Abstract
Neural networks supporting memory function decline with increasing age. Accumulation of amyloid-β, a histopathological finding in Alzheimer's disease, is a likely contributor. Posteromedial cortices (PMCs) are particularly vulnerable to early amyloid pathology and play a role in both encoding and retrieval processes. The extent to which aging and amyloid influence the ability to modulate activity between these processes within the PMC was investigated by combining positron emission tomography-amyloid imaging with functional magnetic resonance imaging in cognitively normal older and young adults. Young subjects exhibited a marked decrease in activity during encoding and an increase during retrieval (also known as encoding/retrieval “flip”). Impaired ability to modulate activity was associated with increasing age, greater amyloid burden, and worse memory performance. In contrast, the hippocampus showed increased activity during both encoding and retrieval, which was not related to these variables. These findings support a specific link between amyloid pathology and neural dysfunction in PMC and elucidate the underpinnings of age-related memory dysfunction.
Keywords: aging, amyloid, encoding, functional MRI, retrieval
Introduction
Neural networks supporting encoding and retrieval of information are known to decline with age (Bäckman et al. 1999; Small 2001). Recent studies have suggested that the accumulation of amyloid-β (Aβ), a major histopathological finding in Alzheimer's disease (AD) (Hardy and Selkoe 2002), is a likely contributor to the observed memory impairments (Naslund et al. 2000). As Aβ pathology can be detected in a substantial proportion of cognitively normal older adults, it is conceivable that both age- and amyloid-related alterations in the networks supporting memory function might underlie late-life cognitive deficits. Understanding how these neurobiological changes impact functional brain activity during normal aging is a major challenge in the field of aging research and could have important implications for disease treatment and prevention. Currently available neuroimaging tools have the potential to advance this objective, providing an opportunity to localize and quantify amyloid deposition in vivo in conjunction with measurement of functional activity during episodic memory tasks. The present study employed such a multimodal neuroimaging approach in a group of clinically normal older individuals to investigate the mechanisms of age-related memory loss.
Recently, there has been significant focus on the posteromedial cortices (PMCs) since these regions are particularly vulnerable to early amyloid pathology (Mintun et al. 2006) and are thought to play a role in both episodic memory encoding and retrieval (Buckner et al. 2005; Vannini et al. 2011b). In a recent study in young subjects, we evaluated the utility of a novel functional magnetic resonance imaging (fMRI) paradigm that incorporated a face–name association memory encoding and retrieval task (Vannini et al. 2011b). We found evidence of an anatomical overlap between brain regions, particularly in the PMC, that deactivate or are suppressed during encoding of novel information (Daselaar et al. 2004; Miller et al. 2008) and activate during retrieval (Wagner et al. 2005). Similar results demonstrating a “toggling” between deactivation during encoding and activation during retrieval have been observed by other groups (Daselaar et al. 2009; Huijbers et al. 2009, 2011) and have been referred to as the “encoding/retrieval flip” or “E/R flip”. Intriguingly, this phenomenon has primarily been observed in a set of brain regions commonly referred to as the “default mode” network (Greicius and Menon 2004). Although the precise mechanism underlying the E/R flip is still under active investigation, one theory suggests that it may represent functionally collaborating brain systems that work together to increase the likelihood of successful information processing (Buckner et al. 2008). The extent to which aging and amyloid influence functional modulation of fMRI activity during encoding versus retrieval, that is, the E/R flip, remains an open question and is of special interest because it might reflect a disruption of modulatory processes within memory.
Recent studies suggest that amyloid preferentially targets brain regions within the default network because of their metabolic and activity properties (Buckner et al. 2009). Evidence in support for this comes from laboratory experiments suggesting that synaptic activity may directly influence production of Aβ-aggregates (Cirrito et al. 2005, 2008), as well as from functional neuroimaging studies demonstrating a link between amyloid burden and disruption of the default network (Hedden et al. 2009, 2012; Sperling et al. 2009; Sheline et al. 2010; Vannini et al. 2011a). Given that daily life involves a constant shift between encoding new information and retrieval of previously processed information, the toggling phenomenon between these 2 processes, which may be particularly metabolically taxing, could be one reason the PMCs are particularly vulnerable to early amyloid deposition. Thus, studying the E/R flip in the context of aging and deposition may serve to elucidate the neural underpinnings of age-related memory alterations and provide an early functional marker of impending cognitive decline in preclinical AD.
In this study, our first objective was therefore to determine whether young and older individuals demonstrate a similar anatomically specific overlapping engagement of the PMC during encoding versus retrieval processes (E/R flip). Secondly, we wanted to investigate whether age preferentially altered the ability to modulate functional activity in the PMC. Finally, given the emerging literature suggesting that the PMC is particularly vulnerable to early amyloid pathology, we sought to further investigate the extent to which amyloid burden might influence the modulation of fMRI activity. The finding of age- and/or amyloid-related functional alteration in the PMC would further support the hypothesis that this region represents an important node in the network that supports episodic memory function and may serve to elucidate the neural underpinnings of memory dysfunction seen in aging and neurodegenerative disease.
Our previous study in young subjects also found an overlap of brain areas in the medial temporal lobe (MTL), including hippocampus, which demonstrated positive activation during both encoding and retrieval (Vannini et al. 2011b). This later finding is in accordance with the “reinstatement” hypothesis, stating that encoding and retrieval processes are strongly interdependent and that successful retrieval of episodic memory is dependent on “reactivation” of parts of the neural patterns or networks associated with encoding that information (McClelland et al. 1995; Rugg et al. 2008). The present study therefore also sought to investigate the overlapping anatomical engagement in the MTL region in our older individuals. Based on our previous findings, we expected that the hippocampus would show a reactivation pattern instead of an E/R flip. More importantly, based on previous neuroimaging studies demonstrating preserved activation in this region with age (e.g. Rand-Giovannetti et al. 2006) and the observation that this region is less vulnerable to early amyloid deposition, we hypothesized that we would not observe functional disruptions with age and amyloid in this region. Such results would implicate functional disruption of the PMC (possibly through its connections to the MTL) as a primary locus of the memory changes observed in aging and preclinical AD.
Materials and Methods
Participants
Forty-one community-dwelling cognitively normal older adults (M = 73.7 years) recruited from ongoing longitudinal studies of cognition and aging at Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH) and 26 healthy (M = 23.3 years) young adults participated in the study (Table 1). All subjects were native English speakers, had normal or corrected-to-normal vision, and were right-handed. No subjects had a history of psychiatric or neurological disorders or were taking medications that significantly affected the central nervous system. Older participants underwent a detailed screening and clinical and neuropsychological evaluation. Inclusion criteria for older participants were a Mini-Mental State Examination (MMSE) (Folstein et al. 1975) score of 28 or above, a Clinical Dementia Rating score of 0 (based on a detailed interview with the subject and with a study partner who has daily contact with the subject), and performance within 1.5 SD of age-adjusted norms on all neuropsychological measures. Informed written consent was obtained from every subject prior to any experimental procedure, and the study was approved by and conducted under the auspices of the Partners Human Research Committee at the BWH and MGH (Boston, MA, USA). Data from the older subjects comparing repeated encoding trials have appeared in another publication (Vannini et al. 2011a). Data from a subset of young subjects, comparing successful encoding and retrieval (Vannini et al. 2011b) and repeated encoding trials (Vannini et al. 2012), have appeared in other publications. Retrieval data and modulation measurement for older subjects have not been published before.
Table 1.
Demographic and neuropsychological characteristics
| Young | All older | PiB− | PiB+ | |
|---|---|---|---|---|
| N | 26 | 41 | 22 | 19 |
| Gender | 13 F/13 M | 28 F/13 M | 15 F/7 M | 11 F/8 M |
| Age (years) | 23.3 ± 2.5 | 73.7 ± 0.5 | 71.5 ± 1.9 | 76.4 ± 1.7 |
| Education | 15.6 ± 0.2 | 15.6 ± 0.5 | 15.1 ± 0.7 | 16.3 ± 0.6 |
| MMSE | N/A | 29.2 ± 0.1 | 29.2 ± 0.2 | 29.2 ± 0.2 |
| AMNART | N/A | 123.9 ± 1.0 | 122.9 ± 1.5 | 125.1 ± 1.3 |
| BNT | N/A | 48.7 ± 2.3 | 50.1 ± 2.9 | 47.0 ± 3.6 |
| BVFDT | N/A | 25.8 ± 1.2 | 26.3 ± 1.5 | 25.4 ± 1.9 |
| Face–name task accuracy (% successful recall) | 76.5 ± 3.5 | 62.2 ± 3.6** | 69.3 ± 5.0 | 53.5 ± 4.3* |
Note: Values are listed as mean ± SD. BNT, Boston Naming Test; BVFDT, Benton Visual Form Discrimination Test.
*P> 0.05, difference between PiB− and PiB+.
**P> 0.01, difference between older (all) and young subjects.
MR Imaging Acquisition
Scanning was performed on a GE Signa 3.0 T MR system (General Electric Healthcare, UK), equipped with an 8-channel head coil. High-resolution T1-weighted structural images were acquired using a 3D magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence: 166 sagittal slices, repetition time (TR) = 6.4 ms, echo time (TE) = 2.8 ms, inversion time (TI) = 900 ms, flip angle (FA) = 8°, field of view (FOV) = 260 mm, and matrix 256 × 256. Blood oxygen level-dependent (BOLD) fMRI data were acquired using a T2*-weighted gradient-echo planar (EPI) sequence: TR, 2000 ms; TE, 30 ms; FA, 90° within an FOV of 220 cm, with a 64 × 64 pixel matrix. Thirty oblique coronal slices with a slice thickness of 5 mm (interslice distance, 1 mm), oriented perpendicular to the anterior–posterior commissural line, were acquired to cover the whole brain. Eight functional runs, each lasting for 5 min, were acquired for each subject with 145 time points per run. The total functional scanning time was 40 min.
Amyloid Imaging Acquisition
Amyloid burden was estimated with N-methyl-[11C]-2-(4-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburg compound B, PiB), prepared as described by Mathis et al. (2002), and acquired at MGH, as described previously (Johnson et al. 2007; Gomperts et al. 2008; Rentz et al. 2010). Following a transmission scan, which was acquired to correct for attenuation, 11C-PiB (10–15 mCi) was injected as a bolus, followed immediately by a 60 min dynamic acquisition. PiB positron emission tomography (PET) data were reconstructed with ordered set expectation maximization and corrected for attenuation, and each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method, with cerebellar cortex as the reference tissue input function, was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) (Price et al. 2005).
PiB PET Statistical Analysis
Individual partial volume-corrected PiB DVR was estimated from a large aggregated cortical region of interest (ROI) consisting of frontal, lateral parietal and lateral temporal, and retrosplenial cortex. This mean cortical region was determined from each individual's high-resolution MP-RAGE image with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/), using a semi-automated parcellation method based on a probabilistic map (Fischl et al. 2004). Regional PiB DVR was calculated within this region from each individual's coregistered PET data. This mean cortical region was chosen as this region has been shown to exhibit substantial elevation of PiB retention in AD patients (Raji et al. 2008) and has been used as a summary measure of PiB uptake in previous studies (Johnson et al. 2007; Hedden et al. 2009). Supporting the use of a mean cortical region as an aggregate measure was the finding of a significant correlation between PiB DVR values in this region with ROIs in the posterior cingulate (r = 0.74, P< 0.001) and isthmus cingulate (r = 0.74, P = 0.001). In the current study, PiB uptake was treated as a continuous variable to ensure that the selection of a cutoff value did not determine the results. However, some analyses (demographics and test scores) were performed using a between-group approach comparing individuals with high and low levels of amyloid. The cutoff value for these analyses was set at PiB DVR = 1.15 based on previous studies (Johnson et al. 2007; Hedden et al. 2009).
fMRI Associative Memory Paradigm
The fMRI paradigm was an event-related design and consisted of a face–name association encoding and retrieval task, as described previously (Vannini et al. 2011b). Briefly, color photographs of faces were shown against a black background with a fictional first name printed in white underneath. Subjects were explicitly told to try to remember the name associated with the face. To provide a deep encoding task that enhances later memory, subjects were asked to press a button indicating whether the name was a good “fit” for the face or not (Sperling et al. 2003). The paradigm consisted of 4 encoding runs alternating with 4 retrieval runs. In each encoding run, 20 face–name pairs were shown to the subjects, each displayed for 2.75 s and presented in pseudorandom order in groups of 4 face–name pairs and interspersed with visual fixation (a white crosshair centered on a black background) periods. During fixation, subjects were told to focus their attention on the crosshair. Within a given encoding run, each of the 20 face–name pairs was presented a total of 3 times (5 groups of 4 face–name pairs comprised the total of 20 stimuli for each encoding run). In total, 80 face–name pairs were presented to the subjects over 4 encoding runs. Each encoding run was immediately followed by a retrieval run, consisting of a cued recall (CR) task followed by a forced-choice recognition (FCR) task for each of the 20 face–name pairs in the preceding encoding run. In the CR task, a face was presented for 5.25 s and the subjects had to respond with a button press whether he/she “remembered” the name associated with the face or had “forgotten” it. Subjects were instructed to press “remember” only for those faces for which they could explicitly bring to mind the name before seeing the names in the FCR phase. During the FCR task, each face was shown (for 3.25 s) with 2 names printed underneath and the subjects were instructed to indicate the correct name associated with that face by pressing 1 of the 2 buttons (correct name was presented in the counterbalanced order across all runs). To ensure that subjects were attentive and understood the instructions, detailed oral instructions were recited prior to each run. All subjects also completed a practice session both outside and inside of the MR scanner before the fMRI data were collected.
The paradigm was designed and generated on an external personal computer using MacStim 2.5 software (WhiteAnt Occasional Publishing, West Melbourne, FL, USA) and projected by means of an MR-compatible goggle system (VisuaStim XGA, Resonance Technology Inc., Los Angeles, CA, USA). Subjects responded with an MRI-compatible fiber-optical key press device with 2 buttons held in their right hand, and responses (accuracy and reaction time) were recorded by a computer interfaced with the optical switch using MacStim software outside the scanner room.
fMRI Preprocessing
fMRI data were preprocessed on a Linux platform running MATLAB version 7.1 (The Mathworks, Inc., Sherborn, MA, USA) with Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm/). The first 5 (additional) images in each run were discarded to allow the magnetization to reach equilibrium. Functional data were motion-corrected using sinc interpolation, by aligning (within-subject) each time series to the first image volume using least-square minimization of a 6-parameter (rigid-body) spatial transformation. No subject exceeded head movement over 3 mm (in the z-axis translation). The data were normalized to the standard SPM2 EPI template and resliced into 3 × 3 × 3 mm3 resolution in Montreal Neurological Institute space. Smoothing was accomplished using an isotropic Gaussian kernel of 8 mm full-width half-maximum. No scaling was implemented for global effects. The coordinates were later converted to Talairach and Tournoux's space (Talairach and Tournoux 1988) using software available online (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
fMRI Analyses
In order to identify the brain regions involved in episodic encoding, second-level t-statistics (using random effects in SPM2) were computed for each group separately. Contrast for each subject comparing fMRI activity during encoding of face–name pairs to a control condition (fixation cross) (Enc > Fix) was used. To investigate the areas that deactivate during this condition, the opposite contrast was used (Fix > Enc). Finally, t-statistics comparing fMRI data during CR with the control condition (Ret > Fix) were used to examine brain regions involved in episodic retrieval of face–name association. The whole-brain statistical maps were threshold at Puncorrected < 0.001 (extent threshold 5 voxels). These analyses were conducted to confirm that the task was activating and deactivating the hypothesized areas of interest in both age groups, thus replicating previous studies (e.g. Rand-Giovannetti et al. 2006; Miller et al. 2008; Pihlajamäki et al. 2008; Vannini et al. 2011b). For each subject, all runs were concatenated and regressors added, in lieu of global scaling, to account for signal differences between runs.
The anatomical overlap of brain regions engaged during the face–name encoding and retrieval task was determined using a conjunction analysis (Price and Friston 1997), that is, the intersection of the 2 statistical maps (random-effects group analysis) for the 2 contrasts was determined by using Imcalc (SPM2). Based on our hypothesis, we conducted 2 conjunction analyses. First, we identified overlapping brain areas activated during both encoding and retrieval (Enc > Fix) AND (Ret > Fix). Secondly, we identified areas that showed an encoding/retrieval flip: deactivated during encoding and activated during retrieval (Fix > Enc) AND (Ret > Fix). The conjunction maps were thresholded at Puncorrected < 0.001 (extent threshold 5 voxels).
To further investigate the magnitude and relation of the BOLD fMRI signals observed during the encoding and retrieval task, we performed an ROI analysis, using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/ for the PMC and the hippocampus. The PMC encompasses the posterior cingulate cortex (BA 23) and isthmus cingulate (BA 31), thus data for these 2 ROIs were collected in the analysis of the PMC. For each individual, significant voxels (defined from the 3 contrasts specified earlier, i.e. Enc > Fix, Fix > Enc, and Ret > Fix) which exceeded an uncorrected threshold of P< 0.05 and residing in the individually defined anatomical region of the PMC and hippocampus, were selected as ROIs. The mean beta-weights for encoding and retrieval trials were then extracted for each subject. We observed no above-threshold voxels in one of the ROIs for some subjects, although all subjects demonstrated activation in a subset of ROIs (the number of subjects included in each analysis is indicated by the degrees of freedom). These values were entered in an analysis of variance (ANOVA) [using STATISTICA 8 (StatSoft Inc., Tulsa, OK, USA)], with subject group (young, PiB−, and PiB+) as between-group factor. Planned comparisons were performed between the groups when a significant group effect was detected. Next, the mean modulation of functional response across cognitive processes (i.e. change in beta weight between the retrieval and encoding in all subjects in the PMC and hippocampus) was calculated. These values were entered in an ANOVA with subject group (young, PiB−, and PiB+) as between-group factor. Planned comparisons were performed between the groups when a significant group effect was detected. Finally, to examine the extent to which functional modulation between encoding and retrieval processes is affected by age, amyloid deposition (controlled for age), as well as performance on the fMRI task, the data were entered in a Pearson's correlation analysis.
Results
Pattern of Brain Regions Involved in the Encoding and Retrieval Task
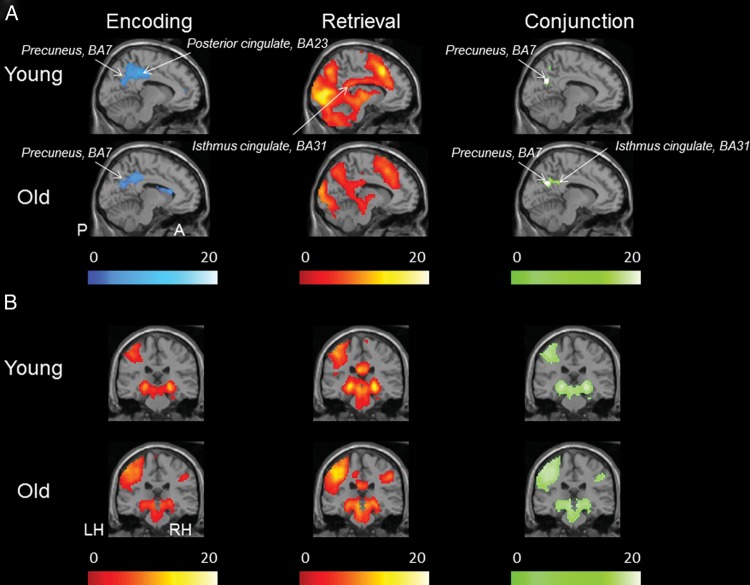
To identify brain regions engaged during the encoding and retrieval task, a series of one-sample t-tests (SPM2) were performed in the young and older subjects separately (Fig. 1).
Figure 1.
Brain regions involved in the memory encoding and retrieval fMRI task. (A) One sample t-test statistical parametric map (random effects) showing deactivation during encoding and activation during retrieval as well as the conjunction for these 2 statistical maps in young and older adults, demonstrating a significant E/R flip in the PMC. Lighter color scale indicates more significant activation (yellow/red), deactivation (blue), or conjunction (green). (B) Activation in encoding and activation during CR as well as the conjunction for these 2 statistical maps, demonstrating a significant overlap in the hippocampus region. The functional maps are thresholded at P< 0.001, minimal extent threshold 5 adjacent voxels and superimposed on a single subject T1 image (X = −9 in the sagittal view and Y = −27 in the coronal view).
Task-Induced Deactivation During Encoding
To investigate task-induced deactivation during the encoding task, the following contrast was used Fix > Enc. This analysis revealed significant deactivation (shown in blue, Fig. 1A) in both groups in the default network, particularly in the parietal lobe, including the precuneus (BAs 7/19) extending into angular gyrus (BA 39), posterior cingulate cortex (BA 23), isthmus cingulate/retrosplenial cortex (BA 31), as well as the superior and inferior lateral parietal cortex (BA 7/39/40) (P< 0.001). Significant deactivation was also found in the bilateral frontal lobes, including the superior and medial prefrontal cortex (BAs 6 and 8), as well as in the superior temporal gyrus (BA 22).
Task-Induced Activation During Encoding
Task-induced activation (shown in orange, Fig. 1B) during encoding (contrast: Enc > Fix) was observed in both groups in the hippocampus bilaterally and in the bilateral parahippocampal gyrus (BA 28) (P< 0.001). Significant activation was also found in the occipital lobe including left primary visual cortex (BA 17) as well as in the frontal lobes in both hemispheres (middle occipital gyrus, BAs 19 and medial frontal gyrus, BA 10).
Task-Induced Activation During Retrieval
Significant task-induced activation (shown in Fig. 1, middle column) during retrieval (contrast: Ret > Fix) demonstrated several areas of activation in the PMC, including the isthmus cingulate, the posterior cingulate (BA 31), bilateral parietal lobe [superior (BA 7/40) and inferior (BA 40)], and the bilateral occipital lobe (e.g. BA 18). Activation was also found in bilateral frontal lobes (e.g. BAs 10 and 46) as well as in the temporal lobe including bilateral hippocampus.
Anatomical Comparison of Identified Activation Maps
Given our main goal to investigate the functional modulation between encoding and retrieval processes (i.e. the E/R flip), we next performed a group-level conjunction analysis (contrast: F > Enc AND Ret > F) in each subject group (young and old) separately to examine the anatomical overlap between the brain regions that demonstrated a deactivation pattern during encoding and an activation pattern during retrieval. Areas demonstrating overlap are shown in green in Figure 1A. A significant overlap between deactivation during encoding and activation during retrieval was found in both groups in bilateral PMC including bilateral precuneus (BAs 7 and 31), extending to isthmus cingulate and posterior cingulate (BA 31). We next performed an additional conjunction analysis to examine the anatomical overlap of regional activity (Enc > F AND Ret > F) across the 2 tasks for each group separately (shown in green, Fig. 1B). Visual inspection of the maps revealed a similar overlapping activation pattern across the groups, including bilateral hippocampus, occipital lobes, as well as several areas in the frontal lobes and superior parietal cortex. Thus, the analyses within our group of older subjects replicate our previous findings in young subjects (Vannini et al. 2011b) and lend further support for the hypothesis that the PMC exhibits a dynamic response to encoding and retrieval processes, whereas the hippocampus reinstates its activity across encoding and retrieval processes.
Relationship Between Functional Modulation of E/R Flip and Memory Performance
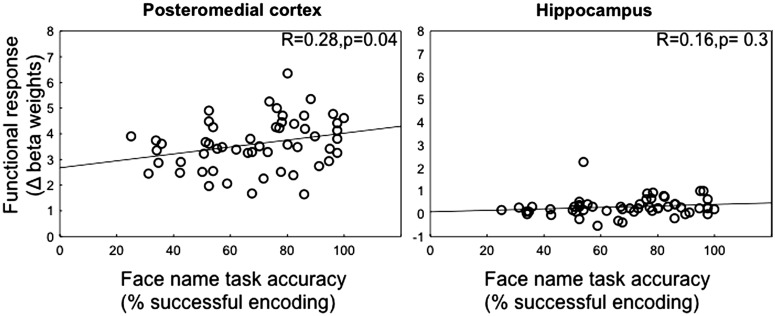
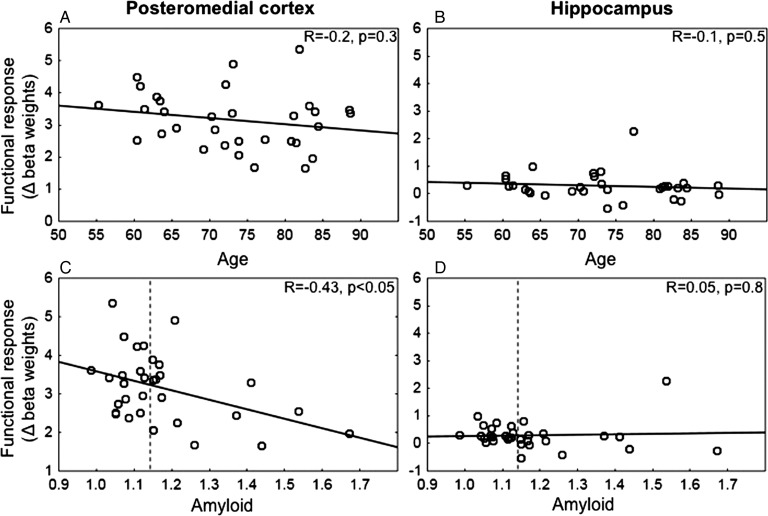
Next, we investigated whether the modulation of functional activity in PMC and hippocampus was related to memory performance on the face–name association task performed during fMRI acquisition. First, we extracted the signal change (beta weights) for each individual and each memory process (encoding and retrieval) separately from 2 regions in the PMC: isthmus cingulate and posterior cingulate as well as the hippocampus (see Materials and Methods). Modulation was then calculated as the change in the functional response during retrieval minus encoding. Across all subjects, we found a significant positive correlation (Fig. 2), between functional modulation and face–name task accuracy in the isthmus cingulate (P = 0.04) [reached trend level in the posterior cingulate (P = 0.08)], such that individuals with better performance showed the greatest modulation between encoding and retrieval (Fig. 2A). The hippocampus did not reveal any significant relationship between functional modulation and memory performance (Fig. 2B).
Figure 2.
Relationship between functional modulation and memory performance in the fMRI experiment. A positive correlation between the functional modulation (change in beta weight between retrieval and encoding) in the isthmus cingulate and accuracy in the face–name task was found across all subjects, such that individuals with better performance showed the best modulation between encoding and retrieval (A). No significant relationship between functional modulation in the hippocampus and task performance was found in the hippocampus (B).
Amyloid Burden Among Older Individuals
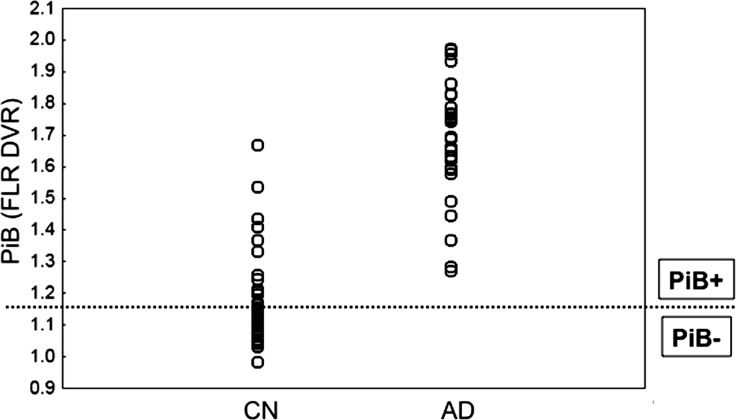
To examine the extent to which aging and amyloid influence modulation of the observed E/R flip, the older individuals were subdivided based on the amyloid deposition quantified within an aggregated ROI (Fig. 3). This mean cortical ROI, including frontal, lateral parietal, temporal, and retrosplenial cortices, was selected on the basis of previous studies to capture the widespread pattern of PiB retention commonly observed in AD patients (Johnson et al. 2007; Raji et al. 2008; Hedden et al. 2009), see also comparison from a preexisting sample of AD patients in Figure 3 (Johnson et al. 2007). The mean cortical PiB DVR values demonstrate that a high proportion of the current sample (ca. 50%) was classified as PiB+ (based on a cutoff value greater than 1.15); however, a relatively small group of subjects demonstrated amyloid burden up in the range seen in AD patients. We divided individuals into PiB– and PiB+ groups in order to investigate age effects by comparing older adults without evidence of substantial amyloid burden (PiB−) with the younger adults. Analyses within the older group involving the impact of fibrillar amyloid burden on the functional response to encoding and retrieval processes were conducted using amyloid as a continuous variable.
Figure 3.
PiB PET amyloid distribution. Amyloid distribution in cognitively normal individuals (N= 41) participating in the study compared with a group of preexisting AD patients (N = 31; Johnson et al. 2007). Each individual's DVR for PiB binding in the mean cortical brain region is plotted. Dotted line represents the classification boundary for negative versus positive PiB. Note that approximately 50% of our older individuals are PiB-positive.
Comparable Demographic and Neuropsychological Test Performance in the PiB– and PiB+ Groups
There were no significant differences between the PiB– and PiB+ individuals on demographic variables (Table 1). No significant difference was found for MMSE or general IQ [as estimated with the American National Adult Reading Test (AMNART); Grober and Sliwinski 1991]. There were also no significant differences on 2 cognitive measures potentially relevant to successful performance in the face–name associative memory paradigm: the Boston Naming Test (Kaplan et al. 1983) and the Benton Visual Form Discrimination Test (Dee and Benton 1970).
Memory Performance on the Face–Name Task in Young, PiB−, and PiB+ Individuals
During the face–name retrieval task, PiB+ individuals performed significantly worse than PiB− individuals [t(38) = 2.32, P = 0.03] and young subjects [t(42) = 4.2, P< 0.001] (Table 1). There was an overall age effect, such that young adults outperformed all older subjects [t(64) = 2.7, P = 0.01]; however, this age effect was completely driven by lower performance in the PiB+ older individuals, as there was no significant difference between the PiB– individuals and the young (P = 0.23).
Functional Modulation of E/R Flip and Relation to Age and Amyloid
To clarify the relationship between age, amyloid, and the E/R flip more in detail, we performed an ANOVA with group (young, PiB−, and PiB+) as between-group factor. This analysis demonstrated a significant main effect of group in both isthmus cingulate and posterior cingulate (Table 2). A nonsignificant main effect of group was found in the hippocampus. Planned comparisons were computed to look at the effect of age and amyloid separately in the PMC regions.
Table 2.
Functional comparison of modulation of response between encoding and retrieval
| ROI | Main effect |
Planned comparisons |
Correlation in the older subjects |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yng versus PiB− |
PiB− versus PiB+ |
Age |
Amyloid* |
|||||||
| F2,52 | P-value | F1,52 | P-value | F1,52 | P-value | R-value | P-value | R-value | P-value | |
| Posteromedial cortex | ||||||||||
| Isthmus cingulate | 12.1 | <0.001 | 9.7 | 0.002 | 2.7 | 0.1 | −0.2 | 0.3 | −0.4 | 0.04 |
| Posterior cingulate | 12.9 | <0.001 | 2.9 | 0.01 | 5.7 | 0.02 | 0.0 | 0.9 | −0.3 | 0.1 |
| Medial temporal lobe | ||||||||||
| Hippocampus | 0.6 | 0.6 | −0.1 | 0.5 | 0.1 | 0.6 | ||||
Note: PiB−, individuals with low amounts of amyloid; PiB+, individuals with high amounts of amyloid; Yng, young subjects.
*Values are controlled for age.
Effects of Age in the Absence of Amyloid
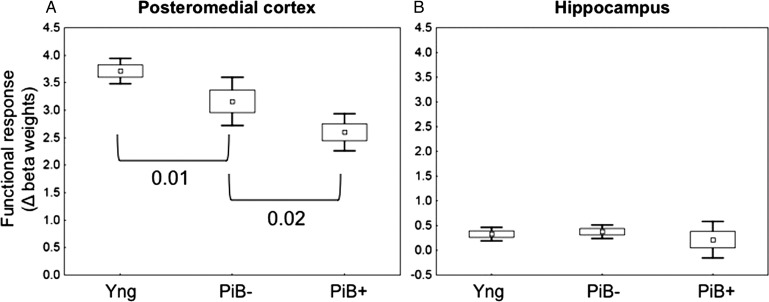
We observed a significant effect of age when comparing the young and PiB− individuals in both the isthmus cingulate (P = 0.002) and the posterior cingulate (P = 0.01), suggesting that older (PiB−) individuals have a restricted dynamic range in the flip between deactivation and activation in these regions (Table 2 and Fig. 4A). The hippocampus did not reveal any significant age effect between the groups (Fig. 4B).
Figure 4.
Functional modulation in young, PiB−, and PiB+ individuals. Mean modulation of functional response across cognitive processes, calculated as the change in beta weight between retrieval and encoding in all subjects in PMC (here showing the posterior cingulate) and hippocampus. (A) A significant effect of age in the PMC, in which PiB− individuals have a restricted dynamic range in the E/R flip. There was an additional significant effect of amyloid, such that individuals with high amounts of amyloid (PiB+) had the worst dynamic range in the flip between deactivation and activation. (B) There was no significant effect between any of the groups in the hippocampus.
Effects of Amyloid
Among all the older individuals (PiB− and PiB+ individuals), we found a significant effect of amyloid on functional modulation in the posterior cingulate (P = 0.02), which reached trend level in the isthmus cingulate (P = 0.1) (Table 2 and Fig. 4A). Furthermore, in the isthmus cingulate, we found a significant correlation between the amount of amyloid and functional modulation, demonstrating that individuals with higher amyloid burden showed the least modulation between encoding and retrieval processes (Table 2 and Fig. 5C). This effect remained significant even after controlling for age (r = − 0.38, P< 0.05). In addition, among all the older subjects, we could not find a significant relationship between the functional modulation and age (Table 2 and Fig. 5A), giving further evidence that the ability to modulate activity between encoding and retrieval is affected primarily by amyloid burden. In contrast, activity in the hippocampus did not reveal any significant relationship with amyloid levels (Table 2 and Figs 4B and 5D), and among all the older subjects, we could not find a significant relationship between age and functional modulation in the hippocampus (Table 2 and Fig. 5B).
Figure 5.
Functional modulation and relation to age and amyloid (controlled for age). Mean modulation of functional response across cognitive processes, calculated as the change in beta weight between retrieval and encoding in all subjects in PMC (here showing isthmus cingulate) and hippocampus. (A and B) Among all the older individuals, a nonsignificant effect was found between age and modulation of functional response. (C) A significant effect of amyloid (controlled for age) was found in the isthmus cingulate, such that individuals with higher amyloid burden showed the least modulation between encoding and retrieval processes. (D) There was no significant effect of amyloid on hippocampal activity.
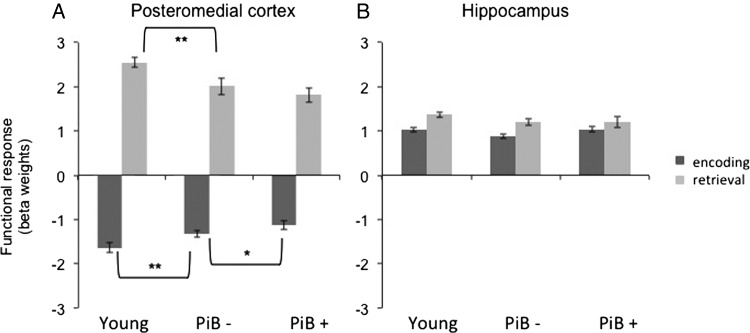
Functional Comparison of Encoding and Retrieval Responses
To further examine the origin of the observed changes in age and amyloid presented earlier, we conducted additional analyses to look at the functional response for encoding and retrieval processes separately. The ANOVA with group (young, PiB−, and PiB+) as between-group factor demonstrated a significant main effect in both isthmus cingulate and posterior cingulate for both encoding and retrieval responses (see Fig. 6 and Supplementary Table S1 for detailed statistics). A significant effect of age (comparison: young vs. PiB− individuals) on the encoding as well as the retrieval response was found in both ROIs. This implies that the observed difference in the E/R flip (Fig. 4A) is driven by significant age-related changes in both encoding and retrieval. We then looked at amyloid effects on the encoding response among all older subjects, which demonstrated a trend level (P = 0.2) difference between the PiB+ and PiB− groups in the isthmus cingulate (nonsignificant in the posterior cingulate). Although the PiB+ individuals also showed a slight decrease in activation during retrieval, this was not statistically significant in either PMC ROIs. Thus, it seems that the observed difference in the E/R flip (Fig. 4A) is driven by amyloid-related changes in both encoding and retrieval, neither of which is significant without examining the E/R flip. A nonsignificant main effect of group was found in the hippocampus.
Figure 6.
Functional response during encoding and retrieval in young, PiB−, and PiB+ individuals. The bar graph displays the extracted functional response for encoding and retrieval responses in young adults and individuals with low (PiB−) and high (PiB+) amounts of amyloid. (A) In the PMC (here showing the isthmus cingulate ROI), a significant age effect was found between the young and PiB− individuals for both encoding and retrieval responses. The effect of amyloid (PiB− vs. PiB+) demonstrated a trend level difference in the encoding response. **P< 0.05 and *P = 0.1. Although the PiB+ individuals also showed a slight decrease in activation during retrieval when compared with PiB− individuals, this did not reach statistical significance. (B) The hippocampus did not demonstrate any significant difference between the groups.
Discussion
The current study demonstrates an anatomical overlap between the neural correlates of encoding and retrieval processes in the PMC of cognitively healthy older individuals. These results extend our previous results in young subjects (Vannini et al. 2011b) as well as other studies (e.g. Daselaar et al. 2009; Huijbers et al. 2009) and provide further evidence that this region plays an important role in episodic memory learning and memory. In the PMC, a significant difference was observed between young and PiB− individuals for the modulation of functional response, suggesting that older individuals have a restricted dynamic range in deactivation and activation in these regions. Among all older subjects, a negative correlation was observed between the modulation of functional response and amyloid deposition in the mean cortical brain region, suggesting that this effect is most evident in individuals with high amyloid burden. As these regions are particularly vulnerable to early amyloid deposition, our findings may serve to elucidate the neural underpinnings of memory dysfunction seen in aging and neurodegenerative disease.
With regard to encoding, our results of a deactivation pattern in the PMC coincide with several previous studies (Daselaar et al. 2004; Miller et al. 2008). In accordance with the default network hypothesis, the suppression of activation has been interpreted to reflect ongoing information processing and to contribute to successful task performance. Support for this idea comes from the findings that the PMC has been shown to exhibit greater deactivation for remembered versus forgotten items (Daselaar et al. 2009). Similarly, our finding of a significant age effect in this region is consistent with previous studies (Miller et al. 2008; Pihlajamäki et al. 2009), demonstrating a reduced ability to deactivate this area during encoding. These results suggest that as you get older, there is a decreased ability to encode the items properly, resulting in poor performance on the task when compared with younger subjects (Miller et al. 2008; Pihlajamäki et al. 2009). Although the older subjects did perform slightly worse in the current task, we did not observe a significant difference in task performance between the PiB– individuals and the young subjects. We speculate that this might be due to the setup of our task paradigm, which allowed the subjects to view and encode the items 3 times before the retrieval task was administered. We are currently working on a modified task paradigm that will give us opportunity to investigate this issue more in detail. More interestingly, we observed an amyloid effect, in that PiB+ individuals demonstrated an even further decrease in the deactivation pattern when compared with PiB– individuals (reaching a trend level difference). This decrease in the deactivation pattern was also accompanied by a significant difference in memory performance between the PiB– and PiB+ individuals. These results are in line with a previous study in cognitively normal older subjects comparing PiB– and PiB+ individuals during a similar encoding task as reported here (Sperling et al. 2009). Similar patterns of dysfunction during encoding have also been observed in patients with early AD (Lustig et al. 2003; Celone et al. 2006) and in individuals with genetic risk of AD (Fleisher et al. 2009; Pihlajamäki et al. 2009). Furthermore, our finding of a nonsignificant difference between the subject groups (Fig. 4B) in the hippocampus region further supports this finding. Taken together, these results support previous studies and give further evidence that early amyloid deposition within the PMC may contribute to the hallmark symptoms of episodic encoding impairment in early AD.
Our finding of a significant age effect during the retrieval task further speaks for the fact that the PMC is particularly challenged in the context of aging. The functional role of this region has been suggested to be that of evaluating information, once it has been retrieved from memory (Wagner et al. 2005). It has further been suggested that the extent or strength of activation in certain parietal regions correlates with successful memory retrieval and with false memory errors (Svoboda et al. 2006). Our finding of a decrease in the activation strength with age and amyloid is in line with these previous studies and suggests that failure of encoding these items might underlie the observed activation changes during retrieval. Thus, our analysis of the functional modulation in this region between these 2 processes may be of particular interest.
The current finding of a significant difference between the modulation of functional response in the young compared with the PiB− individuals suggests that older individuals have a restricted dynamic range in the modulation between deactivation and activation in these regions (Fig. 4A). To test whether the functional modulation of the activation between encoding and retrieval is related to other cognitive processes, we performed several additional analyses to look at the correlation between the E/R flip and other tests of memory or attention performed outside the camera. If found, this would provide further evidence that the functional modulation of activation between encoding and retrieval is related to memory performance. However, although we did find a correlation between the E/R flip and the performance of the task in the scanner (Fig. 2, A), we did not find any correlation between the E/R flip and other tests of memory or attention. This suggests that the modulation between the 2 memory processes is specific to the current fMRI task. Future fMRI studies should investigate this more in detail by looking at the relationship between E/R flip and performance using different functional tasks.
Among the older subjects, amyloid deposition was also inversely correlated with modulation of functional response, such that individuals with high amyloid burden showed the least modulation in memory-related activity (Fig. 5C). Taken together, these findings provide even further evidence that the PMC, which is part of the default network, represents an important brain region in episodic memory function, whose integrity is altered with age and amyloid pathology. Note that we did not find any age- or amyloid-related changes in brain function in the hippocampus region (Figs. 5B and D). These results are in line with previous studies, indicating that early amyloid-associated influences on memory networks may be primarily centered in “hub” regions, especially of the default network (Buckner et al. 2008; Hedden et al. 2009; Sperling et al. 2009; Sheline et al. 2010). For example, using resting-state functional connectivity analysis, Hedden et al. (2009) found evidence that increased amyloid burden in a group of cognitively normal older individuals had significantly reduced functional correlations within the default network relative to individuals with low amyloid burden. Our findings may also help in elucidating the role of amyloid deposition and neuronal integrity in memory impairment in AD. Recent laboratory experiments suggest that amyloid levels might in fact be related to neuronal activity (Cirrito et al. 2005, 2008; Bero et al. 2011). For instance, utilizing in vivo microdialysis together with field potential recordings, Cirrito and collegues were able to demonstrate that amyloid levels in the brain were dynamically and directly influenced by synaptic activity in the brain. In addition, it is also known that high neuronal activity is related to high metabolic activity. Thus, it might not be surprising that the brain regions that show increased amyloid levels are also the areas that exhibit the highest baseline metabolic activity at rest, as measured with PET and fMRI (Gusnard and Raichle 2001; Buckner et al. 2005). Taken together, given our finding of a relationship between altered functional modulation in the default network and performance on the episodic memory task [such that better performance was correlated with more modulation between encoding and retrieval (Fig. 2)], it is conceivable that amyloid pathology might contribute to synaptic dysfunction and eventual neural loss in the PMC, leading to the memory impairment observed. These results are in accordance with previous findings demonstrating that age-related failures to modulate activity in the prefrontal cortex during an executive task were related to poorer performance; suggesting that disruptions of the modulation of neural activity may be an important marker of age-related cognitive change (Hedden et al. 2012). Although the authors found that white matter hyperintensities, rather than amyloid burden, were associated with failures of modulation, they also observed amyloid-related alterations in default network activity (Hedden et al. 2012).
A related issue is the justification for using mean cortical PiB retention from an aggregated cortical region to study the impact on memory function. Today, there is no consensus on the optimal regions for sampling PET amyloid imaging, but the most common approach in previous studies has been to use a global index based on the PiB retention in several regions. The current study also defined the PiB groups based on the PiB retention in a large aggregated cortical ROI consisting of frontal, lateral parietal and lateral temporal, and retrosplenial cortex (sometimes referred to as the FLR region). The rationale for using mean cortical PiB retention was based on the fact that these regions have been shown to exhibit substantial elevation of PiB retention in AD patients (Johnson et al. 2007; Raji et al. 2008; Hedden et al. 2009). Thus, since the current study investigated cognitively normal individuals who may be in very early stages of amyloid accumulation, we choose to sample PiB retention in regions known to be particularly vulnerable to early amyloid deposition (Mintun et al. 2006; Sperling et al. 2009; see also overlap with AD patients in Fig. 3) and which also included areas that are critical for memory function (e.g. Buckner et al. 2005; Vannini et al. 2011a and b; Vannini et al. 2012).
With regard to the current understanding of the default network and the belief that this network is important for information processing, additional interpretations with a cognitive account for the current findings should also be mentioned here. That is, in line with the default network, it has recently been suggested that the activity observed within this network represents different levels of attention. Also termed the orientation of attention hypothesis, this theory states that the decreased activity within this network can be observed during tasks that require attention that is oriented toward the external environment (Buckner et al. 2008), whereas the increased activation observed in the same region would instead represent attention oriented toward the internal environment (Shulman et al. 1997; McKiernan et al. 2003). We have recently argued that this theory can be applicable to our findings of a toggling phenomenon in the PMC (Vannini et al. 2011b), although Huijbers et al.'s (2011) results did not support this hypothesis. Thus, in accordance to the above-mentioned theory, the deactivation patterns observed during encoding would then reflect efficient suppression of internally generated cognitive processes, due to more focussed external attention. In contrast, the observed increased activation in the same area observed during retrieval would then reflect the ability to orient the attention to internal representations of the previously encoded memory. Thus, we believe that the toggling phenomenon observed in this region could in fact represent carefully orchestrated activity within an integrated memory system. That is, by first focussing the attention to novel stimuli to enhance encoding, successful suppression of this region is setting up the stage for activity during retrieval of previous memory processing. With regard to aging and amyloid pathology and in accordance with the above-outlined hypothesis, our observation of an altered functional modulation between these 2 processes would then represent a failure to process the information during encoding, which in turn is leading to a failure of retrieving the information and ultimately to decreased performance of the task at hand. A limitation of the current paradigm is that encoding and retrieval processes take place in different (alternating) runs. Thus, tonic effects of being in an “encoding” or “retrieval” state could have influenced the results presented earlier. Future experiments are using a modified variant of this paradigm to investigate this issue.
The current results were mainly derived from hypothesis-driven ROI analyses. The rationale for this was to maximize power to test our a priori hypothesis that the PMC is an important region for the E/R flip and is primarily affected by amyloid deposition. Thus, given that we were looking at constrained activated voxels lying within an individually defined anatomical region, we believe that a conventional P-value of 0.05, uncorrected was justified. However, one limitation of this approach is how the signal is measured within the ROI. That is, even if the region is significantly active, this activation may only occur in a small proportion of voxels in the ROI. Thus, simply averaging across the entire region could swamp the signal from this small number of voxels with noise from the remaining nonactivated voxels in the specified ROI. The current study used a functional threshold of P = 0.05 to overcome this issue. One constraint of this approach is that even though the applied analysis was sensitive to a specific threshold, it did not take into account the spatial extent of the activation in the ROI. Future studies focused on this region might consider applying a small volume correction to correct for this issue. To ensure that the current paradigm demonstrated an E/R flip within the PMC, the current study used a whole-brain analysis, corrected for multiple comparisons, as well as ROI analysis. Thus, using this approach, we had the ability to maximize power to test our a priori hypothesis using a small number of statistical comparisons, with a whole-brain approach using a much more conservative threshold to look at both expected and unexpected brain regions demonstrating the E/R flip.
In summary, the current study adds to the mounting evidence that the PMC represent a critical brain area in episodic memory function. This region exhibits a dynamic response to encoding and retrieval processes, and the ability to modulate this response was related to memory performance. More importantly, although the ability to modulate functional activity in this region decreased with aging (even in the absence of amyloid pathology), we observed a significant effect of amyloid burden on PMC activity, even when controlling for age. The failure to appropriately modulate neural activity between encoding and retrieval processes in a region particularly vulnerable to early amyloid deposition may provide an important link to very early memory impairment in preclinical AD. In contrast, the hippocampus showed a “reinstatement” of activation during both encoding and retrieval, which was preserved with aging and amyloid pathology.
In order to fully appreciate these findings, there is a need for longitudinal follow-up of these individuals. To confirm whether the pattern that is observed in the individuals with high amounts of amyloid really is a marker of early AD, it would be informative to know how many people progress to mild cognitive impairment (MCI) or AD dementia in the future. In addition, given the small sample size in the current study, there is also a need to further extend these findings in a larger sample of older subjects, preferably stratified by age and demonstrating a more heterogeneous amyloid profile than the current sample. Future studies should also include people with MCI and AD to investigate whether the pattern observed in cognitively normal individuals with high amounts of amyloid resembles the pattern in AD and MCI patients.
Overall, these findings serve to elucidate the neural underpinnings of memory dysfunction related to amyloid deposition and improve our understanding of age-related changes in the absence of one of the most common neurodegenerative diseases associated with aging.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Marie Curie Fellowship: FP7-PEOPLE-2007-4-1-IOF from the European Union [P.V.], the Swedish Brain Foundation and Swedish Society for Medicine [P.V.], the European Molecular Biology Organization: ALTF 318-2011 [W.H], the National Institutes of Health: K24 AG035007 [R.A.S.], R01 AG027435-S1 [R.A.S and K.A.J.], P01AG036694 [R.A.S. and K.A.J.], P50AG00513421 [R.A.S. and K.A.J.], and the American Health Assistance Foundation [R.A.S.].
Supplementary Material
Notes
We are indebted to the volunteers who participated in this study. We thank Janice Fairhurst, George Chiuo, Seung-Schik Yoo, and Istvan Akos Morocz for their help with scan acquisition at the Center for Advanced Imaging at BWH. Conflict of Interest: None declared.
References
- Bäckman L, Small BJ, Wahlin Å, Larsson M. Cognitive functioning in very old age. In: Craik FIM, Salthouse TA, editors. Handbook of cognitive aging. Hillsdale (NJ): Erlbaum; 1999. pp. 499–558. [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee J-M, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-[beta] deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk W, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's Disease: Evidence for a relationship between default activity, amyloid, and memory. J Cogn Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone K, Calhoun VD, Dickerson BD, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang J-E, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-[beta] in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-[beta] levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:1–10. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee L, Benton AL. A cross-modal investigation of spatial performance in patients with unilateral cerebral disease. Cortex. 1970;6:261–272. doi: 10.1016/s0010-9452(70)80015-6. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage. 2009;47:1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHough PR. Mini-Mental State”: a practical method for grading cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gomperts SNMDP, Rentz DMP, Moran EB, Becker JAP, Locascio JJP, Klunk WEMDP, Mathis CAP, Elmaleh DRP, Shoup TP, Fischman AJM. Imaging amyloid deposition in Lewy body diseases SYMBOL. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb Cortex. 2012;22:1038–1051. doi: 10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE. 2011;6:e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CMA, Cabeza R, Daselaar SM. When learning and remembering compete: a functional MRI study. PLoS Biol. 2009;7:e1000011. doi: 10.1371/journal.pbio.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia (PA): Lea & Febiger; 1983. [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang Y, Huang G-F, Debnath ML. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid B-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, DePeau K, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriatr Psychiatr. 2008;16:283–291. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, O'Keefe K, Bertram L, Tanzi RE, Dickerson BD, Blacker D, Albert MS, Sperling RA. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;24:28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Raji C, Becker J, Tsopelas N, Price J, Mathis C, Saxton J, Lopresti B, Hoge J, Ziolko S, DeKosky ST. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer's disease with Pittsburgh compound B. J Neurosci Meth. 2008;172:277–282. doi: 10.1016/j.jneumeth.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older adults. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M, Johnson JD, Park H, Uncapher MR. Encoding–retrieval overlap in human episodic memory: a functional neuroimaging perspective. Prog Brain Res. 2008;169:339–352. doi: 10.1016/S0079-6123(07)00021-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatr. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline. Arch Neurol. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatr. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamäki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme Medical Publishers; 1988. [Google Scholar]
- Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz DM, Johnson KA, Sperling RA. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011a doi: 10.1016/j.neurobiolaging.2011.01.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, O'Brien J, O'Keefe K, Pihlajamäki M, LaViolette PS, Sperling RA. What goes down must come up: role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2011b;21:22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Sullivan C, Sperling RA. Differential functional response in the posteromedial cortices and hippocampus to stimulus repetition during successful memory encoding. Hum Brain Map. 2012 doi: 10.1002/hbm.22011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.