during avian leukosis virus-induced erythroleukemia: extensive internal deletion near the kinase domain activates the fibrosarcoma- and hemangioma-inducing potentials of erbB. J Virol. 1988 Jul;62(7):2444–2452. doi: 10.1128/jvi.62.7.2444-2452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Shu H. K., Pelley R. J., Kung H. J. Dissecting the activating mutations in v-erbB of avian erythroblastosis virus strain R. J Virol. 1991 Nov;65(11):6173–6180. doi: 10.1128/jvi.65.11.6173-6180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. K., Pelley R. J., Kung H. J. Tissue-specific transformation by epidermal growth factor receptor: a single point mutation within the ATP-binding pocket of the erbB product increases its intrinsic kinase activity and activates its sarcomagenic potential. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9103–9107. doi: 10.1073/pnas.87.23.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P. B., Gill G. N. Activation of the purified protein tyrosine kinase domain of the epidermal growth factor receptor. J Biol Chem. 1989 Jul 5;264(19):11346–11353. [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]