Abstract
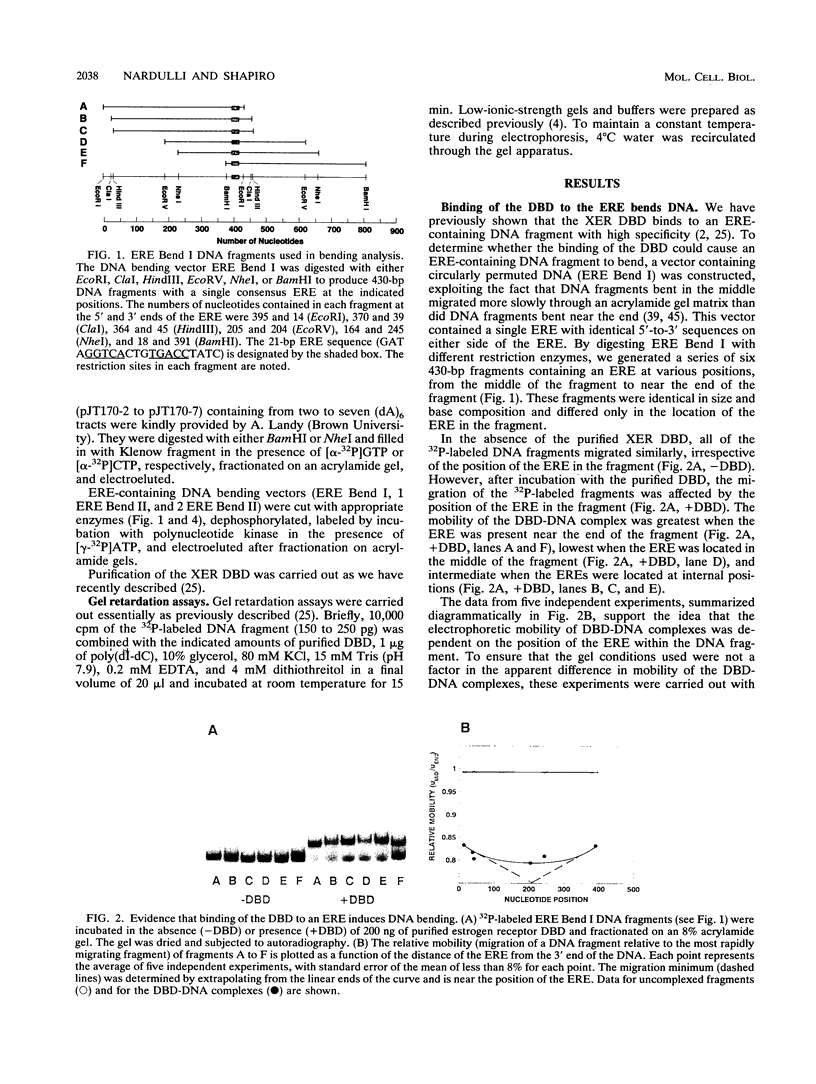
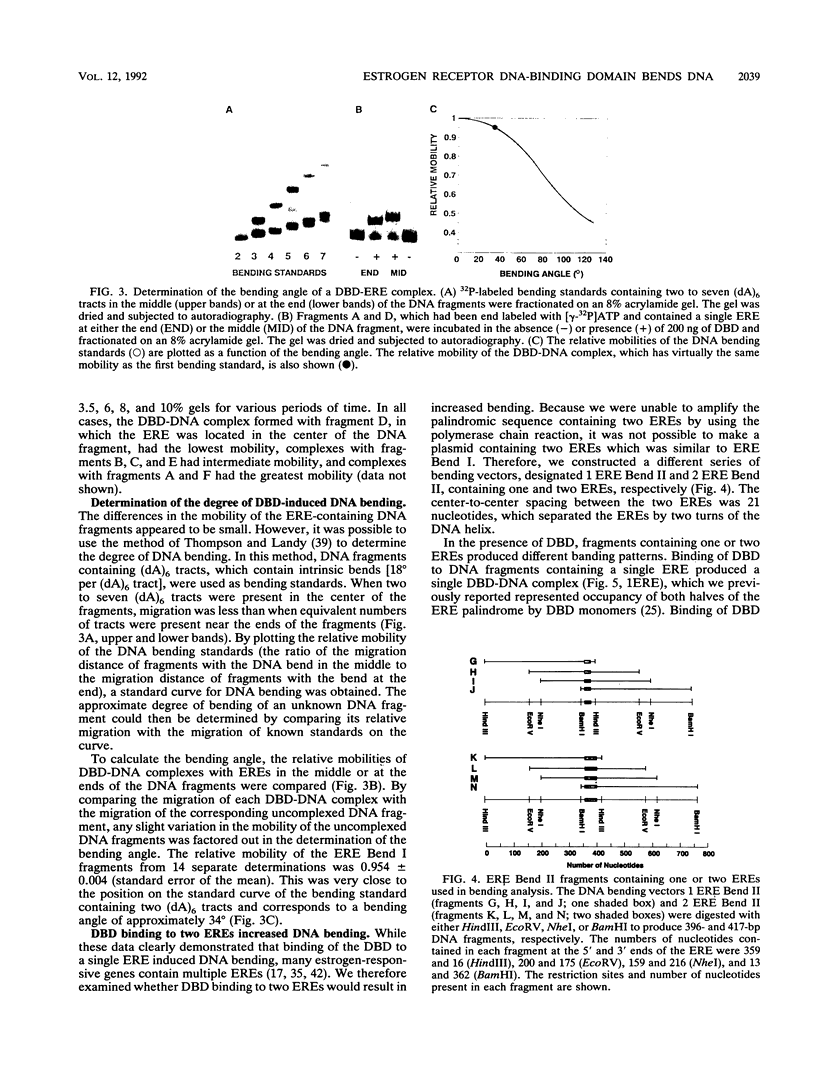
We have used circular permutation analysis to determine whether binding of purified Xenopus laevis estrogen receptor DNA-binding domain (DBD) to a DNA fragment containing an estrogen response element (ERE) causes the DNA to bend. Gel mobility shift assays showed that DBD-DNA complexes formed with fragments containing more centrally located EREs migrated more slowly than complexes formed with fragments containing EREs near the ends of the DNA. DNA bending standards were used to determine that the degree of bending induced by binding of the DBD to an ERE was approximately 34 degrees. A 1.55-fold increase in the degree of bending was observed when two EREs were present in the DNA fragment. These in vitro studies suggest that interaction of nuclear receptors with their hormone response elements in vivo may result in an altered DNA conformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Chang T. C., Shapiro D. J. An NF1-related vitellogenin activator element mediates transcription from the estrogen-regulated Xenopus laevis vitellogenin promoter. J Biol Chem. 1990 May 15;265(14):8176–8182. [PubMed] [Google Scholar]
- Debs R. J., Freedman L. P., Edmunds S., Gaensler K. L., Düzgünes N., Yamamoto K. R. Regulation of gene expression in vivo by liposome-mediated delivery of a purified transcription factor. J Biol Chem. 1990 Jun 25;265(18):10189–10192. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Yoshinaga S. K., Vanderbilt J. N., Yamamoto K. R. In vitro transcription enhancement by purified derivatives of the glucocorticoid receptor. Science. 1989 Jul 21;245(4915):298–301. doi: 10.1126/science.2473529. [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Ampe C., Steitz T. A., Crothers D. M. Molecular characterization of the GCN4-DNA complex. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6034–6038. doi: 10.1073/pnas.87.16.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Taylor A., Kedes L. DNA bending is induced by a transcription factor that interacts with the human c-FOS and alpha-actin promoters. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2162–2166. doi: 10.1073/pnas.86.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Greene G. L., Clark J. H., Tsai M. J., O'Malley B. W. Specific binding of estrogen receptor to the estrogen response element. Mol Cell Biol. 1989 Jan;9(1):43–49. doi: 10.1128/mcb.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Kuhnke G., Theres C., Fritz H. J., Ehring R. RNA polymerase and gal repressor bind simultaneously and with DNA bending to the control region of the Escherichia coli galactose operon. EMBO J. 1989 Apr;8(4):1247–1255. doi: 10.1002/j.1460-2075.1989.tb03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Godowski P. J., Maler B. A., Yamamoto K. R. Glucocorticoid receptor mutants that define a small region sufficient for enhancer activation. Science. 1987 Apr 24;236(4800):423–427. doi: 10.1126/science.3563519. [DOI] [PubMed] [Google Scholar]
- Moskaluk C., Bastia D. DNA bending is induced in an enhancer by the DNA-binding domain of the bovine papillomavirus E2 protein. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1826–1830. doi: 10.1073/pnas.85.6.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardulli A. M., Lew D., Erijman L., Shapiro D. J. Purified estrogen receptor DNA binding domain expressed in Escherichia coli activates transcription of an estrogen-responsive promoter in cultured cells. J Biol Chem. 1991 Dec 15;266(35):24070–24076. [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Piña B., Haché R. J., Arnemann J., Chalepakis G., Slater E. P., Beato M. Hormonal induction of transfected genes depends on DNA topology. Mol Cell Biol. 1990 Feb;10(2):625–633. doi: 10.1128/mcb.10.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponglikitmongkol M., White J. H., Chambon P. Synergistic activation of transcription by the human estrogen receptor bound to tandem responsive elements. EMBO J. 1990 Jul;9(7):2221–2231. doi: 10.1002/j.1460-2075.1990.tb07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik A., Schütz G., Stewart A. F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991 Sep;10(9):2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Salas M. A DNA curvature can substitute phage phi 29 regulatory protein p4 when acting as a transcriptional repressor. EMBO J. 1991 Nov;10(11):3429–3438. doi: 10.1002/j.1460-2075.1991.tb04907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Schreck R., Zorbas H., Winnacker E. L., Baeuerle P. A. The NF-kappa B transcription factor induces DNA bending which is modulated by its 65-kD subunit. Nucleic Acids Res. 1990 Nov 25;18(22):6497–6502. doi: 10.1093/nar/18.22.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe J. W., Neuhaus D., Rhodes D. Solution structure of the DNA-binding domain of the oestrogen receptor. Nature. 1990 Nov 29;348(6300):458–461. doi: 10.1038/348458a0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Walker P., Martinez E., Mérillat A. M., Givel F., Wahli W. Identification of estrogen-responsive DNA sequences by transient expression experiments in a human breast cancer cell line. Nucleic Acids Res. 1986 Nov 25;14(22):8755–8770. doi: 10.1093/nar/14.22.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuey D. J., Parker C. S. Bending of promoter DNA on binding of heat shock transcription factor. Nature. 1986 Oct 2;323(6087):459–461. doi: 10.1038/323459a0. [DOI] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van Weperen W. W., van der Vliet P. C. POU proteins bend DNA via the POU-specific domain. EMBO J. 1991 Oct;10(10):3007–3014. doi: 10.1002/j.1460-2075.1991.tb07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais M. L., Sentenac A. Asymmetric DNA bending induced by the yeast multifunctional factor TUF. J Biol Chem. 1989 May 25;264(15):8463–8466. [PubMed] [Google Scholar]
- Walker P., Germond J. E., Brown-Luedi M., Givel F., Wahli W. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDLII genes. Nucleic Acids Res. 1984 Nov 26;12(22):8611–8626. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. L., Adler S., Nelson C., Greene G. L., Evans R. M., Rosenfeld M. G. A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol Endocrinol. 1988 Jan;2(1):14–21. doi: 10.1210/mend-2-1-14. [DOI] [PubMed] [Google Scholar]
- Weiler I. J., Lew D., Shapiro D. J. The Xenopus laevis estrogen receptor: sequence homology with human and avian receptors and identification of multiple estrogen receptor messenger ribonucleic acids. Mol Endocrinol. 1987 May;1(5):355–362. doi: 10.1210/mend-1-5-355. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Kim J., Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989 May;3(5):606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]