Abstract
We evaluated a clinical “go/no-go” reaction time test (recognition RTclin) that is portable and does not require a computer, and used it to quantify the effect of age on recognition RTclin test scores. Fifty-two healthy adults aged 19 – 83 years completed simple and recognition RTclin testing. Simple RTclin was measured as the elapsed time from initial release of a suspended vertical shaft by the examiner until its arrest by participant pinch grip. Recognition RTclin was similar except that a light on the apparatus randomly illuminated in 50% of the trials to signal the participant to arrest the device. To help interpret the RTclin results we partitioned them into pre-movement time (PMT) and movement time (MT) using an optoelectronic camera system that is not ordinarily part of the RTclin test. Recognition RTclin scores were significantly slower than simple RTclin scores, with 71% of the prolongation attributable to PMT. While simple RTclin test scores correlated with age, recognition RTclin scores did not. A strong negative association between recognition RTclin accuracy and age was found. Recognition RTclin is feasible to measure in healthy adults and appears to represent a portable, computer-independent measure of cognitive processing speed and inhibitory capacity. Potential applications include assessment of brain injury, dementing illness, medication side-effects, fall risk, and driving safety.
Simple reaction time (RT), a measure of how quickly a person performs a uniform response to a specific stimulus, is of clinical relevance with regard to function and health. It is evident that a prolonged reaction time would influence function, for example by increasing risk for accidental falls (e.g., Lord, Clark, & Webster, 1991) and motor vehicle accidents (e.g., McKnight & McKnight, 1999). Less obvious, however, is the strong relationship between simple RT and measures of cognitive and physical health. Simple RT has been linked to intelligence (Jensen & Munro, 1979) and biomarkers such as forced expiratory volume at one second (FEV1), grip strength, visual acuity, and systolic blood pressure (Anstey, Dear, Christensen, & Jorm, 2005). Furthermore, a prolonged simple RT and greater declines in simple RT over a 7-year span have been independently associated with mortality (Shipley, Der, Taylor, & Deary, 2006, 2007).
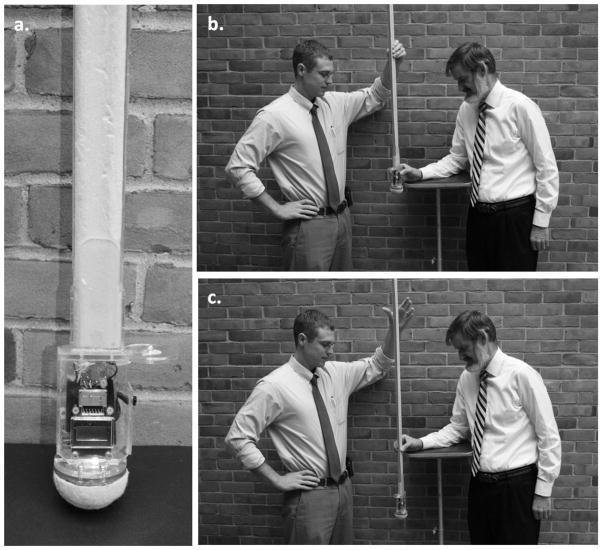
Despite the clear potential of RT as an evaluative tool, it is not commonly used by most clinicians because its measurement typically requires a computer and dedicated software (e.g., CANTAB (Cambridge Cognition Ltd, Cambridge, U.K.) or CogState (CogState Ltd, Melbourne, Australia)). In an effort to make the determination of simple RT available to all health care practitioners, we developed a clinical method of determining RT (RTclin). We defined simple RTclin as the time required to catch a suspended vertical shaft by pinch grip. The device is released at random intervals through the subject’s open hand. As soon as the subject perceives the apparatus to be falling he or she grasps it as quickly as possible (Figure 1). The fall distance is measured and the reaction time is calculated knowing the acceleration due to gravity.
Figure 1.
The clinical reaction time apparatus and testing procedure: (a) the apparatus, (b) pre- drop examiner and test subject positions, (c) post-drop examiner and test subject positions. For the purpose of this illustration, neither the subject nor testing apparatus are instrumented with optoelectronic markers. The light-emitting diode can be seen at far right in (a) as the small light colored hemisphere mounted on the circular shaped transparent plastic top cover to the finger spacer, here seen in side-view.
Previous work has found simple RTclin test scores to be reliable over a wide range of time and their interpretation valid in two distinct populations. In a healthy adult population, short term test-retest and inter-rater reliabilities were ICC=.860 and ICC=.915, respectively (Eckner, Whitacre, Kirsch, & Richardson, 2009). Furthermore, significant correlations were present between RTclin scores and those of a computerized RT measure written in E-Prime (R = .570), as well as with participant age (R = .430). A follow-up study involving collegiate athletes found test-retest reliabilities for simple RTclin test scores of ICC=.645 over a one year retest interval (Eckner, Kutcher, & Richardson, in press). The validity of RTclin test score interpretation was further supported by a second collegiate athlete study, which demonstrated a significant correlation between RTclin test scores and simple RT scores obtained during valid CogState-Sport test sessions (R=.445) (Eckner, Kutcher, & Richardson, 2010). In addition, simple RTclin test scores correlate strongly with the time required to perform a functional head-protective task (R=.725) (Eckner, Lipps, Kim, Richardson, & Ashton-Miller, 2011). Simple RTclin scores also appear to be sensitive to the effects of mild traumatic brain injury. Simple RTclin scores were 13.5% slower in nine concussed collegiate athletes tested within 72 hours of injury, as compared to each athlete’s own baseline during the preseason (Eckner, Kutcher, & Richardson, 2011).
A complex RT occurs when the stimuli and responses vary. There is evidence that complex forms of RT may be of greater clinical utility than simple RT. For example as compared to simple RT, complex RT tasks have been found to relate more strongly with cognition and mortality across the adult age range (Shipley, Der, Taylor, Deary, 2006), a measure of fluid intelligence (Thoma et al., 2006), cognitive ability (Deary, Johnson, & Starr, 2010), death from all causes (Shipley, Der, Taylor, Deary, 2007), and death from cardiovascular diseases, stroke and respiratory diseases (Shipley, Der, Taylor, & Deary, 2008). In addition simple RT is relatively unchanging until the age of 50 years, after which it increases, whereas complex RT tasks increase steadily throughout the lifespan (Der & Deary, 2006) suggesting the latter is a more sensitive marker of change. Lastly, complex RT tasks mirror the complexities of daily life more effectively than does simple RT. For example a driver may have to choose between braking and rapidly turning the wheel in response to an object in the vehicle’s path. In fact, a complex RT has been shown to be a better predictor of on-road driving than simple RT (Mathias & Lucas, 2009).
Given these apparent advantages of a complex RT we modified the simple RTclin apparatus to allow the clinical measurement of a complex RT task, referred to as “recognition RTclin.” In this condition the subject responds, or withholds response, based on the stimuli presented. The modifications to the apparatus include the addition of a light emitting diode (LED), accelerometer, digital timing circuit, and microcontroller (Figure 1a). The LED is programmed to illuminate upon the onset of acceleration after the device is dropped in 50% of trials, with the presentation of those trials being randomized. The subject is instructed to catch the device only when the LED illuminates, but to let the apparatus drop to the floor if the LED remains off.
The essential difference between recognition RTclin and simple RTclin is that the former requires the subject to recognize and interpret additional information not included in the latter, and to use this additional information to decide whether or not to catch the falling device. We anticipate that the additional central nervous system processing required during the recognition RTclin task will increase its utility for evaluating neurocognitive dysfunction. Researchers commonly divide RT into two intervals: the time required by the nervous system to interpret and process the input stimulus and plan a motor response, referred to in this manuscript as pre-movement time (PMT), and the time required to perform the response, referred to here as movement time (MT) (e.g., McMorris, Hill, Sproule, Potter, Swain, Hobson, & Holder, 2005). The reader should note that an alternative definition of “reaction time” is synonymous with “PMT,” as used in this manuscript. Using this definition, “response time” is synonymous with “reaction time,” as used in this manuscript. To distinguish PMT, which includes central processing and decision making (Sternberg 1969; Spirduso 1975), from MT, an optoelectronic camera system quantified the motions of the apparatus and the subject’s fingers. This camera and the measurement of PMT and MT do not ordinarily form part of the recognition RTclin measurement but are used here only for the purposes of testing a specific hypothesis.
The purpose of the study was to determine the feasibility of using the modified RTclin apparatus to measure simple and recognition RTclin across an age spectrum. More specifically, we tested the hypotheses: H1: Recognition RTclin test results would be prolonged as compared to simple RTclin results; H2: The majority of the recognition RTclin result prolongation would be attributable to PMT; H3: Recognition RTclin results would positively correlate with age; H4: Recognition RTclin response accuracy would negatively correlate with age.
Method
We recruited a gender-balanced sample of 52 volunteers (mean age 45.6 ± 21 years, range 19–83 years). The sample was skewed toward a younger population with 16 participants aged 18–27 years, 6 aged 28–37, 4 aged 38–47, 8 aged 48–57, 7 aged 58–67, 7 aged 68–77, and 4 participants age 78 years and above. Healthy, active adults aged 18–85 years were eligible to participate. Potential subjects were excluded if they were pregnant, had corrected vision < 20/40, or had any significant disease or injury limiting the function of their upper extremities. An additional exclusion criterion was current use of any medications known to affect reaction time. All participants provided informed written consent that was approved by the IRB at the authors’ institution prior to participation.
Simple and recognition RTclin scores were measured using an RTclin apparatus (Figure 1a) (Eckner, Lipps, et al., 2011) that was modified from an earlier simple RTclin assessment device (Eckner, et al., 2009). The device is a rigid, lightweight 117 cm elongate bi-concave shaft to the bottom of which is affixed a rectangular thumb-finger “spacer” that houses an accelerometer, timing circuit, microprocessor, and LED. A half tennis ball on the bottom of the housing dissipated energy upon ground contact. The device was programmed so that the LED randomly illuminated in green during 50% of the recognition RTclin trials. The linear accelerometer sensed the onset of device movement and instantaneously triggered the illumination of the LED.
Simple RTclin was assessed first. Participants stood with their dominant forearm resting on an adjustable table such that their hand was positioned at the edge of the surface. The examiner suspended the device vertically from its upper end such that the top of the device “spacer” was collinear in the horizontal plane with the superior-most aspect of the participant’s open first and second digits (Figure 1b). The purpose of the “spacer” was to standardize the initial distance between the participant’s thumb and fingers to be at least 25 mm. Participants were instructed to hold their thumb and fingers so they just did not touch the spacer. The examiner released the apparatus after randomly assigned delay times between 2 and 5 seconds so that participants were unable to anticipate the instant of release. As soon as the device began to fall, the participant used a pinch grip to catch the narrowest portion (measuring 10 mm) of its biconcave-sectioned handle as quickly as possible (Figure 1c). The elapsed time between the instant when, after examiner release, device downward acceleration reached 0.5g until the instant when the subject’s pinch grip slowed device acceleration to 0.5 g again was defined as the total RTclin score. Each participant completed four practice trials followed by eight data acquisition trials.
Next, recognition RTclin scores were measured using a “go/no-go” testing paradigm in a manner identical to simple RTclin, except that participants were instructed to catch the device only during trials when the green LED illuminated. For trials when the LED did not illuminate, participants were instructed to allow the device to fall to the ground. Participants were given two “go” trials during which the LED illuminated and two “no-go” trials during which the LED remained unlit to practice the recognition RTclin task. During data acquisition trials the recognition RTclin task was repeated with the LED randomly illuminating for 50% of trials over a minimum of 16 trials. Trials were then added until the participant successfully completed eight “go” trials.
The movements of the apparatus and the participant’s first and second digits were recorded in three dimensions using optoelectronic markers and an optoelectronic camera system (Certus™; Northern Digital Inc, Waterloo, ON, Canada). It should be noted that these measurements allowed RTclin scores to be divided into PMT and MT components for this study, but are not necessary for RTclin measurement. PMT was defined as the elapsed time between onset of downward device acceleration and the instant when the speed of participant digit movement reached 10 cm/s. MT was defined as the elapsed time from the onset of the participant’s digit movement response until the instant when device acceleration had decreased to 0.5g due to the pinch grip of the participant. Three-dimensional kinematic data were measured at 1 kHz at a resolution of 0.1 mm. Signal processing was performed using MATLAB™ (version 2009B; The Math Works, Natick, MA, U.S.A.).
Trials with ambiguous kinematic data that could not be interpreted were excluded from analysis. This occurred in 4.1 % (34 of 832) of trials. Mean (SD) simple and recognition RTclin values were calculated for each participant, as were the mean (SD) of the PMT and MT sub-intervals, and overall, “go”, and “no-go” recognition RTclin accuracies. Overall accuracy was defined as the number of correct responses divided by the total number of trials. The “go” and “no-go” accuracies were defined as the number of times the device was appropriately caught during “go” trials and the number of times the device was appropriately allowed to fall during “no-go” trials divided by the total number of “go” trials and “no-go” trials, respectively. Visual inspection of the raw data histograms demonstrated right-skewing, so a log transformation was applied to the raw data prior to all parametric analyses. Continuous variables were compared using paired t-tests. Proportions were used to describe the amount of change between total simple and recognition RTclin attributable to PMT. Pearson correlation coefficients were calculated to assess the relationships between continuous variables. A Bonferroni correction was applied to account for multiple comparisons. Statistical analysis was conducted using SAS™ (version 9.1; SAS Institute, Inc., Cary, NC, U.S.A.) and SPSS™ (version 16.0; SPSS Inc., Chicago, IL, U.S.A.).
Results and Discussion
Recognition RTclin test scores were significantly longer than simple RTclin scores (259 ± 23 ms vs. 184 ± 23 ms, P<.001) (H1), as were the PMT and MT sub-intervals (194 ± 26 ms vs. 141 ± 19 ms, P<.001 and 64 ± 14 ms vs. 43 ± 9 ms, P<.001, respectively). The forced choice paradigm prolonged total RTclin by proportionately lengthening both PMT and MT, with PMT accounting for 76.3% and 75.0% of total simple and recognition RTclin, respectively (P=.090). This equates to 71% of the overall prolongation in recognition RTclin scores being attributable to PMT (H2), which includes the time required for central signal processing and decision making. Age was positively associated with simple RTclin test scores (R=.500, P<.001) and their PMT sub-interval (R=.591, P<.001), but not with the corresponding recognition RTclin indices (H3) or simple MT. A weaker association was present between age and the PMT sub-interval of recognition RTclin (R=.315, P=.023) that did not reach statistical significance after Bonferroni correction.
Overall recognition RTclin accuracy was 80.4%. Participants were more successful in catching the falling device during “go” trials than they were in allowing the device to fall during “no-go” trials (91.8% vs. 68.4%, P<.001). This indicates that response inhibition during “no-go” trials was the more difficult task during the forced choice paradigm. All three recognition RTclin accuracy measures decreased with age (R=−.603, P<.001 for overall accuracy; R=- .481, P<.001 for “go” accuracy; R=−.596, P<.001 for “no-go” accuracy) (H4), with the greatest decrement in accuracy observed in participants older than 40 years of age.
This study explored the feasibility of testing a measure of complex RT using a simple and relatively rapid portable method. None of the subjects dropped out, and all were able to complete all the trials suggesting that this form of testing is feasible, at least in a healthy adult population. As hypothesized (H1) the data confirmed prolonged recognition RTclin scores compared to simple RTclin scores. This finding is consistent with prior studies (see Introduction) and thus supports the construct validity of test score interpretation for this novel method of measuring recognition RT. Also as hypothesized (H2), the majority of the recognition RTclin test score prolongation was attributable to PMT, suggesting that recognition RTclin predominantly reflects cognitive processes rather than digital movement.
As expected, and as has been the case in previous work using computerized RT assessment methods (Der & Deary, 2006), simple RTclin test scores were significantly and positively associated with age. In contrast, recognition RTclin latency was not associated with age (H3). However, recognition RTclin accuracy was highly and significantly associated with age (H4) demonstrating that as subjects’ ages increased their decision-making became less accurate. This effect was relatively strong, with age explaining over a third of the variance in error rate.
One possible explanation for these findings involves that fact that the maximal possible response latency for RTclin is the time it takes the apparatus to strike the floor after its release (about 400 ms). In contrast, computerized complex RT tests allow for a prolonged or even indefinite latency. Therefore, during recognition RTclin testing older subjects may have felt that they did not have sufficient time to make an accurate decision. In support of this, other work demonstrates that older subjects require approximately 190 ms to inhibit perceptual and motor impulses, whereas younger subjects take 35 ms (Mendelson, Redfern, Nebes, & Jennings, 2010). Given the natural tendency to catch a falling object, there is a clear need for the subjects being tested with recognition RTclin to inhibit the impulse to catch the apparatus during the “no-go” trials. When the 190 ms of inhibition time for older persons is added to the mean simple RTclin score of 185 ms the sum is nearly equal to 400 ms, at which point the apparatus will strike the floor. However, the sum of simple RTclin scores and response inhibition for younger persons is about 220 ms, which would leave sufficient time to make an accurate decision and execute a response before the apparatus strikes the floor. This idea is supported by a significant negative correlation between the PMT component of simple RTclin and recognition RTclin response accuracy (R=−.424, P=.002), suggesting that subjects who needed little time to initiate motion for simple RTclin had greater time during recognition RTclin testing to make an accurate decision.
If this reasoning is correct, then recognition RTclin accuracy may be evaluating inhibitory function. One domain of inhibitory function is “inhibition of a prepotent response (the ability to withhold the most obvious reaction to a stimulus)” (Boonstra, Kooij, Oosterlaan, Sergeant, & Buitelaar, 2010, p. 209). This appears to apply to recognition RTclin given the natural tendency to catch the device, a point underscored by the fact that “no-go” accuracy was significantly lower than “go” accuracy. Inhibitory function is of clinical relevance given that it is recognized as being fundamental to effective executive function, and in the view of some affects all other executive domains (Barkley, 1997). Accordingly, impairment of inhibition is thought to underlie cognitive deficiencies in older persons (Andres & Van der Linden, 2000), be responsible for some kinds of psychopathology (Patterson & Newman, 1993), and be one of the earliest and most prominent findings in Alzheimer’s type dementia (Collette, Schmidt, Scherrer, Adam, & Salmon, 2009). Abnormal inhibitory responses have also been noted in stimulant users and alcohol-dependent subjects (Lawrence, Luty, Bogdan, Sahakian, & Clark, 2009; Monterosso, Aron, Cordova, Xu, & London, 2005), as well as in traumatic brain injury patients (Leblanc et al., 2005; Stewart & Tannock, 1999). Moreover, recent findings suggest that inhibitory proficiency is critical to the ability of older persons to maintain balance while receiving multiple sensory inputs (Mendelson, et al., 2010). Therefore, a clinical tool that can quantify inhibitory function quickly may have clinical utility.
However, further work is needed before recognition RTclin can be applied in the clinical setting. The present study included a relatively small number of healthy subjects, and so further testing is indicated. Moreover, the investigators were not blinded and were aware of the hypotheses. This is likely rendered less relevant given that the outcomes were in the ms range and were determined using objective kinematic measurement techniques. Furthermore validation with a proven method for determining recognition RT and testing in specific populations with pathologies of clinical concern are necessary before application can be considered. In addition, recognition RTclin scores should be compared to simple RTclin scores to determine if the former provides incremental diagnostic precision or greater clinical inference and, if so, with regard to which clinical populations. Lastly, the optimal number of trials necessary to make clinically relevant distinctions should be explored.
In conclusion, this pilot study demonstrated that a novel, clinically available technique for evaluating recognition RT is feasible in a healthy population across a broad adult age range. The study also suggests that when using this technique recognition RTclin accuracy, rather than latency, is of greater importance with regard to the cognitive consequences of aging. When the results are taken in the context of previous work, they suggest that recognition RTclin may be a measure of inhibitory capacity, an internal act of control that broadly affects executive function. An inexpensive bedside or office tool that can relatively precisely measure short latency cognitive processes might have a wide range of clinical applicability.
Contributor Information
James T Eckner, Department of Physical Medicine and Rehabilitation, University of Michigan.
James K Richardson, Department of Physical Medicine and Rehabilitation, University of Michigan.
Hogene Kim, Department of Biomedical Engineering, University of Michigan.
David B Lipps, Department of Biomedical Engineering, University of Michigan.
James A Ashton-Miller, Departments of Biomedical Engineering and Mechanical Engineering, University of Michigan.
References
- Andres P, Van der Linden M. Age-related differences in supervisory attentional system functions. The journals of gerontology Series B, Psychological sciences and social sciences. 2000;55(6):P373–380. doi: 10.1093/geronb/55.6.p373. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Dear K, Christensen H, Jorm AF. Biomarkers, health, lifestyle, and demographic variables as correlates of reaction time performance in early, middle, and late adulthood. The Quarterly journal of experimental psychology A, Human experimental psychology. 2005;58(1):5–21. doi: 10.1080/02724980443000232. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Kooij JJ, Oosterlaan J, Sergeant JA, Buitelaar JK. To act or not to act, that”s the problem: primarily inhibition difficulties in adult ADHD. Neuropsychology. 2010;24(2):209–221. doi: 10.1037/a0017670. [DOI] [PubMed] [Google Scholar]
- Collette F, Schmidt C, Scherrer C, Adam S, Salmon E. Specificity of inhibitory deficits in normal aging and Alzheimer”s disease. Neurobiology of aging. 2009;30(6):875–889. doi: 10.1016/j.neurobiolaging.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychology and aging. 2010;25(1):219–228. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychology and aging. 2006;21(1):62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Eckner JT, Kutcher JS, Richardson JK. Pilot evaluation of a novel clinical test of reaction time in national collegiate athletic association division I football players. Journal of athletic training. 2010;45(4):327–332. doi: 10.4085/1062-6050-45.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner JT, Kutcher JS, Richardson JK. Effect of concussion on clinically measured reaction time in nine NCAA Division I collegiate athletes: a preliminary study. PM & R: the journal of injury, function, and rehabilitation. 2011;3:212–218. doi: 10.1016/j.pmrj.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner JT, Kutcher JS, Richardson JK. Between season test-retest reliability of clinically measured reaction time in NCAA Division I collegiate athletes. Journal of athletic training. doi: 10.4085/1062-6050-46.4.409. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner JT, Lipps DB, Kim H, Richardson JK, Ashton-Miller JA. Can a clinical test of reaction time predict a functional head-protective response? Medicine and science in sports and exercise. 2011;43(3):382–387. doi: 10.1249/MSS.0b013e3181f1cc51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner JT, Whitacre RD, Kirsch N, Richardson JK. Evaluating a clinical measure of reaction time: An observational study. Perceptual and motor skills. 2009;108:717–720. doi: 10.2466/PMS.108.3.717-720. [DOI] [PubMed] [Google Scholar]
- Jensen A, Munro E. Reaction time, movement time and intelligence. Intelligence. 1979;3(2):121–126. [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207(1):163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N, Chen S, Swank PR, Ewing-Cobbs L, Barnes M, Dennis M, et al. Response inhibition after traumatic brain injury (TBI) in children: impairment and recovery. Developmental neuropsychology. 2005;28(3):829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. Journal of the American Geriatrics Society. 1991;39(12):1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Lucas LK. Cognitive predictors of unsafe driving in older drivers: a meta-analysis. International psychogeriatrics. 2009;21(4):637–653. doi: 10.1017/S1041610209009119. [DOI] [PubMed] [Google Scholar]
- McKnight AJ, McKnight AS. Multivariate analysis fo age-related driver ability and performance deficits. Accident Analysis and Prevention. 1999;31:445–454. doi: 10.1016/s0001-4575(98)00082-7. [DOI] [PubMed] [Google Scholar]
- McMorris T, Hill C, Sproule J, Potter J, Swain J, Hobson G, Holder T. Supra-maximal effort and reaction and movement times in a non-compatible response time task. Journal of Sports Medicine and Physial Fitness. 2005;45(1):127–133. [PubMed] [Google Scholar]
- Mendelson DN, Redfern MS, Nebes RD, Jennings JR. Inhibitory processes relate differently to balance/reaction time dual tasks in young and older adults. Neuropsychology, development, and cognition Section B, Aging, neuropsychology and cognition. 2010;17(1):1–18. doi: 10.1080/13825580902914040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and alcohol dependence. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: toward a psychological mechanism for the syndromes of disinhibition. Psychological review. 1993;100(4):716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and all-cause mortality across the entire adult age range: health and lifestyle survey. Psychosomatic medicine. 2006;68(1):17–24. doi: 10.1097/01.psy.0000195867.66643.0f. [DOI] [PubMed] [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Association between mortality and cognitive change over 7 years in a large representative sample of UK residents. Psychosomatic medicine. 2007;69(7):640–650. doi: 10.1097/PSY.0b013e31814c3e7c. [DOI] [PubMed] [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and mortality from the major causes of death: the Health and Lifestyle Survey. Journal of psychosomatic research. 2008;65(2):143–152. doi: 10.1016/j.jpsychores.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Spirduso WW. Reaction and movement time as a function of age and physical activity level. Journal of Gerontology. 1975;30(4):435–440. doi: 10.1093/geronj/30.4.435. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: Mental processes revealed by reaction time experiments. American Scientist. 1969;57(4):421–457. [PubMed] [Google Scholar]
- Stewart JA, Tannock R. Inhibitory control differences following mild head injury. Brain and cognition. 1999;41(3):411–416. doi: 10.1006/brcg.1999.1141. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Yeo RA, Gangestad S, Halgren E, Davis J, Paulson KM, et al. Developmental instability and the neural dynamics of the speed-intelligence relationship. Neuroimage. 2006;32(3):1456–1464. doi: 10.1016/j.neuroimage.2006.05.016. [DOI] [PubMed] [Google Scholar]