Abstract
Objective
Autopsy and biopsy studies have shown that there is significantly more fibrosis in hearts of patients with hypertensive heart disease compared to normal hearts. Fibrocytes, a population of circulating bone marrow-derived cells, have been shown to home to tissues and promote scar formation in several diseases, but their role in human hypertensive heart disease has not been investigated to date. Our objective was to determine whether fibrocyte levels are elevated in individuals with hypertensive heart disease.
Methods
We measured peripheral blood fibrocyte levels and their activated phenotypes in 12 individuals with hypertensive heart disease as determined by increased left ventricular mass on noninvasive imaging and compared them to fibrocyte levels from 19 healthy normal controls and correlated them to cardiac MRI findings.
Results
Compared to normal controls, individuals with hypertensive heart disease had significantly higher circulating levels of total fibrocytes [median (interquartile range); 149000 (62200–220000) vs. 564500 (321000–1.2900e+006), P < 0.0001, respectively] as well as activated fibrocytes [15700 (6380–19800) vs. 478500 (116500–1.2360e+006) P < 0.0001]. Moreover, the fibrocyte subsets expressing the chemokine markers CXCR4 (P < 0.0001), CCR2 (P < 0.0001), CCR7 (P < 0.0001) and coexpression of both CXCR4 and CCR2 (P < 0.0001) were significantly elevated in patients with hypertensive heart disease compared to controls. Lastly, in patients with hypertensive heart disease there was a strong correlation between left ventricular mass index and total fibrocytes (r = 0.65, P = 0.037) and activated fibrocytes (r = 0.70, P = 0.016).
Conclusion
Our data suggest that bone marrow-derived circulating fibrocytes are associated with the presence and extent of left ventricular hypertrophy in patients with hypertensive heart disease.
Keywords: cardiac MRI, fibrocyte, fibrosis, hypertensive heart disease, left ventricular hypertrophy
INTRODUCTION
Hypertensive heart disease (HHD), defined as the presence of left ventricular hypertrophy (LVH), occurs in a third of individuals with hypertension and is among the most common causes of death and morbidity from hypertension [1]. Importantly, HHD has an unpredictable course and can progress despite optimal blood pressure control [2]. The pathogenesis of HHD involves remodeling of the myocardium with fibrosis of the muscle and perivascular space, medial hypertrophy of intramyocardial coronary vasculature and cardiomyocyte hypertrophy [3,4]. The mechanisms responsible for the development, progression and pattern of LVH are incompletely defined but include the severity and duration of hypertension, effects of growth factors, cytokines and neurohormones and genetic predisposition [5,6].
Autopsy studies indicate 30% more collagen in the hearts of hypertensive individuals as compared to normal hearts [7,8], with intense perivascular and interstitial remodeling and deposition of extracellular matrix [9,10]. Myocardial fibrosis predisposes patients to diastolic and systolic dysfunction, myocardial ischemia and atrial and ventricular arrhythmias [11]. Understanding the factors that regulate the fibrotic process within the myocardium is necessary for developing new treatment strategies for HHD.
Traditionally, resident cardiac fibroblasts were thought to be activated by proinflammatory processes to proliferate and synthesize collagen that is secreted and deposited in the interstitial space [12]. However, recent studies describe an important role for circulating bone marrow-derived cells in both physiological and pathological fibrosis in diverse diseases. These cells, referred to as fibrocytes, home from the bone marrow to the blood and then to injured tissues, differentiate into fibroblasts and myofibroblasts and contribute to fibrosis [13–15]. Fibrocytes have been implicated in physiologic wound healing [16] as well as in many fibrotic diseases, including pulmonary fibrosis [17–20], mouse models of renal fibrosis [21], myocardial infarction [22,23] and cardiomyopathy [24].
Fibrocytes express surface markers of leukocytes and hematopoetic stem cells but also collagen-I [16,25,26]. When cultured in vitro without cytokines or growth factors, isolated peripheral blood fibrocytes retain expression of collagen-1 and gain expression of the myofibroblast marker α-smooth muscle actin (SMA), consistent with differentiation into fibroblasts and myofibroblasts [25–28], the primary effector cells in the pathogenesis of tissue fibrosis [29,30]. Because chemokines are critical to the homing of fibrocytes to tissue, and prior literature have shown that subsets of human fibrocytes express the chemokine receptors CCR2, CCR7 and CXCR4 [18,26,31], we measured circulating fibrocytes expressing these receptors as well as the activated α-SMA fibrocyte subset.
Data on the role of fibrocytes in HHD is limited to mouse models [32,33]. We hypothesized that circulating fibrocyte levels are elevated in patients with HHD compared to healthy controls. To this end, we performed a pilot study to examine the association of circulating fibrocytes and their activated phenotypes with left ventricular mass as determined by cardiac MRI (CMR), the gold standard for evaluating left ventricular mass [34], in a cohort of patients with HHD.
METHODS
Study participants
Study participants were recruited from the echocardiography laboratory, cardiac catheterization laboratory and cardiology clinics at the University of Virginia. The project was approved by the University of Virginia Institutional Review Board, and participants gave informed consent. For patients with HHD, the inclusion criteria was age older than 18 years with a history of hypertension and evidence of LVH on 12-lead electrocardiogram or echocardiography or CMR. Exclusion criteria were estimated glomerular filtration rate less than 45 ml/min per 1.73 m2 based on a serum creatinine drawn within 30 days of the CMR; acute kidney injury; history of paraproteinemia syndrome; hepatorenal syndrome; liver transplantation; metallic implants including pacemakers and defibrillators, cerebral aneurysm clips, and cochlear implants; severe claustrophobia; inability to provide informed consent; presence of acute myocardial infarction or acute coronary syndrome; SBP less than 90 mmHg; pregnancy; severe congestive heart failure (inability to lie flat) and diagnosis of a known fibrotic disease including interstitial lung disease, cirrhosis, scleroderma and myxomatous mitral valve degeneration. Control individuals were healthy volunteers older than 18 years of age without a known history of hypertension or other comorbidities. Between 1 November 2010, and 1 August 2011, 27 volunteers were recruited.
Assessment of left ventricular hypertrophy
Two-dimensional echocardiography
For screening purposes, a two-dimensional echocardiogram was performed and using a standard parasternal long axis view, two-dimensional-guided left ventricular mass was measured based on the Penn criteria [35]. The presence of LVH on echocardiography was defined as left ventricular mass more than 150 g, or more than 88 g/m2 in women, and left ventricular mass more than 200 g, or more than 102 g/m2 in men. Participants in whom the presence of LVH was verified on echocardiography underwent CMR for further evaluation of left ventricular mass, volumes, function and presence of focal fibrosis.
Cardiac MRI
After peripheral intravenous access was obtained and ECG leads for gating and monitoring were placed, the participant was placed in the center of the magnet with a phased array surface coil overlying the chest. Scout imaging was performed to localize the heart, followed by images of heart morphology and function. Left ventricular volumes and function were determined using steady state free precession cine imaging with the following sequence parameters: repetition time (TR) 2.7 ms, echo time (TE) 1.3 ms, flip angle 73°, field of view (FOV) 300–350 mm and resolution 1.8 × 1.4 × 8.0 mm. The temporal resolution of the image acquisition depended on underlying heart rate but ranged from 40–50 ms. A stack of short axis images that were 8 mm thick with 2 mm gap covered the left ventricle from apex to base. Three long-axis images were obtained (four-chamber, two-chamber and three-chamber). Analyses were performed by an observer blinded to patient fibrocyte data (R.J.) using Argus software (Siemens Healthcare, Princeton, New Jersey, USA) on a Leonardo workstation (Siemens Healthcare, Erlangen, Germany). End-diastolic and end-systolic endocardial and epicardial cavity areas were semiautomatically planimetered for each short-axis slice with care taken to exclude papillary muscles and trabeculae from the endocardial contours and the total left ventricular mass, end-diastolic volume, end-systolic volume, stroke volume and cardiac output was calculated for each individual and indexed for body surface area at each time point. Late gadolinium enhanced (LGE) images were obtained 10–15 min after injection of 0.15 mmol/kg using a phase-sensitive inversion recovery pulse sequence with the following sequence parameters: TR 7.1 ms, TE 3.4 ms, flip angle 25°, FOV 300–340 mm, resolution 1.8 × 1.3 × 8 mm, inversion time adjusted to null normal myocardium). The images were visually analyzed for the presence of focal LGE.
Measurement of circulating fibrocytes
After informed consent was obtained and before the CMR was performed, 10 ml of heparinized venous blood was obtained for the measurement of circulating fibrocytes. Quantitative fluorescence-activated cell sorting (FACS) analysis for fibrocytes (defined as CD45+Col1+ cells), α-SMA+ cells(CD45+ α-SMA+Col1+), and fibrocytes expressing chemokines receptors (CXCR4, CCR2, CCR7 and in combination) were measured as described [19,25,36]. All antibodies were purchased from BD Biosciences (San Jose, California, USA) except anti-CCR2 PerCP, anti α-SMA PE (R&D Systems, Minneapolis, Minnesota, USA), and anti-Collagen 1 (Col1) (Rockland, Gilbertsville, Pennsylvania, USA). All the antibodies were purchased conjugated except anti-Col1. Anti-Col1 or control antibody was conjugated to fluorescein isothiocyanate using DyLight conjugation kits (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Using the manufacturer’s instruction cells were processed as previously described [19,25,36] and read on a FACS Canto II flow cytometer using BD Diva software (San Jose, California, USA).
Statistical methods
Data were analyzed on a Macintosh computer using PRISM statistical software (Version 4.0a, GraphPad Software, Inc. La Jolla, California, USA). Comparisons between healthy controls and patients with HHD were performed with the unpaired two-tailed Mann–Whitney (nonparametric) test. In patients with HHD, we assessed whether there was a correlation between the total concentration of circulating fibrocytes and activated fibrocytes with left ventricular mass indexed to body surface area. Correlation coefficient (r) was calculated using Spearman (nonparametric) correlation. Probability values (two-sided) were considered statistically significant if they were less than 0.05.
RESULTS
Baseline characteristics of the 19 healthy controls and the 12 patients with HHD including their medications and CMR variables are listed in Table 1. More than half of the patients with HHD were taking an antihypertensive medical regimen that included angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker and a diuretic.
TABLE 1.
Baseline characteristics study participants
| Variable | Hypertensive heart disease (n = 12) |
Healthy controls (n =19) |
P |
|---|---|---|---|
| Demographic characteristics | |||
| Age, year ± SD | 52 ± 16 | 41 ± 4 | 0.043 |
| Sex, n (%) | |||
| Women | 8 (67%) | 17 (89%) | 0.174 |
| Race | |||
| African American | 11 (92%) | 19 (100%) | 0.387 |
| SBP (mmHg) | 151 ± 14 | – | |
| DBP (mmHg) | 82 ± 12 | – | |
| Hypertension, n (%) | 12 (100%) | 0 | |
| Diabetes mellitus, n (%) | 2 (17%) | 0 | |
| Hyperlipidemia, n (%) | 7 (58%) | 0 | |
| Coronary artery disease, n (%) | 1 (8%) | 0 | |
| Medications | |||
| ACE-I/ARB, n (%) | 8 (66%) | 0 | |
| B-blocker, n (%) | 4 (33%) | 0 | |
| Calcium channel blocker, n (%) | 6 (50%) | 0 | |
| Diuretic, n (%) | 7 (58%) | 0 | |
| Statin, n (%) | 6 (50%) | 0 | |
| CMR variables | |||
| Left ventricular ejection fraction | 60.7 ± 12.9 | – | |
| End diastolic volume index | 60.2 ± 18.7 | – | |
| End systolic volume index | 24.3 ± 14.9 | – | |
| Left ventricular mass index | 76.6 ± 18.8 | – | |
| Stroke volume index | 35.7 ± 12.6 | – | |
| Cardiac index | 2.52 ± 0.74 | – | |
ACE-I, angiotensin converting-enzyme inhibitor; ARB, angiotensin receptor blocker; CMR, cardiac MRI. Values are mean ± SD, percentage (%).
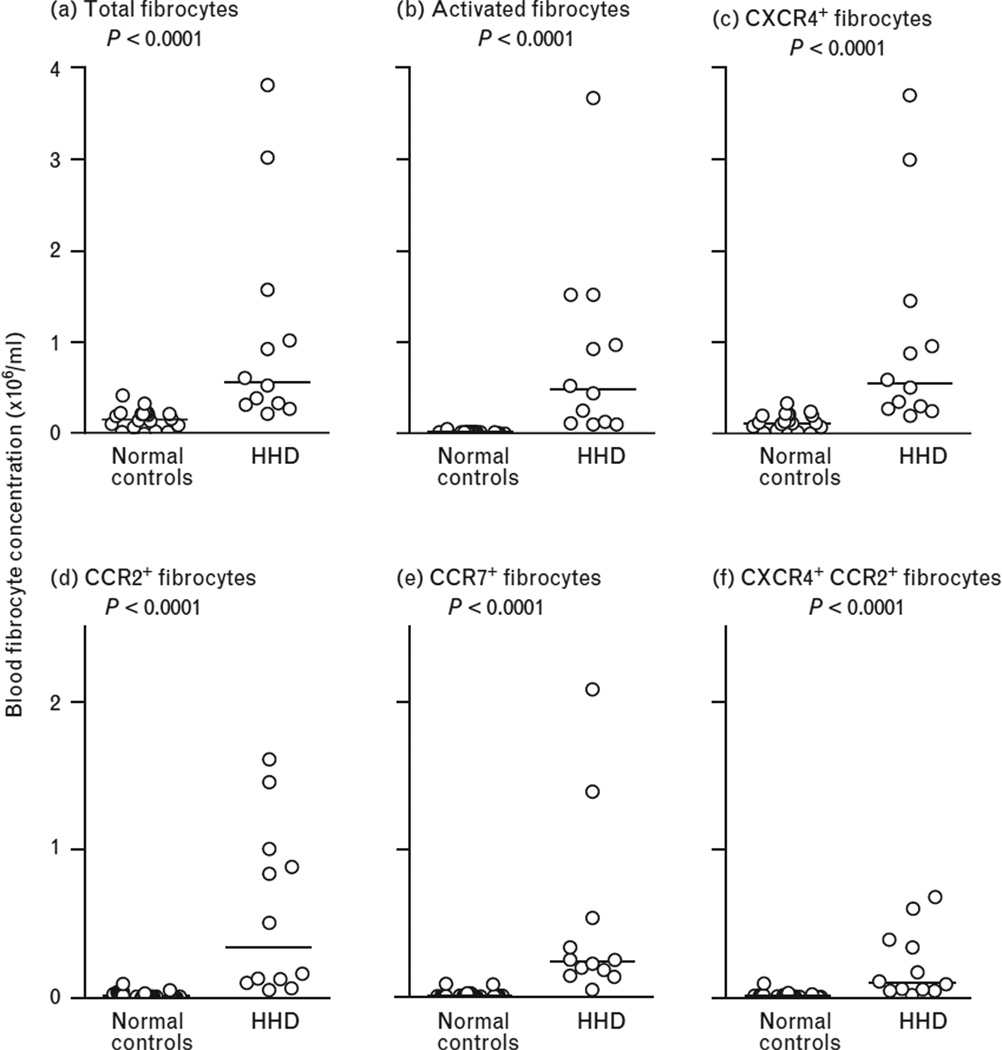
Compared to normal controls, patients with HHD had significantly higher absolute concentration of total fibrocytes (CD 45+Col1+ cells in the peripheral blood) [median (interquartile range)] [149000 (62200–220000) vs. 564500 (321000–1.2900e+006), P < 0.0001, respectively] (Fig. 1, panel A). In addition, the absolute concentration of activated fibrocytes (CD45+Col1+ α-SMA+) was also higher [15700 (6380–19800) vs. 478500 (116500–1.2360e+006) P < 0.0001], with median values that were 30-fold greater than in normal individuals (Fig. 1, panel B). Chemokines are critical to homing of blood fibrocytes to fibrotic tissues, and the chemokine receptors CCR2, CXCR4 and CCR7 have been implicated in fibrocyte trafficking in prior studies [15]. We, therefore, compared the concentration of the subsets of blood fibrocytes that express these receptors in patients with HHD and normal controls. As found in prior literature [18], most fibrocytes in both HHD and control individuals expressed CXCR4, with smaller subsets expressing CCR2 and CCR7; there was a small subset of fibrocytes that coexpressed CCR2 and CXCR4. We found that, compared to normal controls, there was a statistically significant increase in CD45+Col1+CXCR4+ fibrocytes [114000 (53300–200000) vs. 548000 (288500–1.2015e+006), P < 0.0001] (Fig. 1, panel C); CD45+Col1+CCR2+ fibrocytes [16800 (8890–26400) vs. 340000 (114000–948500) P < 0.0001], (Fig. 1, panel D); CD45+Col1+CCR7+ fibrocytes [11800 (6400–21300) vs. 240000 (166500–438000) P < 0.0001] (Fig. 1, panel E) and CD45+Col1+CXCR4+CCR2+ fibrocytes [11100 (4440–20000) vs. 99850 (51150–364000) P < 0.0001] (Fig. 1, panel F) in patients with HHD.
FIGURE 1.
Median values of circulating blood fibrocyte concentrations (× 106/ml) in normal controls compared to individuals with hypertensive heart disease (HHD). Panel (a) shows total fibrocytes (P < 0.0001). Panel (b) shows activated fibrocytes (P < 0.0001). Panel (c) shows CXCR4+ fibrocytes (P < 0.0001). Panel (d) shows CCR2+ fibrocytes (P < 0.0001). Panel (e) shows CCR7+ fibrocytes (P < 0.0001). Panel (f) shows CXCR4+CCR2+ fibrocytes (P < 0.001).
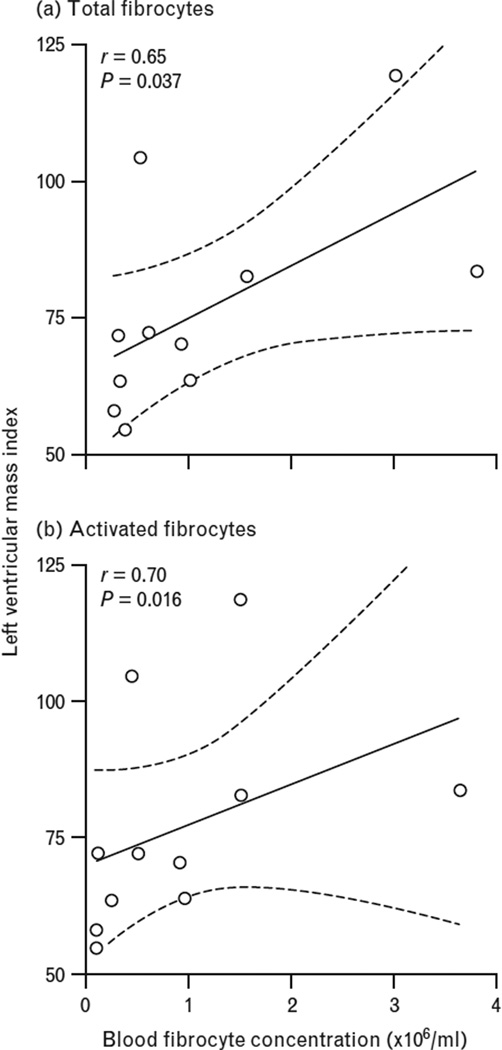
Eleven of the 12 patients with HHD underwent CMR imaging (one patient did not due to claustrophobia); none had focal LGE by visual assessment. We found that both the total fibrocyte concentration and that of the activated subset significantly correlated with left ventricular mass indexed to body surface area: total (CD45+Col1+) fibrocytes (r = 0.65, P = 0.037) (Fig. 2, panel a), and the activated subset (CD45+Col1+ α-SMA+) (r = 0.70, P = 0.016) (Fig. 2, panel B).
FIGURE 2.
Correlation coefficient with 95% confidence intervals of left ventricular mass index (g/m2) as assessed by cardiac MRI with blood fibrocyte concentrations (× 106/ml) in patients with hypertensive heart disease. Panel (a) shows total fibrocytes (r = 0.65, P = 0.037). Panel (b) shows activated fibrocytes (r = 0.70, P = 0.016).
DISCUSSION
The main findings of our study is that compared to healthy controls circulating fibrocyte levels are elevated in the plasma of patients with HHD, and that in these individuals, increased fibrocyte levels correlate with increased left ventricular mass.
Previous studies have shown that fibrocytes comprise 0.1–0.5% of nucleated cells in the peripheral blood of healthy individuals [25,26,37], and that in patients with fibrotic diseases, the ratio of fibrocytes in the peripheral blood compared to healthy individuals has been shown to increase 5–10% [19,20] and correlate with disease activity and mortality [17,19,20]. In addition to finding a similar increase in the absolute concentration of total fibrocytes, we found a marked increase in the activated subset of fibrocytes in patients with HHD. The activated fibrocytes, identified on the basis of expression of α-SMA, are thought to have acquired the myofibroblast phenotype; tissue myofibroblasts deposit extracellular matrix including collagen and are critical to driving the fibrotic process. In contrast to normal controls, we found that a large proportion of circulating fibrocytes in patients with HHD had the activated phenotype, supporting the notion that these cells are relevant to the pathogenesis of HHD.
Chemokines are critical to the homing of fibrocytes to tissue, and prior literature has shown that subsets of human fibrocytes express the chemokine receptors CCR2, CCR7 and CXCR4 [18,26,31]. As noted above, most human and mouse fibrocytes express CXCR4, with smaller subsets expressing the other receptors. The contribution of specific chemokine receptors to tissue infiltration of fibrocytes differs in different diseases and may differ between mouse models and human disease. Although there have been no published reports in human HHD, in a mouse model of arterial hypertension, CCR2 and one of its ligands, CCL2, have been implicated in the development of cardiac fibrosis via a fibrocyte-oriented mechanism; in this study, the absence of CCR2 prevented angiotensin II-induced cardiac fibrosis through suppression of fibrocyte infiltration into the heart [33]. Moreover, CCR7, CXCR4 and CCL21 (a ligand of CCR2 in mice but not humans) have been implicated in a renal fibrosis model [21]. In our study we found all subsets of human fibrocytes expressing chemokine receptors to be elevated in patients with HHD compared to healthy controls, but the difference was most marked between the CXCR4+ subset.
Our study has several limitations. First, the correlative findings reported here are consistent with, but do not constitute evidence of, a causal relationship between elevated blood fibrocytes and development of HHD. Second, our sample size was small, bringing into question the generalizability of our findings. On the contrary, the very small P values and correlation with left ventricular mass indexed to body surface area using the gold standard in assessing left ventricular mass provided precise measures with minimal variation and make type I error less likely. Third, more than half the patients with HHD were taking antihypertensive medications that may have influenced fibrocyte levels. Fourth, elevated fibrocyte levels associated with other disease processes may impact on the specificity of fibrocytes as a reliable biomarker of HHD. Fifth, although the healthy controls did not have a known history of hypertension, blood pressure and CMR measurements were not performed. Lastly, although fibrosis is an important component of hypertensive LVH, our CMR measures reflect overall left ventricular mass and not just the fibrotic component. T1 mapping techniques to measure diffuse fibrosis in hypertensive LVH are actively being developed and tested by our group and others [38].
In conclusion, our results suggest that bone marrow-derived circulating fibrocytes and their activated subsets are associated with hypertensive LVH. If validated in larger studies of patients with HHD undergoing CMR, fibrocyte levels may serve as a novel biomarker of cardiac fibrosis. As in animal models elevation of blood fibrocytes temporally precedes detectable tissue fibrosis, this may represent a potentially useful clinical application. In addition, compared to traditional biomarkers that indirectly represent the pathophysiologic process, fibrocytes may directly contribute to cardiac fibrosis and the abnormal myocardial mechanics seen in HHD. Additional studies are needed to more fully characterize the potential role of fibrocytes in human HHD as a simple blood test that can predict the development of fibrosis and/or its progression over time, thus leading to a paradigm shift in the treatment of HHD.
Reviewers’ Summary Evaluations.
Referee 1
The study has shown that circulating fibrocytes and myofibroblasts are increased in patients with hypertensive heart disease (HHD) and correlate with the extent of the indexed left ventricular mass. Although several limitations of the study are acknowledged by the authors, the results raise many important questions: Is myocardial fibrosis the result of bone marrow activation? Is the increased level of circulating fibrocytes a predecessor of myocardial fibrosis? Should the hypothesis of a predominant role of the renin–angiotensin system in fibrosis development be re-considered? Is bone marrow a possible new therapeutic target in patients with hypertension and HHD? If the presented data are confirmed, heart-bone marrow interactions will deserve to be investigated further.
Referee 2
The paper by Keeley et al. supports the view that peripheral fibrocyte levels are markedly increased in patients with hypertensive heart disease, as assessed by MRI, in comparison to normotensive controls. Furthermore, the authors report a strong linear correlation between circulating fibrocytes and left ventricular mass index. Since these results are obtained in a very small selected sample of middle-aged hypertensive patients without co-morbidities associated with a marked activation of circulating fibrocytes (such as fibrotic lung disease, asthma, pulmonary hypertension and systemic collagen disorders) they cannot be extrapolated to the general hypertensive population. Thus, future larger investigations are needed to explore the clinical role of circulating fibrocytes as a reliable marker of hypertensive heart disease.
ACKNOWLEDGEMENTS
Funding: This work was supported by the National Institutes of Health (HL097074 to E.C.K., HL08710 and HL098526 to J.J.F., HL098526 and HL098329 to B.M., and AHA Grant-in-Aid 11GRNT7370022 to C.M.K.).
Abbreviations
- CMR
cardiac MRI
- HHD
hypertensive heart disease
- LGE
late gadolinium enhanced
- LVH
left ventricular hypertrophy
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics: 2011 update – a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 3.Diez J, Gonzalez A, Lopez B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216. doi: 10.1038/ncpcardio0158. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 5.Diez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1–8. doi: 10.1161/HYPERTENSIONAHA.109.141887. [DOI] [PubMed] [Google Scholar]
- 6.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 7.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J. 1986;55:575–581. doi: 10.1136/hrt.55.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huysman JA, Vliegen HW, Van der Laarse A, Eulderink F. Changes in nonmyocyte tissue composition associated with pressure overload of hypertrophic human hearts. Pathol Res Pract. 1989;184:577–581. doi: 10.1016/S0344-0338(89)80162-1. [DOI] [PubMed] [Google Scholar]
- 10.Pearlman ES, Weber KT, Janicki JS, Pietra GG, Fishman AP. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Invest. 1982;46:158–164. [PubMed] [Google Scholar]
- 11.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens. 1990;3:735–740. doi: 10.1093/ajh/3.10.735. [DOI] [PubMed] [Google Scholar]
- 12.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeley EC, Mehrad B, Strieter RM. The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair. 2011;4:2. doi: 10.1186/1755-1536-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010;42:535–542. doi: 10.1016/j.biocel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost. 2009;101:613–618. [PMC free article] [PubMed] [Google Scholar]
- 16.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 20.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 21.Sakai N, Wada T, Matsushima K, Bucala R, Iwai M, Horiuchi M, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 22.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 24.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan H, Abraham A, Phillips JR, Pringle JH, Walker RA, Jones JL. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003;56:271–276. doi: 10.1136/jcp.56.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 29.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 31.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 32.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301:H538–H547. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janardhanan R, Kramer CM. Imaging in hypertensive heart disease. Expert Rev Cardiovasc Ther. 2011;9:199–209. doi: 10.1586/erc.10.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 36.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 37.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]