Abstract
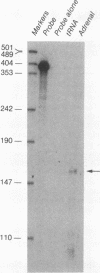
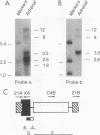
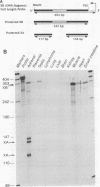
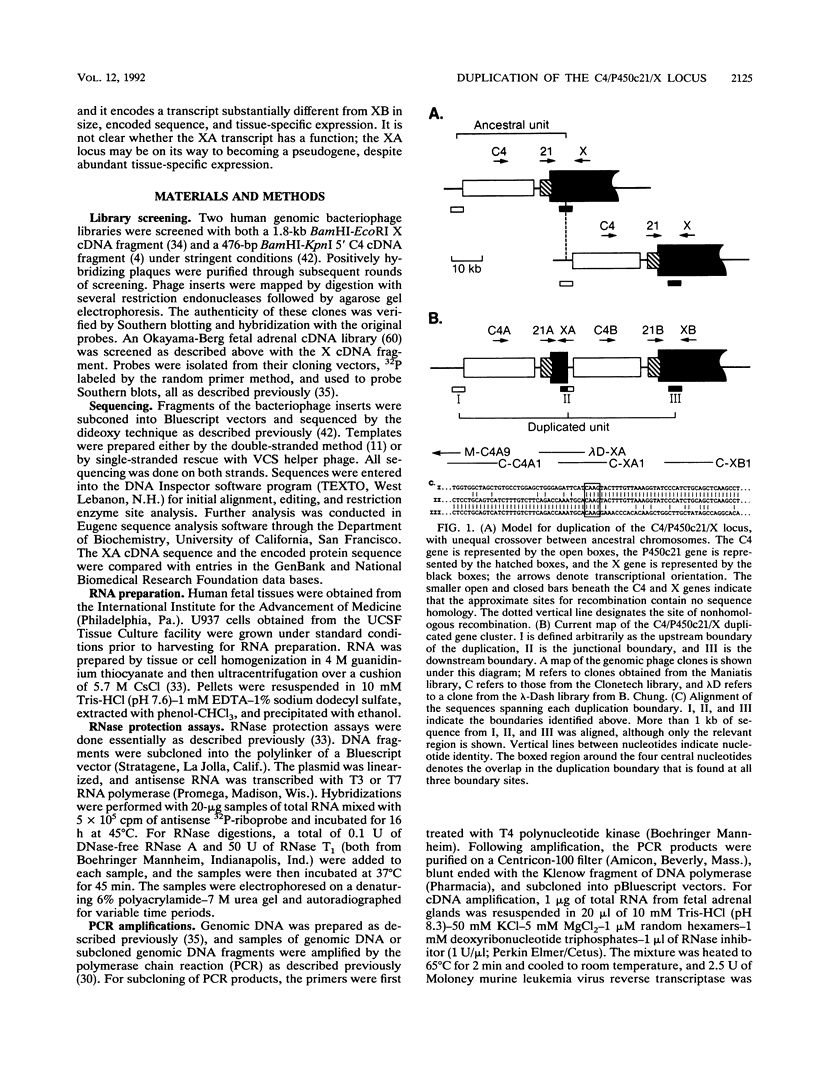
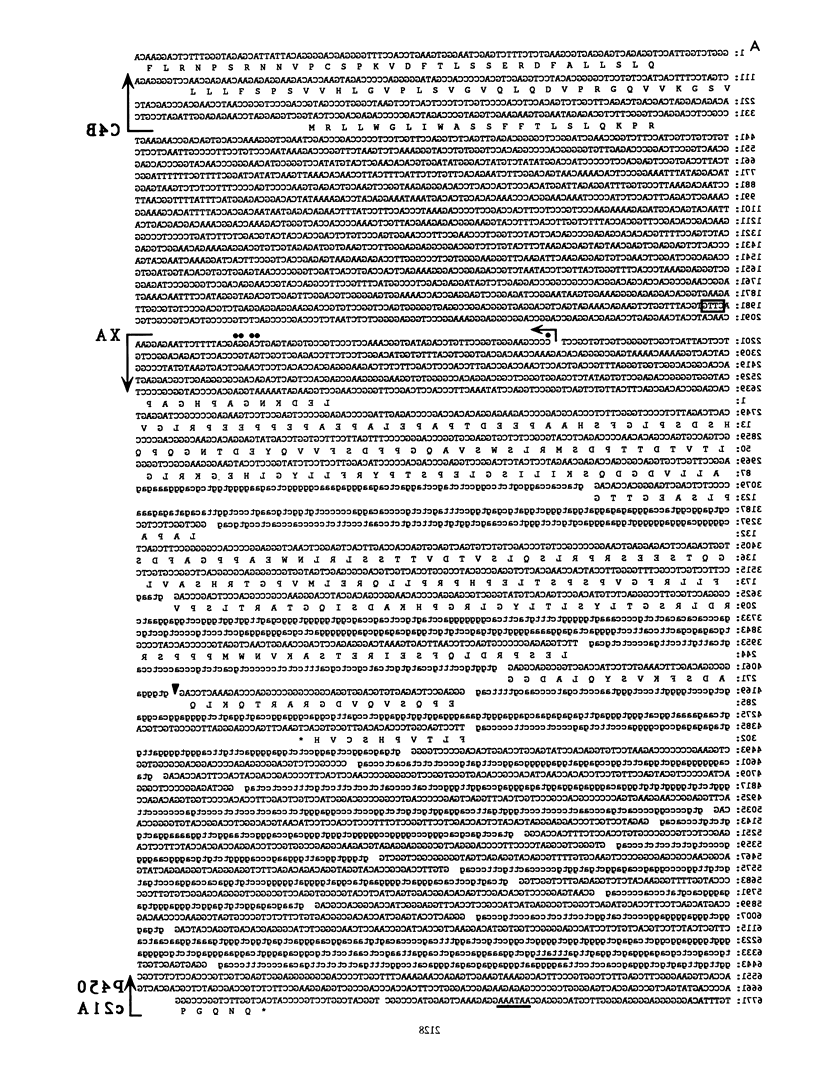
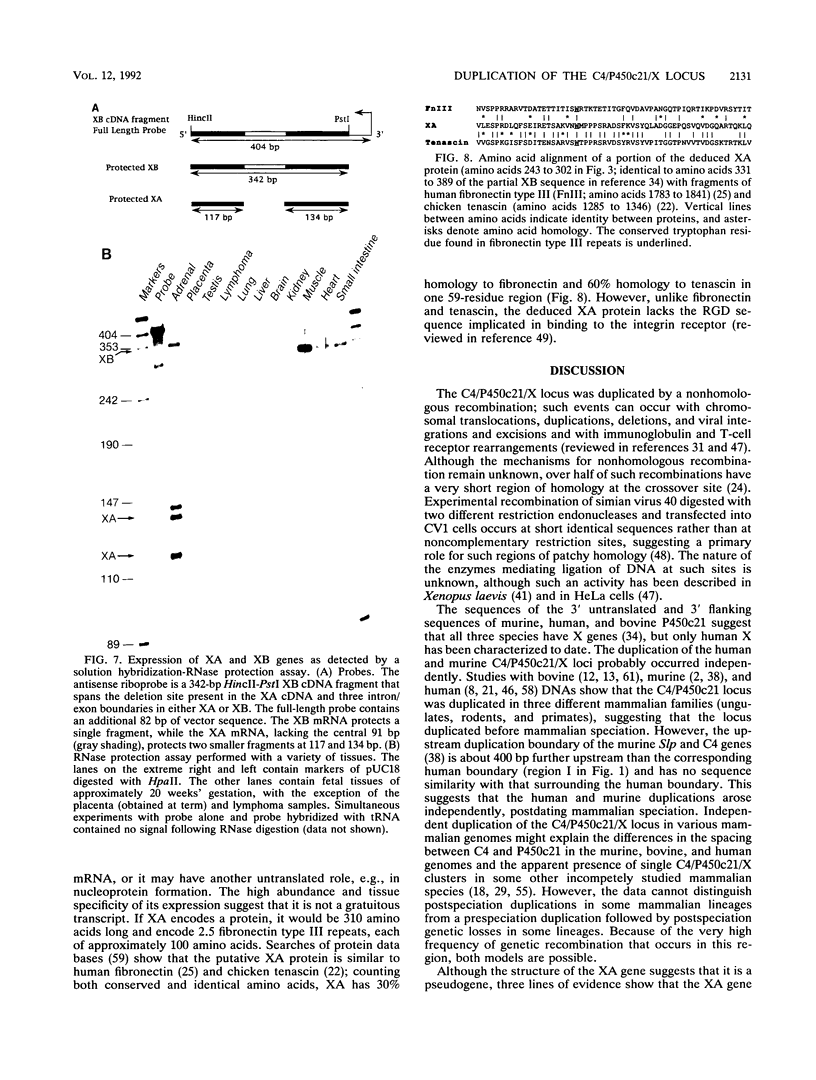
The adjacent C4 and P450c21 genes encode the fourth component of serum complement and steroid 21-hydroxylase respectively, and are tandemly duplicated in the human, murine, and bovine genomes. We recently cloned a cDNA for another duplicated gene, operationally termed X, which overlaps the 3' end of human P450c21 and has the opposite transcriptional orientation. Thus, the organization of the locus is 5'-C4A-21A-XA-C4B-21B-XB-3' (Y. Morel, J. Bristow, S. E. Gitelman, and W. L. Miller, Proc. Natl. Acad. Sci. USA 86:6582-6586, 1989). To determine how this locus was duplicated, we sequenced the DNA at the duplication boundaries and the 7 kb between P450c21A and C4B comprising the XA locus. The sequences located the duplication boundaries precisely and indicate that the duplication occurred by nonhomologous recombination. The boundaries are substantially different from those of the corresponding duplication in the mouse genome, suggesting that similar gene duplications may have occurred independently in ancestors of rodents and primates after mammalian speciation. Compared with XB, the XA gene is truncated at its 5' end and bears a 121-bp intragenic deletion causing a frameshift and premature translational stop signal. Nevertheless, XA is transcribed into a stable 2.6-kb polyadenylated RNA that is expressed uniquely in the adrenal gland.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Amor M., Tosi M., Duponchel C., Steinmetz M., Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4453–4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei M. S., Zemel S., Tilghman S. M. Parental imprinting of the mouse H19 gene. Nature. 1991 May 9;351(6322):153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Bernards A., de la Monte S. M. The ltk receptor tyrosine kinase is expressed in pre-B lymphocytes and cerebral neurons and uses a non-AUG translational initiator. EMBO J. 1990 Jul;9(7):2279–2287. doi: 10.1002/j.1460-2075.1990.tb07399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990 Jan;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Katzman P., Alicot E. M., Koller B. H., Geraghty D. E., Orr H. T., Strominger J. L., Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8535–8539. doi: 10.1073/pnas.84.23.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Galbraith L. J., Seidman J. G., White P. C., Parker K. L. Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9601–9605. doi: 10.1073/pnas.83.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Cloning and characterization of the bovine gene for steroid 21-hydroxylase (P-450c21). DNA. 1985 Jun;4(3):211–219. doi: 10.1089/dna.1985.4.211. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Structure of a bovine gene for P-450c21 (steroid 21-hydroxylase) defines a novel cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4243–4247. doi: 10.1073/pnas.83.12.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Sinnott P. J., Dyer P. A., Price D. A., Harris R., Strachan T. Pulsed field gel electrophoresis identifies a high degree of variability in the number of tandem 21-hydroxylase and complement C4 gene repeats in 21-hydroxylase deficiency haplotypes. EMBO J. 1989 May;8(5):1393–1402. doi: 10.1002/j.1460-2075.1989.tb03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham I., Sargent C. A., Trowsdale J., Campbell R. D. Molecular mapping of the human major histocompatibility complex by pulsed-field gel electrophoresis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7237–7241. doi: 10.1073/pnas.84.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffrotin C., Chardon P., de Andres-Cara D. F., Feil R., Renard C., Vaiman M. The swine steroid 21-hydroxylase gene (CYP21): cloning and mapping within the swine leucocyte antigen complex. Anim Genet. 1990;21(1):1–13. doi: 10.1111/j.1365-2052.1990.tb03202.x. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hauptmann G., Tappeiner G., Schifferli J. A. Inherited deficiency of the fourth component of human complement. Immunodefic Rev. 1988;1(1):3–22. [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Konopka A. K. Compilation of DNA strand exchange sites for non-homologous recombination in somatic cells. Nucleic Acids Res. 1988 Mar 11;16(5):1739–1758. doi: 10.1093/nar/16.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Strauss M., Tosi M., Steinmetz M., Klein J., Meo T. Multiple duplications of complement C4 gene correlate with H-2-controlled testosterone-independent expression of its sex-limited isoform, C4-Slp. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1746–1750. doi: 10.1073/pnas.82.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Gitelman S. E., Saenger P., Miller W. L. Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J Clin Invest. 1991 Dec;88(6):1955–1962. doi: 10.1172/JCI115520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi-Strauss M., Carroll M. C., Steinmetz M., Meo T. A previously undetected MHC gene with an unusual periodic structure. Science. 1988 Apr 8;240(4849):201–204. doi: 10.1126/science.3353717. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genet. 1989;23:371–393. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- Moore C. C., Brentano S. T., Miller W. L. Human P450scc gene transcription is induced by cyclic AMP and repressed by 12-O-tetradecanoylphorbol-13-acetate and A23187 through independent cis elements. Mol Cell Biol. 1990 Nov;10(11):6013–6023. doi: 10.1128/mcb.10.11.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel Y., André J., Uring-Lambert B., Hauptmann G., Bétuel H., Tossi M., Forest M. G., David M., Bertrand J., Miller W. L. Rearrangements and point mutations of P450c21 genes are distinguished by five restriction endonuclease haplotypes identified by a new probing strategy in 57 families with congenital adrenal hyperplasia. J Clin Invest. 1989 Feb;83(2):527–536. doi: 10.1172/JCI113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel Y., Bristow J., Gitelman S. E., Miller W. L. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6582–6586. doi: 10.1073/pnas.86.17.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel Y., Miller W. L. Clinical and molecular genetics of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Adv Hum Genet. 1991;20:1–68. doi: 10.1007/978-1-4684-5958-6_1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Kimura H., Yeul Y. D., Yokoyama S., Nakayama K., Takahashi M. Identification of the 5'-flanking regulatory region responsible for the difference in transcriptional control between mouse complement C4 and Slp genes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7883–7887. doi: 10.1073/pnas.83.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Sepich D. S. Murine sex-limited protein: complete cDNA sequence and comparison with murine fourth complement component. J Immunol. 1985 Dec;135(6):4239–4244. [PubMed] [Google Scholar]
- Parker K. L., Chaplin D. D., Wong M., Seidman J. G., Smith J. A., Schimmer B. P. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7860–7864. doi: 10.1073/pnas.82.23.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988 Feb 11;16(3):907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J., Miller W. L. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA. 1987 Oct;6(5):439–448. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice H. L., Schneider P. M., Strominger J. L. C4B gene polymorphism detected in a human cosmid clone. Immunogenetics. 1986;23(4):274–276. doi: 10.1007/BF00373024. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Dunham I., Yu C. Y., Carroll M. C., Porter R. R., Campbell R. D. Molecular characterization of the HLA-linked steroid 21-hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J. 1987 Jun;6(6):1653–1661. doi: 10.1002/j.1460-2075.1987.tb02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Campbell R. D. Identification of multiple HTF-island associated genes in the human major histocompatibility complex class III region. EMBO J. 1989 Aug;8(8):2305–2312. doi: 10.1002/j.1460-2075.1989.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Trowsdale J., Campbell R. D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Womack J. E., Petresh J. M., Miller W. L. Synteny mapping of the genes for 21 steroid hydroxylase, alpha A crystallin, and class I bovine leukocyte antigen in cattle. DNA. 1988 Apr;7(3):143–149. doi: 10.1089/dna.1988.7.143. [DOI] [PubMed] [Google Scholar]
- Spies T., Blanck G., Bresnahan M., Sands J., Strominger J. L. A new cluster of genes within the human major histocompatibility complex. Science. 1989 Jan 13;243(4888):214–217. doi: 10.1126/science.2911734. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilliaert R., Palsdottir A., Arnason A. Analysis of the C4 genes in baleen whales using a human cDNA probe. Immunogenetics. 1990;32(2):73–76. doi: 10.1007/BF00210443. [DOI] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- White P. C., Chaplin D. D., Weis J. H., Dupont B., New M. I., Seidman J. G. Two steroid 21-hydroxylase genes are located in the murine S region. 1984 Nov 29-Dec 5Nature. 312(5993):465–467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Doolittle R. F. Presence of a vertebrate fibrinogen-like sequence in an echinoderm. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2097–2101. doi: 10.1073/pnas.87.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Morohashi K., Sogawa K., Yamane M., Kominami S., Takemori S., Okada Y., Omura T., Fujii-Kuriyama Y. Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P-450(C21) which catalyzes steroid 21-hydroxylation in bovine adrenal cortex. J Biol Chem. 1986 Mar 25;261(9):4106–4109. [PubMed] [Google Scholar]