Abstract
Rett syndrome (RTT), caused by mutations in the methyl-CpG binding protein 2 gene (MECP2), is a debilitating autism spectrum developmental disorder predominantly affecting females. Mecp2 mutant mice have reduced levels of brain-derived neurotrophic factor (BDNF) in the brain; conditional deletion and overexpression of BDNF in the brain accelerates and slows, respectively, disease progression in Mecp2 mutant mice. Thus we tested the hypothesis that 7,8-dihydroxyflavone (7,8-DHF), a small molecule reported to activate the high affinity BDNF receptor (TrkB) in the CNS, would attenuate disease progression in Mecp2 mutant mice. Following weaning, 7,8-DHF was administered in drinking water throughout life. Treated mutant mice lived significantly longer compared with untreated mutant littermates (80 ± 4 and 66 ± 2 days, respectively). 7,8-DHF delayed body weight loss, increased neuronal nuclei size and enhanced voluntary locomotor (running wheel) distance in Mecp2 mutant mice. In addition, administration of 7,8-DHF partially improved breathing pattern irregularities and returned tidal volumes to near wild-type levels. Thus although the specific mechanisms are not completely known, 7,8-DHF appears to reduce disease symptoms in Mecp2 mutant mice and may have potential as a therapeutic treatment for RTT patients.
Keywords: Mecp2 mutant mice, irregular respiratory pattern, Trkb, tidal volume
rett syndrome (RTT) is a debilitating autism spectrum disorder that predominantly affects females (14, 15). RTT patients have normal birth and continue developing normally for ∼6 mo, but suffer a rapid regression thereafter (24). Major symptoms of RTT include a small brain, autistic features, loss of expressive language, stereotypic hand movement, motor function deterioration, and respiratory distress, including profound ventilatory instability during wakefulness with milder irregularities occurring during the night (6, 33).
Mutations in the methyl-CpG binding protein 2 (MECP2) gene have been identified as the cause of classic RTT (1, 27, 31, 34). MeCP2 binds to methyl-DNA to modulate chromatin structure and gene transcription (4). Shortly after the identification of MECP2 as the disease gene, mouse models of RTT were generated by engineering Mecp2 gene deletions (9, 13, 25). Male Mecp2 mutant mice that lack the essential methyl-DNA binding domain recapitulate several key pathological features of RTT patients, including reduced brain weight, hindlimb clasping, impaired locomotor function (9), and respiratory abnormalities (19), and are frequently used to study the pathogenesis of RTT attributable to the reproducibility and timeliness (death within 8–10 wk of age) of these clinical signs.
Brain-derived neurotrophic factor (BDNF) was first implicated in RTT when MeCP2 was found to directly bind to the Bdnf promoter in cultured neurons (10). A subsequent in vivo study (8) demonstrated that BDNF protein levels are reduced in Mecp2 mutant brains, and certain phenotypic similarities exist between the Mecp2 mutants and Bdnf conditional mutant mice, suggesting that BDNF deficiency may mediate some RTT-like symptoms. Moreover, complete Bdnf deletion from the mutant brain exacerbates disease phenotypes, whereas BDNF overexpression in the Mecp2 mutant brain reverses the disease phenotypes (8). Several additional studies (21, 22, 32) further link decreased BDNF expression with functional deficits in Mecp2 mutant mice and demonstrate that these defects could be ameliorated by restoring normal BDNF levels. However, the therapeutic utility of BDNF is limited because of problems with delivering proteins to the central nervous system (23). Hence, small molecule ligands of the high affinity BDNF receptor, TrkB, have significantly greater therapeutic potential (20).
7,8-DHF is reported to be a TrkB agonist that potentially crosses the blood-brain barrier after oral administration (18). Because 7,8-DHF is reported to mimic certain BDNF functions in learning and memory (3, 11), we tested the hypothesis that 7,8-DHF would also attenuate disease symptoms in a RTT mouse model (9), similar to that seen with BDNF overexpression (8). Although the precise mechanisms remain unclear, our data suggest that continuous 7,8-DHF administration in drinking water significantly extends life span, delays body mass loss, increases the size of neuronal nuclei, enhances voluntary wheel running, and partially improves irregular breathing patterns in Mecp2 mutant mice.
MATERIALS AND METHODS
Animals, 7,8-DHF administration, and body mass measurement.
All procedures were performed in accordance with the University of Wisconsin's Animal Care and Use Committee. The Mecp2 mutant mice (MT) and their wild-type littermates (WT) were maintained in the C57BL/6 background (Mecp2tm1.1/Jae) and genotyped as previously described (9). On the basis of the preliminary dose-response studies, 7,8-DHF was first dissolved in DMSO to generate a stock solution at the concentration of 100 mg/ml. Stock solution (80 μl) was added to 100 ml of Hydropac water containing 1% of sucrose (pH = 7.4), and given to mice as drinking water. The 7,8-DHF containing drinking water was replaced every 3–4 days. Mice were monitored daily, and body mass was recorded every 3–4 days. The water bottle was weighed every 3–4 days to monitor water intake. Four groups of mice were studied: mutants with either 0 or 80 mg/l 7,8-DHF in their drinking water (MT0, MT80) and wild-type mice with either 0 or 80 mg/l 7,8-DHF in their drinking water (WT0, WT80). Survival curves were generated, and a log rank test (16) was performed to assess the statistical significance in the difference between the curves. A two-way ANOVA was performed to compare body mass between groups (WT0, WT80, MT0, MT80) at each week (week 0/weaning, 1, 2, 3, and 4 wk of treatment). Although every attempt was made to randomize investigations in mutant and wild-type mice, parts of the studies were not blinded because the Mecp2 mutant mice can be easily distinguished from the wild type mice because of their obvious phenotypes. However, the investigators conducting the running wheel assay and measuring the neuronal nuclei size did not have knowledge of the genotypes of the mice or the brain sections. When plethysmography recordings were performed, the investigators were not blinded so that mice could be randomly assigned to different plethysmography chambers.
Neuronal nuclei size measurement in the CA1 region of the hippocampus.
For CA1 neuronal nuclei size measurement, brains were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), postfixed in 4% paraformaldehyde overnight at 4°C, washed in PBS, processed by a TISSUE-TEK VIP machine (MilesCo Scientific, Princeton, MN), and embedded in paraffin. Serial coronal sections (5 μm) were taken through the whole brain. Sections of the comparable rostral-caudal position were stained with cresyl violet. Digital pictures were taken for the CA1 region of the hippocampus. The nuclei area (μm2) of projection neurons was determined by tracing the outline of the nuclei, using ImageJ (NIH, Bethesda, MD). At least 20 neurons per image were analyzed. At least two independent images were scored per mouse. Thus 177 neurons from 4 mice (at least 40 neurons per mouse) in the WT0 group, 126 neurons from 3 mice in the WT80 group, 305 neurons from 7 mice (at least 40 neurons per mouse) in the MT0 group, and 298 neurons from 7 mice (at least 40 neurons per mouse) in the MT80 group were included in the double-blind analysis. Nuclear sizes between groups were compared using a one-way ANOVA.
Voluntary running wheel activity.
At 8 wk of age (∼4 wk following treatment start), total spontaneous wheel running distance over a 24-h period was recorded in a wheel running cage connected with a Mini-counter (Mini Mitter, Bend, OR) in each experimental group (WT0: n = 4; MT0: n = 10; WT80: n = 3; MT80: n = 10). Wheel circumference was 72 cm/revolution. Distances were compared between groups with a one-way ANOVA.
Plethysmography recording and data analysis.
At 6 wk of age, ventilatory responses were assessed in unanesthetized, unrestrained Mecp2 mutant mice and their wild-type littermates administered either 0 mg/l (WT0, MT0) or 80 mg/l 7,8-DHF (WT80, MT80) in their drinking water using a whole body flow through plethysmograph (BUXCO Electronics, Troy, NY). Thus, four groups of mice were studied: WT0 (n = 9), WT80 (n = 6), MT0 (n = 12), and MT80 (n = 8). Whole body plethysmography allows for quantitative measurement of ventilation in unanesthetized, free-behaving animals while simultaneously controlling inspired gas concentrations. A pressure calibration signal, the plethysmograph temperature, mouse body temperature, ambient and chamber pressures, and mouse body mass were then used by the computer software to continuously calculate breath-by-breath ventilatory variables using the equations described by Drorbaugh and Fenn (12) as modified by Jacky (17). Data were rejected if there was evidence of pressure fluctuations caused by gross body movements or sniffing behavior. Mice were weighed, body temperatures recorded, and then placed into the plethysmograph chamber while breathing room air (21% oxygen, balance nitrogen). Mice were allowed to acclimate to the chamber. Once the mice were quiet but awake, ventilatory measurements were recorded continuously for 30 min during baseline conditions (21% oxygen, 0% carbon dioxide, balance nitrogen), followed by consecutive 10 min periods of hypoxia (11% oxygen, balance nitrogen) and hypercapnia. Statistical analyses were performed on data collected during the most quiet 3-min baseline period and the last 3 min of each exposure period. At the conclusion of the study, mice were removed from the chambers and body temperatures recorded.
Ventilatory variables in 6-wk-old mice were averaged over 3 min of baseline, hypoxia, and hypercapnia. Measurements included breathing frequency, tidal volume (Vt), and minute ventilation (the product of breathing frequency and tidal volume, V̇e). Breathing volumes (Vt and V̇e) were normalized per 100 g of body mass. The total respiratory cycle duration (TTOT) was measured for 100 continuous breaths during baseline breathing and averaged in each mouse to reflect differences in stability of the breathing patterns based on the distribution of TTOT and the respective 95% confidence intervals (CI). The coefficient of variation (CV) was calculated by dividing the standard deviation of TTOT by TTOT. After TTOT, CI, and CV were determined for each mouse, mean TTOT, CI, and CV were calculated for each group. Statistical comparisons of baseline ventilatory variables were made between groups (WT0, MT0, WT80, MT80) at each condition (baseline, hypoxia, and hypercapnia) with a two-way ANOVA. Baseline variability analyses (TTOT, CV) were compared between groups (WT0, MT0, WT80, MT80) with a one-way ANOVA.
Statistical analyses.
All statistical analyses were performed using SigmaStat 3.0 (SPSS Chicago, IL). Differences were considered statistically significant at P < 0.05 and Student-Neuman-Keuls post hoc tests were performed when applicable. Except for survival curves, all data are presented as means ± SE.
RESULTS
Survival and body mass.
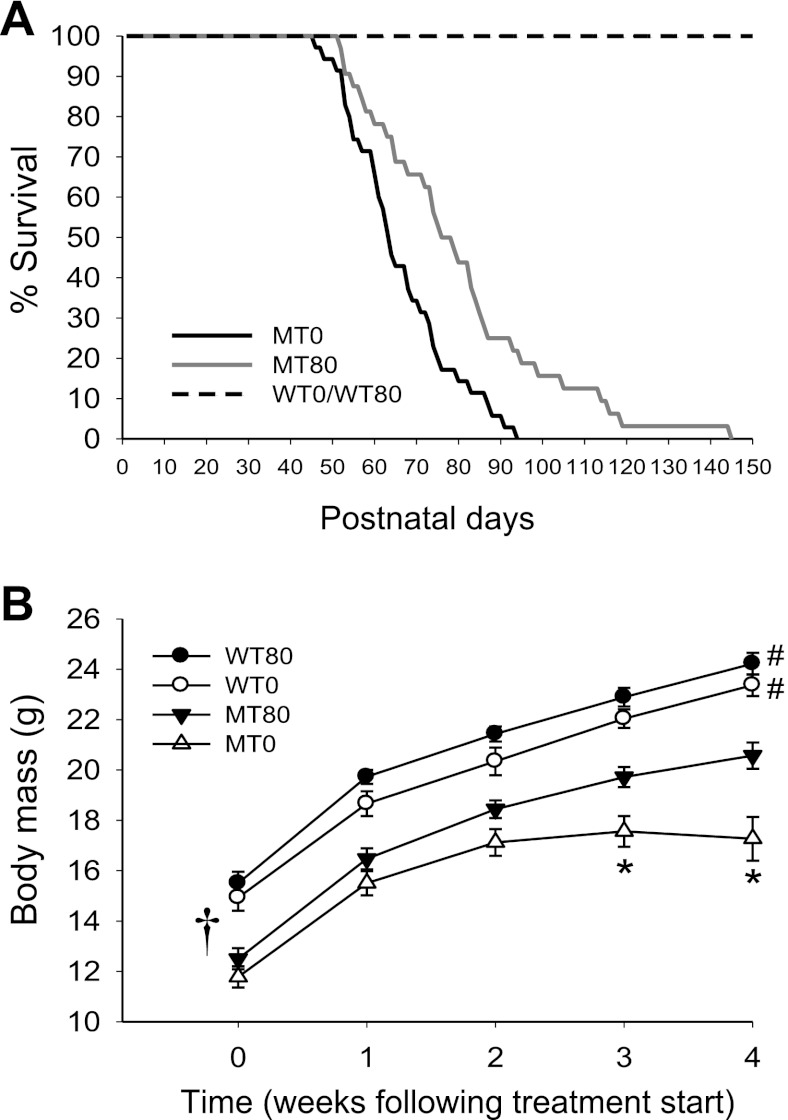
7,8-DHF (80 mg/l) was administered to Mecp2 mutant mice and their wild-type littermates in drinking water from weaning (∼ 4 wk of age) and continued until the end of the study. The average water intake per mouse per day was 2–3 ml. Compared with Mecp2 mutant mice receiving no 7,8-DHF (MT0, n = 35), Mecp2 mutant mice receiving 7,8-DHF in drinking water (MT80, n = 32) lived significantly longer (average survival times: MT0 = 66 ± 2 days, MT80 = 80 ± 4 days, Fig. 1A, P = 0.003). The beneficial effect of 7,8-DHF on the life span of the Mecp2 mutant mice was similar to genetic overexpression of BDNF in the brains of Mecp2 mutant mice (8). Because both wild-type littermates receiving no 7,8-DHF (WT0) and 7,8-DHF (WT80) in drinking water had a normal life span, their survival curves were combined in Fig. 1A (n = 55).
Fig. 1.
7,8-dihydroxyflavone (7,8-DHF) treatment extends the life span and promotes general growth in Mecp2 mutant mice. A: survival curves of the WT0, WT80, MT0, and MT80 mouse groups comparing the percentage of live mice and postnatal days. The black line is the survival curve of the Mecp2 mutant mice receiving no 7,8-DHF (MT0, n = 35), and the gray line is the survival curve of the Mecp2 mutant mice receiving 80 mg/l 7,8-DHF in drinking water (MT80, n = 32). The dashed black line represents wild-type mice (WT0 and WT80, n = 55). MT80 mice lived significantly longer than the MT0 group (66 ± 2 vs. 80 ± 4 days, log rank test; P = 0.003); all WT mice had normal survival. B: growth curve of the treated and nontreated mutant and wild-type groups comparing the average body mass and the weeks following 7,8-DHF treatment. When data are combined over time, all 4 experimental groups were significantly different from each other (†, all P < 0.007). For 2 wk following the start of treatment, there were no significant differences between MT0 and MT80 groups (P = 0.215 and P = 0.069, respectively). However, MT80 mice had significantly larger body mass at 3 and 4 wk following the start of treatment compared with their untreated mutant littermates (P = 0.002 and P < 0.001, respectively). The MT0 and MT80 groups had significantly lower body mass than the WT0 and WT80 groups at every time point (all P < 0.05), resulting in a significant interaction between group and time (P = 0.033). *P < 0.05 vs. MT80 group, #P < 0.05 vs. both MT0 and MT80 group for each point in growth curve.
We also compared the body mass of the four mouse groups from ∼4 wk of age until 8 wk of age (4 wk of treatment). When all data are combined over time, all four experimental groups were significantly different from each other (all P < 0.007; Fig. 1B) with a significant interaction between group and time (Fig. 1B; P = 0.033). Before treatment (week 0) and 1–2 wk after treatment with 7,8-DHF, no significant difference was detected between the MT0 and MT80 groups (Fig. 1B; P = 0.351, P = 0.215, and P = 0.069, respectively). However, at 3 and 4 wk following the start of treatment, the MT80 weighed significantly more than the MT0 group (Fig. 1B; P = 0.002 and P < 0.001, respectively), whereas the WT0 and WT80 groups never differed from each other (all P > 0.05). Mutant mice (MT0 and MT80) weighed significantly less than wild-type mice (WT0 and WT80) at each time point studied (Fig. 1B; all P < 0.05). In a separate two-way ANOVA comparing only the WT0 and WT80 groups, there was no significant interaction term (P = 0.966), nor were there differences at individual times post-treatment (P = 0.363, 0.071, 0.065, 0.160, and 0.197, for time points 0, 1, 2, 3, and 4, respectively), suggesting that the treated wild-type mice were simply slightly larger than the untreated wild-type mice for unexplained reasons. These data suggest that 7,8-DHF only partially rescued the growth deficit in Mecp2 mutant mice because mutants always had smaller body mass than the wild types. However, MT80 mice continued to gain weight at a similar rate as the WT0 and WT80 groups (linear slopes: MT80 = 1.9, WT0 = 2.0, WT80 = 2.1), suggesting that 7,8-DHF helped to maintain the increase in body mass over time as seen in young WT mice.
Voluntary wheel-running activity.
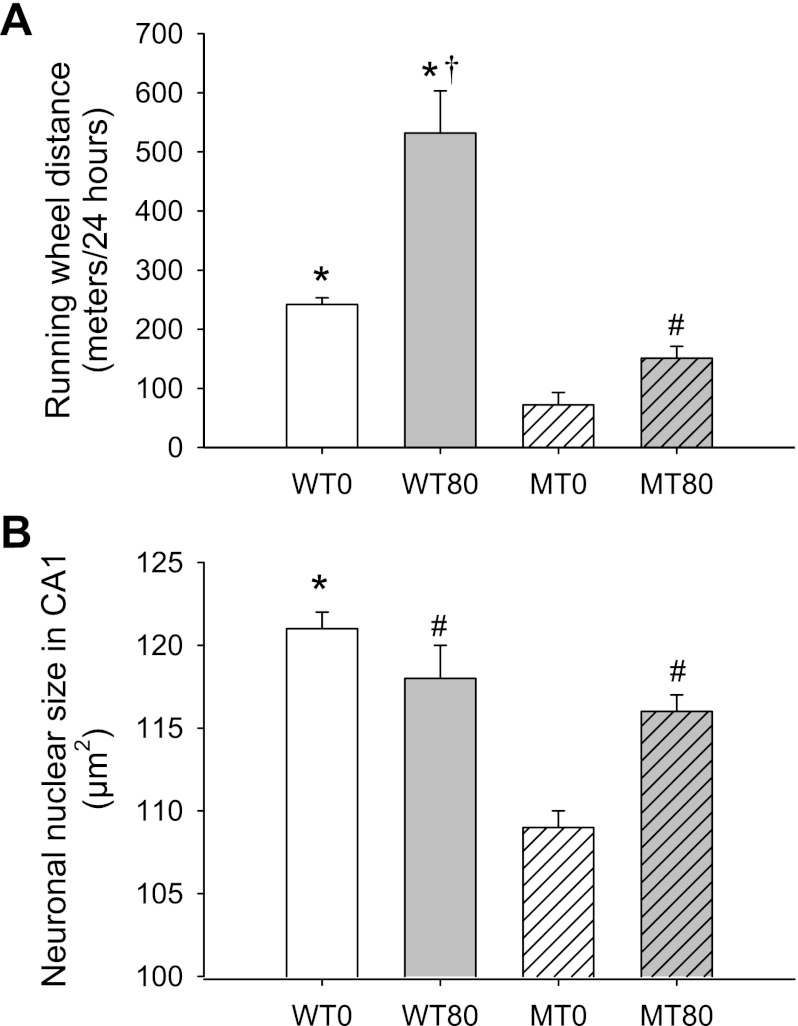
Because impaired motor function has been observed in RTT patients (6) and Mecp2 mutant mice (8), we examined the effect of 7,8-DHF treatment on voluntary locomotor distance in Mecp2 mutant mice. In a voluntary running wheel assay that measures spontaneous wheel distance run per night, we found that the MT80 group ran significantly longer distances than the MT0 group at 8 wk of age (∼4 wk after the start of 7,8-DHF treatment; 151 ± 20 vs. 72 ± 21 meters/24 h; P = 0.008; Fig. 2A). The improvement in voluntary wheel running in Mecp2 mutant mice attributable to 7,8-DHF treatment was also reminiscent of the rescue observed following BDNF overexpression in an earlier study (8). However, this rescue is only partial, because the running wheel distances in both the MT80 and MT0 groups remained significantly lower than the WT0 mice (242 ± 11 m/24 h; P = 0.019 and P < 0.001, respectively; Fig. 2A). WT80 mice ran quantitatively more than all other groups (532 ± 71 m/24 h; all P < 0.001; Fig. 2A). However, the proportional increase in voluntary wheel running associated with 7,8-DHF administration was similar in WT and MT groups (1.2- and 1.1-fold increase from nontreated mice, respectively).
Fig. 2.
7,8-DHF treatment improves voluntary distance run and increases the size of neuronal nuclei in Mecp2 mutant mice. A: a running wheel assay was performed to assess the effect of 7,8-DHF treatment on the voluntary locomotor distance run of the Mecp2 mutant mice at 8 wk of age (∼4 wk after the start of treatment). In a 24-h period, WT0 and WT80 mice ran significantly more than both the treated (MT80; P = 0.019) and nontreated mutants (MT0; P < 0.001). However, MT80 mice also ran significantly greater distances than the MT0 group (P = 0.008), indicating a partial improvement in voluntary distance run with 7,8-DHF administration. Although WT80 mice ran quantitatively more than all other groups (all P < 0.001), the proportional increase in voluntary wheel running was similar in WT and MT groups (1.2- and 1.1-fold increase from nontreated mice, respectively). *P < 0.05 vs. MT0 and MT80 groups, #P < 0.05 vs. MT0 group, †P < 0.05 vs. WT0 group. B: the area occupied by clearly stained (cresyl violet) neuronal nuclei in WT0, WT80, MT0, and MT80 mice. WT0 mice had significantly larger neuronal nuclei than the MT0 group (P < 0.001) as well as the MT80 group (P = 0.014). The MT80 group also had significantly larger nuclei compared with the untreated MT group (MT0; P < 0.001). WT80 mice had significantly large nuclei than the MT0 group (P < 0.001) but did not differ significantly than the WT0 (P = 0.150) and MT80 (P = 0.361) groups. *P < 0.05 vs. MT0 and MT80 groups, #P < 0.05 vs. MT0 group.
Hippocampal neuronal nuclei size.
RTT patients and Mecp2 mutant mice exhibit smaller brain (6, 9), hippocampal neuron soma (8, 9), and neuronal nuclei (2) sizes. We examined the size of neuronal nuclei in the hippocampal CA1 region from 8- to 10-wk-old mice. Nuclear sizes in mice from the WT0 and WT80 groups were not statistically different (121 ± 1 and 118 ± 2 μm2; P = 0.15). The MT80 group (116 ± 1 μm2) had significantly larger neuronal nuclei than the MT0 mice (109 ± 1 μm2; P < 0.001; Fig. 2B), and the MT80 group did not differ from the WT80 group (P = 0.361), thus confirming results from the aforementioned studies. WT0 mice (120 ± 1 μm2) had significantly larger neuronal nuclei than the MT0 group (P < 0.001) as well as the MT80 group (P = 0.0150; Fig. 2B). Together, these data suggest significant, but not complete recovery of neuronal nuclei size by 7,8-DHF treatment.
Ventilatory capacity and breathing pattern.
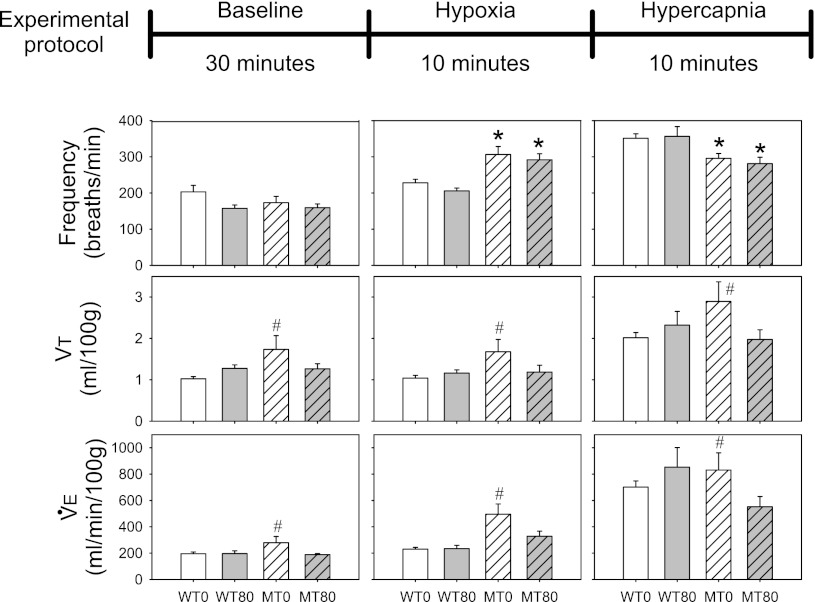
Respiratory rhythm generation and pattern formation within the brain stem are severely affected in RTT patients (6, 33) and Mecp2 mutant mice (19). We performed whole body plethysmography to assess breathing in unanesthetized mice. We made measurements in 6-wk-old unanesthetized and unrestrained mice to obtain quantitative ventilatory measurements under conditions of 1) baseline (21% oxygen, balance nitrogen), 2) hypoxia (11% oxygen, balance nitrogen), and 3) hypercapnia (21% oxygen, 7% carbon dioxide, balance nitrogen). Although there were no significant differences among the MT0, MT80, WT0, and WT80 groups in breathing frequency under baseline conditions (P = 0.235; Fig. 3), frequency was significantly elevated in MT0 and MT80 groups vs. WT0 (P < 0.001 and P = 0.004, respectively) and WT80 groups during hypoxia (P < 0.001 and P = 0.001, respectively; Fig. 3). In contrast, breathing frequency was significantly decreased in MT0 and MT80 mice vs. WT0 (P = 0.005 and P = 0.004, respectively) and WT80 during hypercapnia (P = 0.020 and P = 0.011, respectively; Fig. 3). Thus there was a significant interaction between mouse group (MT0, MT80, WT0, WT80) and condition (baseline, hypoxia, hypercapnia) on breathing frequency (interaction: P < 0.001). There were no significant interactions between group and condition in tidal volume (Vt) or minute ventilation (V̇e = Vt × f) (P = 0.977 and P = 0.462, respectively; Fig. 3). However, combining measures across conditions (baseline, hypoxia, hypercapnia) revealed significant increases in Vt and V̇e in the MT0 group vs. the WT0 and MT80 groups (Vt: P = 0.002 and P = 0.013, respectively; V̇e: both P = 0.007; Fig. 3), suggesting that 7,8-DHF treatment (MT80 group) attenuated Vt and V̇e responses in the Mecp2 mutant mice, and returned values nearly to wild-type levels when all conditions were considered.
Fig. 3.
Effects of 7,8-DHF treatment on baseline, hypoxic, and hypercapnic breathing in 6-wk-old mice. Top: schematic diagram of the conditions used in the plethysmography recording experiments. Bottom: bar graphs of breathing parameters recorded in each group of mice under the 3 conditions illustrated on top. Open bars: WT0 (n = 9); open shaded bars: WT80 (n = 6); hatched open bars: MT0 (n = 12); hatched grey bars: MT80 (n = 8). There were no significant differences among experimental groups in breathing frequency under baseline conditions (P = 0.235). However, frequency was significantly elevated in MT0 and MT80 groups during hypoxia (vs. WT0: P < 0.001 and P = 0.004, respectively; vs. WT80: P < 0.001 and P = 0.001, respectively). In contrast, breathing frequency was significantly decreased in MT0 and MT80 groups during hypercapnia (vs. WT0: P = 0.005 and P = 0.004, respectively; vs. WT80: P = 0.020 and P = 0.011, respectively). Thus, there was a significant interaction between mouse group (MT0, MT80, WT0, WT80) and condition (baseline, hypoxia, hypercapnia) on breathing frequency (interaction: P < 0.001). Combining measures across conditions (baseline, hypoxia, hypercapnia) revealed significant increases in tidal volume (Vt) and minute ventilation (V̇e) in the MT0 group vs. the WT0 and MT80 groups (Vt: P = 0.002 and P = 0.013, respectively; V̇e: both P = 0.007), suggesting that treatment attenuated Vt and V̇e responses in the Mecp2 mutant mice. *P < 0.05 vs. both WT groups during hypoxia and hypercapnia (significant interaction term between group and condition). #P < 0.05 vs. WT0 and MT80 groups when all conditions are combined (no significant interaction term).
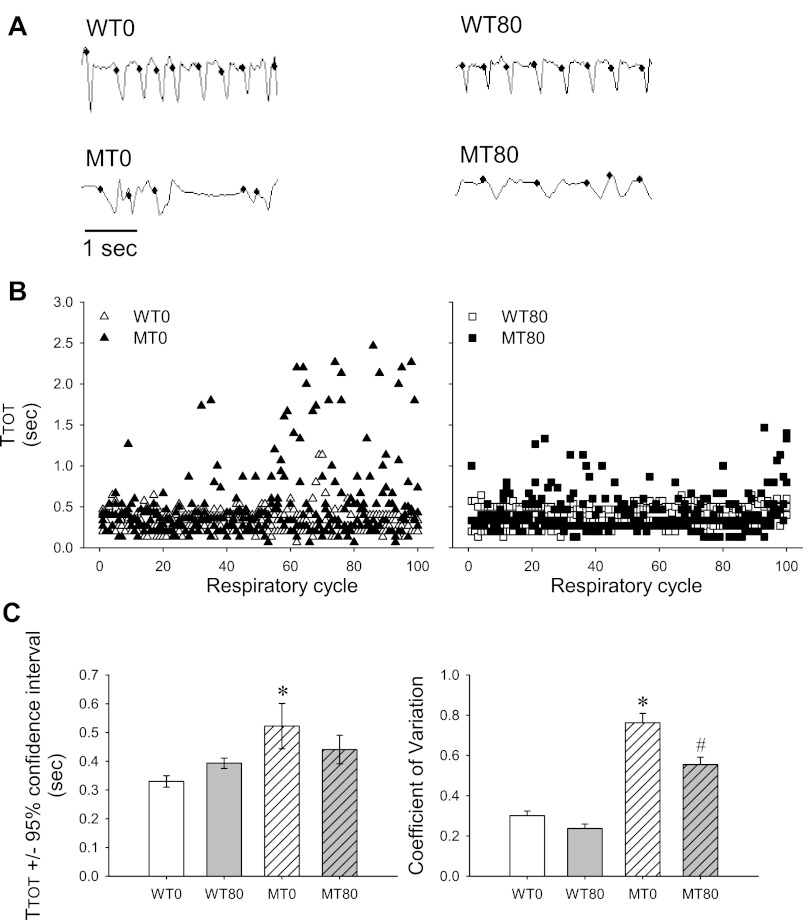
Highly variable breathing patterns with prominent apneas are characteristic of Mecp2 mutant mice (29). Therefore, we analyzed the variability of breathing pattern in 6-wk-old mice by quantifying the total duration of each breath (TTOT), the 95% CI of TTOT, and the CV of TTOT for each mouse and then for each group. Representative plethysmography traces from a single 6-wk-old mouse from each experimental group are shown in Fig. 4A; three representative 6-wk-old mice from each group are incorporated into the scatterplots for TTOT in Fig. 4B. Under baseline conditions, the MT0 group had significantly longer TTOT than all other groups (WT0: P < 0.001, WT80: P = 0.014 and MT80: P = 0.038; Fig. 4, A and B, left, and C). Similarly, the MT0 group also showed greater variability in breathing pattern as the CI and CV were significantly greater than for all other groups (all P < 0.001; Figs. 4, A and B, left, and C). These deficits were partially rescued by 7,8-DHF because TTOT in the MT80 group was not significantly different from the WT0 or WT80 groups (P = 0.080 and P = 0.349, respectively; Figs. 4, A–C). However, the CV of MT80 remained significantly higher than the WT0 and WT80 groups (both P < 0.001, Figs. 4, A–C), as did the CI (P < 0.001 and P = 0.002, respectively; Figs. 4, A–C), suggesting that although 7,8-DHF reduced breathing variability in mutant mice (lower CV and CI in the MT80 group vs. the MT0 group), it did not return breathing stability completely to normal.
Fig. 4.
7,8-DHF treatment improves baseline breathing stability in 6-wk-old Mecp2 mutant mice. A: representative plethysmography traces from a single mouse within each group showing the highly irregular breathing pattern in the MT0 group compared with the regular breathing patterns in the WT0 and WT80 groups and the partial rescue of this irregularity in the MT80 group. The small dots indicate an accepted breath. B: scatterplots of TTOT from 100 continuous respiratory cycles of 3 representative mice from each group, showing the high variability in the MT0 group and reduced variability in the MT80 group compared with the WT0 and WT80 groups. C: bar graphs of TTOT ± 95% confidence interval (CI) and coefficient of variation (CV) in each group. Open white bars: WT0 (n = 12). Open shaded bars: WT80 (n = 8); hatched white bars: MT0 (n = 9); hatched gray bars: MT80 (n = 6). TTOT was significantly increased in the MT0 group compared with all other groups (WT0: P < 0.001, WT80: P = 0.014, and MT80: P = 0.038) and TTOT in the MT80 groups was not significantly different from the WT0 (P = 0.080) or WT80 (P = 0.349) groups. Similarly, the CV and CI was significantly greater in the MT0 vs. all other groups (all P < 0.05). The CV of MT80 remained significantly higher than the WT0 and WT80 groups (both P < 0.001). These data suggest that the untreated Mecp2 mutants had highly variable breathing patterns that were partially (but not completely) restored by treatment with 7,8-DHF. *P < 0.05 vs. WT0, WT80 and MT80 groups. #P < 0.05 vs. WT0 and WT80 groups. TTOT data presented as means ± 95% CI, whereas CV data presented as means + SE.
DISCUSSION
Here we demonstrate that 7,8-DHF administered in drinking water significantly decreases the severity of pathologies in Mecp2 mutant mice, including improvements in life span, body mass, voluntary running wheel distance, hippocampal neuronal nuclei size, and breathing abnormalities. Interestingly, although there are abundant literature reports that increased voluntary or induced physical activity increase BDNF levels in the hippocampus (for review, see Ref. 28), we are unaware of converse reports whereby TrkB receptor activation increases voluntary wheel running in rodents. However, our laboratory has observed that genetic overexpression of BDNF in mice increases running wheel activity to a similar extent (Chang et al., unpublished data) as does 7,8-DHF administration.
Although restoring BDNF in mutant Mecp2 mice appears to slow disease progression (8, 21, 22, 32), BDNF is unlikely to become a systemic treatment for RTT because of its inability to cross the blood-brain barrier (23). 7,8-DHF has been implicated as a TrkB agonist (18) and has similar effects as BDNF in certain forms of learning and memory (3, 11). Intraperitoneal or oral 7,8-DHF administration is reported to activate TrkB in the mouse brain, indicating either that this compound itself or its metabolites may cross the blood-brain barrier and access CNS TrkB receptors (18). In the current study, we tested the therapeutic efficacy of 7,8-DHF in Mecp2 mutant mice to reduce pathologies such as respiratory and somatic motor impairment, breathing irregularities, and growth/viability deficiencies. Although additional pharmacological actions of 7,8-DHF are possible, its effects on these hallmark pathological features of RTT disease are similar to the effect of genetic overexpression of BDNF in Mecp2 mutant mice. In the future, it will be of interest to study the effects of 7,8-DHF on female Mecp2 heterozygous mutant mice as most RTT patients are female and to directly test the hypothesis that its actions are via TrkB receptor activation. Regardless, the present study demonstrates therapeutic efficacy in males, where disease is more severe (usually lethal at a young age).
Consistent with previous reports (5, 30), our study found greater ventilatory responses to hypoxia in Mecp2 mutant mice. We further report that Mecp2 mutant mice have significantly larger tidal volumes than wild-type mice when the baseline, hypoxic, and hypercapnic conditions were combined. Previous reports (22, 30) detected a similar trend, although those reports did not show statistical significance. Without measurements of metabolic rate or arterial blood gases, it is not possible to determine if the increased tidal volume (and minute ventilation) reflect a true hyperventilation, or if the increase is appropriate to match a greater metabolic demand. Unlike a previous study (22), we did not observe elevated breathing frequencies at rest in Mecp2 mutant mice. Because genetic background affects ventilatory control in mice (7, 35), it is worth noting that Ogier et al. (22) used the Mecp2tm1.1/Jae mice in a mixed background of 129Sv/Jae, C57BL/6, and BALB/c, whereas we used Mecp2tm1.1/Jae mice in a pure C57BL/6 background. In a different RTT mouse model (Mecp2tm1.1/Bird), Voituron et al. (30) did not detect elevated breathing frequency at baseline. Developmental age also affects breathing pattern and frequency between P30 and P60 (30), potentially explaining (at least in part) different baseline frequencies in these studies. Finally, our P42 Mecp2 mutant mice had highly variable breathing patterns. If breathing frequency data were taken during a period of low breathing frequency with prolonged apneas and TTOT, the overall frequency will be slower vs. baseline breathing during an episode of high breathing frequency. Although every attempt to avoid sampling bias was made and breaths were averaged over 3 min, sampling variation could have affected the mean values of breathing frequency reported here. Interestingly, breathing frequencies were higher in MT mice during hypoxia but were lower in MT mice during hypercapnia when compared with wild-type groups. Because chemosensory pathways from the peripheral carotid body chemoreceptors are primarily responsible for breathing changes in response to hypoxemia, whereas central chemoreceptors mainly respond to changes in carbon dioxide, these chemosensory pathways may be differentially regulated in Mecp2 mutant mice.
7,8-DHF administration tended to return the augmented tidal volumes (but not frequency) and irregular respiratory rhythms seen in mutant mice toward wild-type breathing patterns. Because 7,8-DHF was systemically delivered in the drinking water, the site of action is unknown. However, likely locations include integrative centers in the brain stem that regulate breathing pattern. Nonetheless, we cannot rule out other sites of action (e.g., direct actions on chemoreceptors, bulbospinal pathways to phrenic motor neurons, neuromuscular junctions on respiratory muscles) as tidal volumes were abnormally increased in mutant mice, but returned toward wild-type levels with 7,8-DHF administration.
The actions of BDNF (presumably via TrkB receptor activation) on the brain stem respiratory control network are complex. BDNF decreases respiratory frequency and enhances glutamate-evoked currents of pre-Bötzinger neurons in postnatal day 1–4 mice (26). BDNF also reduces the amplitude of evoked inward currents in nucleus tractus solitarius (NTS) neurons of postnatal day 35–48 Mecp2 mutant mice (21). Although previous reports suggest that 7,8-DHF activates TrkB receptors in the CNS (similar to BDNF), our study does not address the pharmacological mechanisms of 7,8-DHF actions; further investigations are needed.
In summary, 7,8-DHF significantly extends life span, improves growth (body mass), increases the size of neuronal nuclei, increases voluntary wheel running, and partially corrects breathing instability. The effects of 7,8-DHF on these hallmark pathological features of RTT disease are similar to genetic overexpression of BDNF in Mecp2 mutant mice. Our results support 7,8-DHF as a candidate drug that may eventually be tested in RTT patients once mechanistic studies are confirmed. Because drinking water appears to be an acceptable route for delivering 7,8-DHF in mice, oral administration of this compound in humans may be possible. Moreover, because noninvasive assessment of breathing stability can be readily performed in RTT patients, improvement in ventilatory abnormalities may provide valid endpoint measures to test the therapeutic efficacy of 7,8-DHF.
GRANTS
This work was partially supported by International Rett Syndrome Foundation Grant 2540 to Q. Chang, National Institutes of Health (NIH) P30HD03352 to the Waisman Center, HL-69064, and NS-057778 to G. S. Mitchell, and NIH NIDCD R01 DC-010204 to K. Ye. R. A. Johnson was supported by Grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. H. Li was supported by a predoctoral fellowship from the Stem Cell and Regenerative Medicine Center at the University of Wisconsin-Madison.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.A.J., K.Y., G.S.M., and Q.C. conception and design of research; R.A.J., M.L., A.M.P., H.L., and B.R.L. performed experiments; R.A.J., M.L., A.M.P., H.L., B.R.L., G.S.M., and Q.C. analyzed data; R.A.J., H.L., K.Y., G.S.M., and Q.C. interpreted results of experiments; R.A.J. and Q.C. prepared figures; R.A.J. and Q.C. drafted manuscript; R.A.J., K.Y., G.S.M., and Q.C. edited and revised manuscript; R.A.J., K.Y., G.S.M., and Q.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Shruti Marwaha for technical support.
REFERENCES
- 1. Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Ananiev G, Williams EC, Li H, Chang Q. Isogenic pairs of wild type and mutant induced pluripotent stem cell (iPSC) lines from Rett syndrome patients as in vitro disease model. PLos One 6: e25255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry 168: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bird AP, Wolffe AP. Methylation-induced repression—belts, braces, and chromatin. Cell 99: 451–454, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bissonnette JM, Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding 2 (Mecp2) deficient mice. Pediatr Res 59: 513–518, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron 56: 422–437, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chai S, Gillombardo CB, Donovan L, Strohl KP. Morphological differences of the carotid body among C57/BL6 (B6), A/J, and CSS B6A1 mouse strains. Respir Physiol Neurobiol 177: 265–272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49: 341–348, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27: 327–331, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Depression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302: 885–889, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci USA 107: 2675–2680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955 [PubMed] [Google Scholar]
- 13. Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Hagberg B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand 74: 405–408, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Hagberg B, Hagberg G. Rett syndrome: epidemiology and geographical variability. Eur Child Adolesc Psychiatry 6, Suppl 1: 5–7, 1997 [PubMed] [Google Scholar]
- 16. Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika 69: 553–566, 1982 [Google Scholar]
- 17. Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol 45: 644–647, 1978 [DOI] [PubMed] [Google Scholar]
- 18. Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA 107: 2687–2692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 168: 101–108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 66: 395–403, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 30: 5303–5310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci 27: 10912–10917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pardridge WM. Molecular trojan horses for blood-brain barrier drug delivery. Discov Med 6: 139–143, 2006 [PubMed] [Google Scholar]
- 24. Samaco RC, Neul JL. Complexities of Rett Syndrome and MeCP2. J Neurosci 31: 7951–7959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35: 243–254, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz DM. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Bötzinger complex of neonatal mice. J Neurosci 23: 7685–7689, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van den Veyver IB, Zoghbi HY. Methyl-CpG-binding protein 2 mutations in Rett syndrome. Curr Opin Genet Dev 10: 275–279, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair 19: 283–295, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci 25: 11521–11530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol 168: 109–118, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, Amir RE, Budden S, Naidu S, Pereira JL, Lo IF, Zoghbi HY, Schanen NC, Francke U. Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet 65: 1520–1529, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci 26: 10911–10915, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett syndrome during nighttime in-home recordings. Pediatr Pulmonol 43: 1045–1060, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Xiang F, Buervenich S, Nicolao P, Bailey ME, Zhang Z, Anvret M. Mutation screening in Rett syndrome patients. J Med Genet 37: 250–255, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamauchi M, Kimura H, Strohl KP. Mouse models of apnea: strain differences in apnea expression and its phamacologic and genetic modification. Adv Exp Med Biol 669: 303–307, 2010 [DOI] [PubMed] [Google Scholar]