Abstract
Orexins/hypocretins are hypothalamic peptides involved in arousal and wakefulness, but also play a critical role in drug addiction and reward-related behaviors. Here, we review the roles played by orexins in a variety of animal models of drug addiction, emphasizing both commonalities and differences for orexin’s involvement in seeking of the major classes of abused drugs, as well as food. One common theme that emerges is an involvement of orexins in drug seeking triggered by external stimuli (e.g., cues, contexts or stressors). We also discuss the functional neuronal circuits in which orexins are embedded, and how these circuits mediate addiction-related behaviors, with particular focus on the role of orexin and glutamate interactions within the ventral tegmental area. Finally, we attempt to contextualize the role of orexins in reward by discussing ways in which these peptides, expressed in only a few thousand neurons in the brain, can have such wide-ranging effects on behavior.
Keywords: cues, glutamate, cocaine, opiates, nicotine, ethanol, food, motivation
Introduction and overview
The brain neuropeptides orexin A and orexin B (also denoted hypocretin 1 and hypocretin 2) originate exclusively in hypothalamus and target neurons throughout the central nervous system via two G-protein-coupled receptors (orexin 1 and 2 receptors (OX1R and OX2R), also denoted hypocretin 1 and 2 receptors), as described in other chapters in this volume (Mieda and Sakurai, Gotter et al 2012). Although compelling evidence shows a role for orexins in arousal and maintenance of the waking state (de Lecea, 2012), additional evidence supports an important and specific role in reward processes and drug abuse as well (e.g. Baimel and Borgland, 2012). This chapter will review this evidence, summarize the current understanding of roles played by orexins in addiction, and discuss some of the brain mechanisms involved.
Orexin neurons are located exclusively in the hypothalamus. They are distributed mediolaterally from the dorsomedial hypothalamic nucleus (DMH) through the perifornical area (PFA) into the lateral hypothalamus (LH) proper, ranging from about 2.5 to 3.5 mm caudal of bregma in the rat (de Lecea et al., 1998; Sakurai et al., 1998). Animal studies, many of which are described below, frequently find that reward functions are associated specifically with orexin neurons in LH but not in PFA or DMH. In contrast, stress-, arousal-, and wake-promoting functions of orexin are instead more consistently linked with DMH and PFA orexin neuron activation. These observations led us to propose a functional dichotomy between these populations of orexin neurons, where LH orexin cells are involved in reward processes and the more medial orexin cells are instead involved with waking and stress responses (Harris and Aston-Jones, 2006). Thus, evidence supports a role of orexin in both arousal and reward.
Another dichotomy in orexin function appears to be related to the two orexin receptors, where arousal is most closely associated with activation of the OX2R and reward with OX1R activation (Akanmu and Honda, 2005; Aston-Jones et al., 2010; Marcus et al., 2001; Smith et al., 2009b; Willie et al., 2003). This may indicate that these two functions can be differentially targeted by drugs that interact with one of the two receptors. Therefore, development of specific OX1R and OX2R compounds to treat addiction versus sleep disorders, respectively, seems a potentially promising pharmacotherapeutic strategy (Gotter et al., 2012).
One of the many remaining questions regarding orexin function is in understanding the relationship between arousal and reward mediated by these neighboring orexin cell groups. Rewards are often arousing, and arousing stimuli are often rewarding—but does this well-known relationship somehow reflect interactions between the lateral/medial cell groups or OX1 and OX2 receptors? In fact, some pharmaceutical companies have started developing combined OX1R/OX2R compounds, in the view that manipulating both receptors simultaneously may prove to be more effective for some purposes than manipulating either one alone (Winrow et al., 2010). One hypothesis we will explore below is whether both arousal and reward functions can be viewed as parts of a role for the entire orexin system in assigning salience to stimuli in the service of facilitating motivated behavior.
Common functional themes are emerging for the orexin system. As we discuss below, these neurons are engaged by stimuli associated with rewards, including food, sex, and abused drugs (but not novelty reward) (Cason et al., 2010; Di Sebastiano et al., 2010, 2011; Harris et al., 2005; Sakurai et al., 1998), and interference with OX1R neurotransmission blocks the ability of discrete or contextual stimuli to reinstate extinguished drug- or food-seeking behaviors. In contrast, these cells are involved in the primary reinforcing properties of some but not all drug types—for example, OX1R antagonists reduce heroin but not cocaine self-administration. As discussed below, this constellation of common and differential effects across rewards may in part reflect the ability of orexin to enhance responses of ventral tegmental area (VTA) dopamine neurons to afferent (perhaps especially glutamate) inputs.
Physiological data show that orexin not only activates its target neurons, but also has substantial modulatory effects that augment glutamate neurotransmission in target neurons (as discussed in more detail below and by Baimel and Borgland, 2012). This property may help to explain the accumulating evidence that indicates that the function of orexin neurons depends upon the target that they innervate, as is commonly the case for neuromodulatory systems.
In general, research on the orexin system since its discovery 14 years ago has indicated that these neurons play important roles in fundamental brain and behavioral processes. In fact, it is hard to identify another neuropeptide system that is as strongly linked to wide-ranging behavioral effects as the orexin system. However, work in this nascent field has generated perhaps as many questions as it has answered. It seems clear that continuing studies will reveal ever more complex and intriguing properties of this key brain system.
Here, we seek to review the roles of the orexin system in drug addiction. Most of the extant literature is based upon animal studies modeling various aspects of addiction and addiction-related behaviors. Therefore, we first review some of the most commonly used animal models used to test addiction-related phenomena, and then summarize the mounting literature using these models to test the roles of orexins in addiction.
Animal models of addiction
To investigate the underlying mechanisms of addiction, it is vital to have reliable animal models that capture key features of the human phenomenon. Fortunately, there are a wide variety of behavioral paradigms that model various aspects of human drug addiction with considerable face validity, which is not necessarily the case for other psychiatric disorders. Addiction models can be used to examine brain correlates of addiction-related behaviors, such as neuronal activation as measured by electrophysiology, or immediate early genes like c-fos and its protein product Fos. Additionally, these models can be used to test the effects of experimental interventions on these behaviors, using lesions of brain regions, or pharmacologic or genetic manipulations. In the following section, we summarize some of the most commonly used paradigms in rodent addiction research and lay out advantages and disadvantages of each. Following this overview, we will discuss the involvement of orexin in addiction-related processes measured with the various paradigms.
Acute withdrawal
Withdrawal is a key clinical symptom of drug dependence (APA, 2000), and desire to avoid withdrawal is a major factor motivating human addicts to continue using drugs (Koob and Le Moal, 1997). In animals, withdrawal can be elicited by either cessation of chronic drug exposure (spontaneous withdrawal) or administration of an antagonist for the receptors at which the drug acts (precipitated withdrawal). Acute withdrawal effects in animals are typically measured by quantifying the physical withdrawal symptoms for drugs like opiates (such as piloerection, hunched posture, and diarrhea), and/or the psychological withdrawal symptoms that occur with most abused drugs (such as anxiety and negative affect).
However, it is clear that the phenomenon of addiction is much larger than avoidance of acute withdrawal symptoms. Addiction is a chronic disorder, such that up to 60% of addicts relapse to drug taking within 12 months of quitting (Brandon et al., 2007; NIDA, 2009). The fact that these individuals relapse even after enduring the acute withdrawal period indicates that addiction fundamentally involves long-lasting alterations to brain circuitry that persist for years (or perhaps permanently), even after cessation of drug use. Therefore, many additional models have been developed (outlined below) that measure these persistent effects, in an attempt to investigate the long-term neuroadaptations underlying addiction.
Sensitization
One factor that is considered to be crucial to the development of addiction is repeated exposure to drugs, often with escalated dosing over time due to the tolerance that occurs for certain effects of the drug (e.g., euphoria). However, some drug effects instead show sensitization over time, such that the same dose of drug causes increased effect sizes with repeated exposures. One effect that sensitizes with repeated exposure to all major addictive drugs is locomotor activation (Clarke and Kumar, 1983; Downs and Eddy, 1932; Kalivas and Stewart, 1991; Robinson and Becker, 1986; Vezina and Stewart, 1989). For example, following repeated administration of cocaine in a locomotion testing chamber, the hyperlocomotor properties of cocaine are increased with each subsequent injection (Downs and Eddy, 1932). Interestingly, locomotor sensitization is strongest in novel environments and is markedly context dependent (Hinson and Poulos, 1981; Robinson et al., 1998; Stewart and Vezina, 1991). In addition, locomotor sensitization is remarkably persistent and may last for months or years, even if no drug has been administered in the interim (Robinson and Becker, 1986; Segal et al., 1981).
Robinson and Berridge have argued that locomotion can be used as an indirect proxy for incentive salience, or the attractive, noticeable quality of rewards and associated cues that drives motivation to seek them (also called “wanting”) (Robinson and Berridge, 1993, 2008). This “incentive sensitization” also occurs with repeated dosing, and may account for the increased difficulty in resisting drugs once they have been taken repeatedly. Indeed, locomotor/incentive sensitization has been correlated to other addiction-like behaviors observed in humans and animals (Di Ciano, 2008; Leyton, 2007; Piazza et al., 1990a,b; Steketee and Kalivas, 2011; Vezina, 2004).
Conditioned place preference
The conditioned place preference (CPP) paradigm measures Pavlovian approach toward an environment previously associated with reward. The CPP apparatus consists of two chambers with distinct visual, tactile, and/or olfactory cues, separated by a solid partition (or sometimes a third, neutral chamber with partitions on either side). In a typical CPP procedure, animals receive pairings of one chamber with drug, and the other chamber with control vehicle injections, during 30-min sessions. Following multiple pairings with each chamber, animals are tested for CPP by allowing free access to both chambers during a 15-min drug-free session. If they spend more time on the drug-paired side, they are said to show a CPP. If they avoid this side, they show a conditioned place aversion. Humans can also learn CPPs for drugs (Childs and de Wit, 2009).
CPP is one of the most commonly employed paradigms for studying drug reward, partially due to the fact that the experimental procedure is short in duration (about 1 week) and relatively simple. It can be used to study most drugs abused by humans and to determine how the acquisition and/or expression of this learned behavior is affected by experimental manipulations. However, controversy exists as to whether preference/avoidance reflects drug seeking/aversion, or some other aspect of conditioning. Most drugs that cause euphoric effects in humans elicit a positive preference in the CPP paradigm, and most that have unpleasant subjective effects evoke a place aversion, giving credence to the seeking/avoidance interpretation. This issue is dealt with in considerable detail elsewhere (Bardo and Bevins, 2000).
Self-administration
One limitation of both sensitization and CPP paradigms is that drugs are administered to the animals by the experimenter. However, when drugs are experienced instead via voluntary self-administration, they instigate different changes to the brain (as compared to yoked noncontingent administration), and these differences may be critical to understanding some aspects of the addiction process (Dumont et al., 2005; Hemby et al., 1997; Jacobs et al., 2005; Suto et al., 2010). One factor that likely underlies this difference is that during self-administration, both instrumental and Pavlovian contingencies are learned [e.g., pressing a lever delivers drug, and a discrete cue (e.g., a tone/light stimulus) temporally predicts drug]. These learning-related factors are crucial for addiction, which may critically depend upon learning processes—some of which can cause the pursuit of drugs to transition into an automatic, inflexible habit (Berke and Hyman, 2000; Everitt et al., 2008; Pierce and Vanderschuren, 2010). This adds to the face validity of the self-administration model (Katz and Higgins, 2003; O’Connor et al., 2011; Panlilio and Goldberg, 2007). However, self-administration studies in animals usually fail to model potential consequences of drug taking, and therefore, animals have few disincentives for taking drugs. For this reason, some researchers have begun adding consequences for drug taking in animal models, such as a shock or shock-associated cues (Belin et al., 2008; Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004).
Self-administration experiments are typically conducted in an operant chamber, in which animals learn to make an instrumental action (e.g., lever press, nose poke, wheel turn, chain pull) to receive rewards, such as drug infusions delivered via a chronic intravenous catheter, or food or ethanol dispensed into a receptacle. Drugs are often paired with discrete tone/light cues, which can later trigger drug seeking (discussed below).
Fixed ratio (FR) schedules of self-administration require relatively little effort for an animal to receive drug, so specialized paradigms have been developed to determine motivation for drug in particular; this is typically measured as the amount of effort an animal will exert to receive a specific dose of drug. Under low FR schedules (e.g., FR-1), animals can easily maintain a preferred blood level of drug (Koob, 1992; Pickens and Thompson, 1971; Wise, 1987, 1997). Under a progressive ratio (PR) schedule of reinforcement, animals must expend increasing (exponential) amounts of effort to obtain each subsequent drug infusion. The level at which animals cease responding (breakpoint) is taken to reflect the upper limit of motivation for an animal to receive drug. A related paradigm employs behavioral economics principles (Bickel et al., 2010; Oleson and Roberts, 2009) and requires animals to make a fixed number of responses (e.g., FR-1) for decreasing doses of drug over time. At first, animals readily achieve desired blood drug levels with only a few infusions. However, as doses decrease over the course of the session, more effort (responding) is required to achieve blood drug levels preferred by the animal. The cutoff point at which animals eventually cease responding for these very small doses is referred to as the maximal price, or Pmax, which is thought to reflect the value an animal ascribes to (or price it is willing to pay for) the drug.
Finally, a model of drug self-administration common to ethanol research is voluntary home cage oral intake. In a typical setup, animals have access to two bottles—one containing ethanol and one containing water alone. Rats and mice are trained to drink ethanol in this paradigm either by allowing only intermittent access to the ethanol bottle or by transitioning over many days from a solution containing pure sucrose to solutions containing increasing ethanol concentrations (e.g., Simms et al., 2008). In general, the degree of consumption is taken as a measure of rewarding effects of, or motivation for, ethanol.
Relapse to drug seeking
Due to the chronic relapsing nature of addiction, several paradigms have been developed to model the relapse event, when drug seeking is triggered after a period of abstinence and/or explicit extinction training (for an excellent review of the history of reinstatement models, see Shaham et al., 2003). Three main risk factors are known to trigger drug craving and relapse in humans—exposure to drug-associated stimuli/contexts, the drug itself, and stressors (Breiter et al., 1997; Ferguson and Shiffman, 2009; Haney et al., 2001; O’Brien et al., 1992; Sinha and Li, 2007; Sinha et al., 2011). These triggers also cause drug seeking in animals and are typically studied using an extinction–reinstatement paradigm after drug self-administration training (though drug priming and stress are effective at reinstating extinguished CPP as well). In this paradigm, animals are given a period of self-administration (e.g., 10–30 days), followed by an extinction period during which operant responses no longer produce drug or cues (1–3 weeks). Once responding has decreased to a specific criterion, drug seeking is reinstated using one of the three reinstatement triggers, as discussed below. It is important to study the mechanisms underlying each type of reinstatement, given that each involves at least some unique neurocircuitry.
Drug-associated cues are any environmental stimuli that have been associated in a Pavlovian fashion with drug rewards. For cue-induced reinstatement of drug seeking in animals, discrete cues (such as light or tone) are reintroduced after extinction, delivered upon performance of the behavior that once yielded drug+cues (Meil and See, 1996; Shaham et al., 2003). Alternatively, contextual stimuli (e.g., the sight, smell, and tactile cues of an operant chamber) can also cause reinstatement of drug seeking (Bouton and Bolles, 1979; Crombag et al., 2008; Feltenstein and See, 2008). Related models of cue-induced drug seeking are conditioned reinforcement (CR) and discriminative stimulus (DS) tasks. In CR (sometimes called second-order conditioning), animals learn to perform a novel response (such as a nose poke) to receive the cue that was previously associated with drug delivery via another response (such as a lever press) (Di Ciano and Everitt, 2005; Schindler et al., 2002). In DS tasks, noncontingently delivered cues signal the availability of drug during self-administration sessions (“occasion setters”), which can then be used to drive reinstatement by reintroducing the DS after extinction (Ciccocioppo et al., 2001; Root et al., 2009; Yun and Fields, 2003).
Another factor that reinstates drug seeking is drug “priming,” which models the phenomenon in humans where a lapse(e.g. a single drink, cigarette, etc.), often turns into a full-blown relapse to excessive use. In animals, reinstatement of extinguished drug seeking can be elicited by an experimenter-delivered priming dose, even though instrumental responding is reinforced by neither drug nor cues (de Wit and Stewart, 1981, 1983; Stewart et al., 1984).
The third factor that can reinstate drug seeking is stress. Two common methods for eliciting stress-induced reinstatement are a brief exposure (15 min) to intermittent, unpredictable electrical footshock, or a period of food deprivation (Shaham et al., 2000, 2003; Shalev et al., 2010). In addition, certain pharmacologic agents can elicit stress- or panic-like states that also reinstate drug seeking (See and Waters, 2010). One common pharmacological stressor is yohimbine, which appears to trigger reinstatement primarily through its actions at serotonin and dopamine receptors, and not via its well-known actions as an antagonist of noradrenergic alpha-2 receptors (Brown et al., 2009; Dzung Le et al., 2009; Millan et al., 2000; Nair et al., 2009; Smith and Aston-Jones, 2011). It is important to note that these are different circuits than those underlying, for example, footshock stress, and so it is unlikely that these different stress modalities are entirely equivalent.
Other models of addiction
A variety of other paradigms have been developed to model aspects of addiction. For example, some investigations have focused on the persistent neural and behavioral changes that occur after periods of abstinence from either experimenter-delivered drugs or self-administration (without explicit extinction training) (Aston-Jones and Harris, 2004; Fuchs et al., 2006). Beyond the acute withdrawal stage described above, further changes occur in reward- and stress-related brain circuits that appear to increase with longer periods of abstinence. This “protracted withdrawal” phenomenon is associated with increased motivation for drug rewards in particular (Harris and Aston-Jones, 2003, 2007) and may be related to the phenomenon of “incubation of craving,” where appetitive responses to drug cues grow stronger over time (Grimm et al., 2001; Pickens et al., 2011).
Another paradigm used to measure the rewarding or aversive effects of drugs involves examining the effects of drugs on intracranial self-stimulation (ICSS) behavior. In this paradigm, rats (or humans) perform instrumental behaviors to receive electrical stimulation of specific brain regions (especially those involving the medial fore-brain bundle), but only when the current is delivered at or above a certain stimulation intensity, known as the threshold (Heath, 1996; Wise, 1996; Yeomans, 1990). Changes to ICSS threshold are often interpreted as reflecting shifts in the hedonic state of the animals. Addictive drugs and other rewarding manipulations lower the stimulation threshold, potentially suggesting that they elicit positive hedonic states, whereas stressful or aversive manipulations (including drug withdrawal) increase thresholds, suggesting that they cause negative hedonic states (Koob and Le Moal, 2006; Markou et al., 1993; Wee and Koob, 2010).
In the following section, we discuss how these different paradigms have been used to determine the role that the orexin system plays in various aspects of the addiction process for different types of drug and food rewards.
Orexin roles in drug seeking
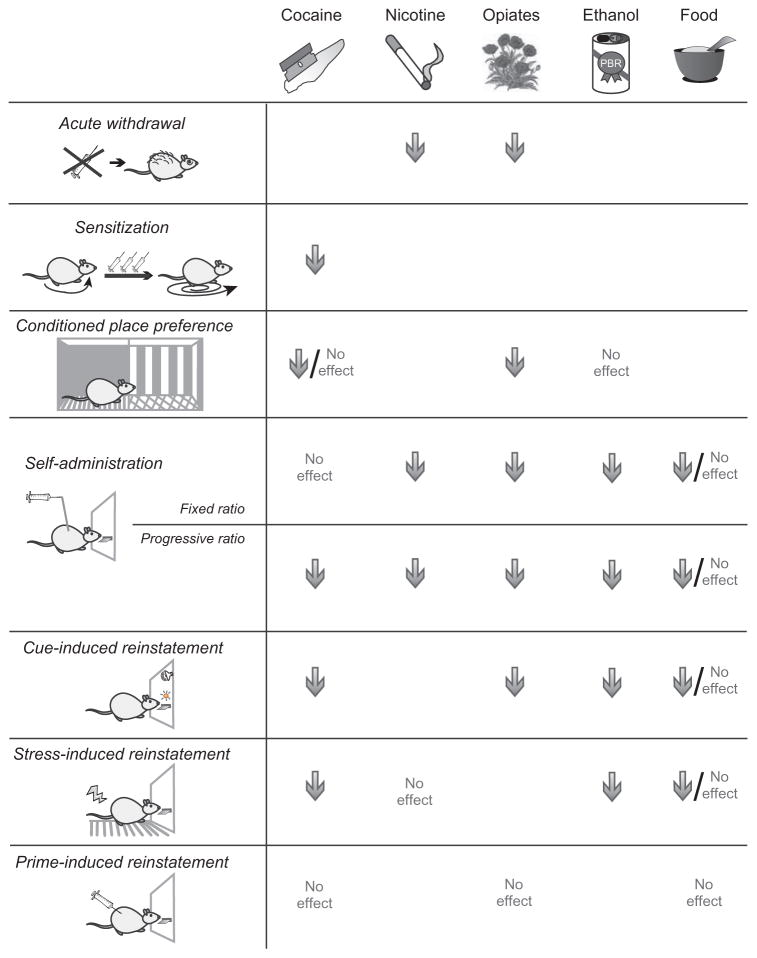
Numerous studies have shown that orexin plays important roles in drug seeking in animal models of addiction. However, the exact nature of these roles has remained elusive. This is most likely due to a complex involvement of orexin in several aspects of drug seeking, such as aversive as well as appetitive motivation, interactions with Pavlovian and/or instrumental learning processes, and hedonic states induced by drugs. This is further complicated by the fact that there are two orexin peptides that act at two receptors, which are distributed differently throughout the brain. Orexin also plays somewhat different roles in addiction to different substances (summarized in Fig. 1). In the following section, we review findings regarding orexin’s roles in reward and addiction for different types of drugs, as well as food.
Fig. 1.
Summary of orexin roles in addiction. Effects of systemic orexin 1 receptor antagonism on addiction-related behaviors according to reward type and animal model, summarized from studies described in this chapter. Arrows correspond to reduction or blockade of behavior by an orexin 1 receptor antagonist, “no effect” means that the behavior was unchanged, arrow/“no effect” indicates mixed results between studies using different procedures, and a blank space indicates that orexin antagonist effects on that behavior have not yet been reported for that drug. PBR, Progress in Brain Research. (For color version of this figure, the reader is referred to the online version of this chapter.)
Cocaine and amphetamines
The role of orexin in psychostimulant addiction has been relatively well studied. In general, orexin seems to increase the incentive motivational properties of conditioned cues and to enhance highly motivated seeking of stimulants, but not to affect the direct reinforcing properties of these drugs themselves (Fig. 2).
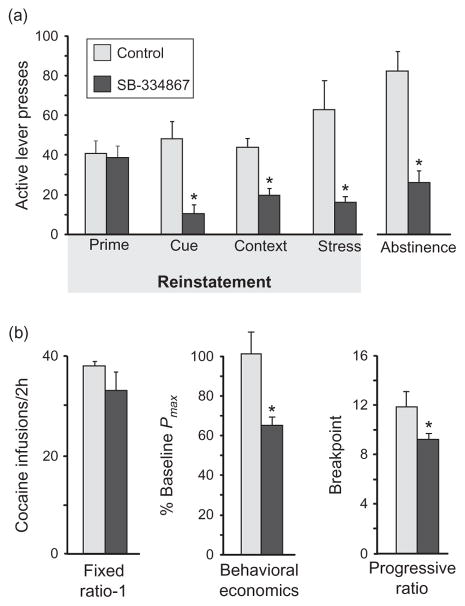
Fig. 2.
Effects of orexin 1 receptor antagonism on cocaine seeking and self-administration. (a) Evoked cocaine seeking. Effects of blocking orexin 1 receptors with systemic SB-334867 (30 mg/kg, i.p.) on instrumental cocaine seeking elicited by external stimuli are shown. SB-334867 blocks reinstatement of cocaine seeking driven by discrete cues, contextual cues, and stress, but not by a priming dose of cocaine. SB-334867 also reduces cocaine seeking after a period of abstinence, without intervening extinction training. Stress-induced reinstatement data are reproduced with permission from Boutrel et al. (2005). (b) Cocaine self-administration. SB-334867 reduces instrumental cocaine self-administration when high levels of motivation are required (progressive ratio), and decreases the value of cocaine in a decreasing dose behavioral economics paradigm, but fails to affect cocaine taking when low levels of effort are required (fixed ratio-1). Behavioral economics and progressive ratio data are reproduced with permission from Espana et al. (2010). *Indicates significant difference from control. (For color version of this figure, the reader is referred to the online version of this chapter.)
Orexin is involved in the development of stimulant-induced locomotor sensitization, and, in some cases, the expression of this sensitization as well. Administration of the OX1R antagonist SB-334867 systemically or into VTA blocked the acquisition of cocaine sensitization, but not the expression of sensitization in animals that were tested immediately after sensitization training (no abstinence period; Borgland et al 2006). In contrast, when a period of abstinence was given after amphetamine sensitization training, administration of SB-334867 or the OX1R/OX2R antagonist N-biphenyl-2-yl-1-[[(1-methyl-1H-benzimidazol-2-yl)sulfanyl]acetyl]-l-prolinamide (DORA) blocked the expression of sensitization (Quarta et al., 2010; Winrow et al., 2010). DORA also blocked plasticity-related gene expression in VTA caused by repeated amphetamine (Winrow et al., 2010).
To further understand how orexin is involved in the changes that occur following acute versus chronic (sensitizing) administration of psychostimulants, several research groups have investigated alterations in neuronal activation and protein expression levels. Acute methamphetamine or amphetamine increased Fos protein levels (a marker of neuronal activation) in DMH/PFA orexin neurons, but not in LH orexin neurons (Estabrooke et al., 2001; Fadel and Deutch, 2002; McPherson et al., 2007). Acute caffeine also increased Fos expression in these orexin neurons, indicating a common effect across stimulants (Murphy et al., 2003), and potentially reflecting the arousal-promoting effects of these drugs, as opposed to their reinforcing properties. However, McPherson et al. (2007) found that a sensitizing regimen of amphetamine increased Fos expression in both DMH/PFA and LH orexin neurons. Although psychostimulant exposure induces Fos in orexin neurons, expression levels of the orexin peptide are not changed. Acute or sensitizing regimens of cocaine had no effect on expression levels of orexin peptide or OX1R, although an escalating “binge” regimen of home cage cocaine exposure paradoxically decreased orexin mRNA levels in hypothalamus (Zhang et al., 2007; Zhou et al., 2008). Finally, a sensitizing regimen of cocaine, but not acute cocaine, upregulated OX2Rs in nucleus accumbens (NAc) for up to 60 days but did not affect OX2R protein levels in prefrontal cortex (PFC), VTA, hippocampus, or dorsal striatum (Zhang et al., 2007). In sum, orexin plays a role in sensitized locomotor responses to stimulants, and, therefore, potentially in incentive sensitization as well.
Orexin appears to play a consistent role in the expression of CPP for psychostimulants. Systemic administration of OX1R antagonists SB-334867 or GSK-1059865 attenuated cocaine and amphetamine CPP expression in rats (Gozzi et al., 2011; Hutcheson et al., 2011; Sartor and Aston-Jones, 2012), although one study failed to find an effect of a moderate dose of SB-334867 (20 mg/kg) on cocaine CPP in mice (Sharf et al., 2010a). Further evidence for an important involvement of orexin in drug preference comes from findings showing a correlation between CPP scores and the degree of Fos expression in LH, but not DMH/PFA, orexin neurons (Harris et al., 2005). Cocaine CPP was also associated with a decrease in LH orexin mRNA expression, indicating there may be some compensatory feedback to gene expression following strong neuronal activation (Zhou et al., 2008).
In stimulant self-administration paradigms, orexin is involved in cue-induced drug seeking and motivation to obtain cocaine when high levels of effort are required to obtain the drug, but not in the primary reinforcing properties of cocaine itself. For example, systemic or intracranial injections of SB-334867 have no effect on cocaine self-administration under low FR schedules of reinforcement (such as FR-1; Espana et al., 2010; Hutcheson et al., 2011; Smith et al., 2009b) (Fig. 2b). Orexin administration into the VTA or cerebral ventricles (ICV) also had no effect on cocaine intake under an FR-1 schedule (Boutrel et al., 2005; Espana et al., 2011). In addition, the priming effect of cocaine in an extinction/reinstatement model was not affected by either systemic or intra-VTA SB-334867 (Mahler et al., 2012) (Fig. 2a). Finally, a recent report showed that neither ICSS of LH nor the ability of cocaine to reduce ICSS thresholds was affected by systemic SB-334867 in mice (Riday et al., 2011). Together, these results suggest that neither the reinforcing nor the priming effects of cocaine are mediated by orexin.
However, orexin does seem to be involved in high-effort motivation for seeking stimulant drugs during self-administration. For example, systemic or intra-VTA SB-334867 reduced breakpoints for cocaine under a PR schedule of reinforcement (Borgland et al., 2009; Espana et al., 2010) and reduced the Pmax value of cocaine at high prices in a behavioral economics paradigm (Espana et al., 2010) (Fig. 2b). Conversely, intra-VTA orexin increased breakpoint in a PR task for cocaine (Espana et al., 2011). Additionally, intra-VTA SB-334867 reduced, and intra-VTA orexin increased, cocaine self-administration under a discrete trials (DT) reinforcement schedule, where animals have intermittent, 24-h access to cocaine (FR-1 schedule; three 10-min access periods/h, one infusion per access period; Espana et al., 2010, 2011). These findings may be explained by changes in motivation under this reinforcement schedule or by the influence of circadian or diurnal factors on orexin function (Estabrooke et al., 2001; Gompf and Aston-Jones, 2008; McGregor et al., 2011; Mignot et al., 2002; Moorman and Aston-Jones, 2010; Yoshida et al., 2001).
In general, orexin is also crucial for cocaine seeking triggered by external stimuli, such as stressors or cues. Systemic SB-334867 blocked footshock-induced reinstatement, and ICV orexin increased ICSS thresholds, suggesting that orexin can modulate stress and/or negative affect-related pathways in the brain (Boutrel et al., 2005) (Fig. 2a). ICV infusion of orexin reinstated cocaine seeking, and systemic administration of antagonists for cortico-tropin-releasing factor (CRF) or norepinephrine blocked this effect (Boutrel et al., 2005). However, the site of action for this stress-like effect is not likely to be VTA, because reinstatement triggered by intra-VTA orexin is not blocked by coadministration of a CRF antagonist, and footshock-induced reinstatement is not affected by intra-VTA SB-334867 (Wang et al., 2009). Systemic administration of SB-334867 also blocked reinstatement of extinguished cocaine seeking elicited by either discrete cues or contextual stimuli in male rats (Smith et al., 2009b, 2010) (Fig. 2a), though a recent paper found that discrete cue-induced reinstatement is not blocked by SB-334867 in female rats (Zhou et al., 2011). SB-334867 also reduced cocaine seeking following 1 day or 2 weeks of abstinence from self-administration with no extinction training, indicating that orexin may be involved in habitual drug seeking as well as reinstatement (Smith et al., 2010) (Fig. 2a). In contrast, systemic administration of the OX2R antagonist 4-PT failed to affect cue-induced reinstatement (Smith et al., 2009b). OX1R-mediated effects appear to require the VTA, as local administration of SB-334867 there attenuated cocaine seeking reinstated by either discrete cues or a DS (James et al., 2011; Mahler et al., 2012).
Regarding the role of orexin in the acquisition of conditioned cocaine cues, one study found that systemic SB-334867 attenuated cocaine CR, regardless of whether SB-334867 was administered during the CR task or during the initial FR-1 self-administration training (Hutcheson et al., 2011). These findings not only support a role for orexin in cue-induced drug seeking but also suggest that orexin may play a role in learning to ascribe motivational significance to cues during self-administration. However, previous results from our laboratory showed that SB-334867 had no effect on the acquisition of cues paired with cocaine during a single Pavlovian session in the self-administration chamber (Smith et al., 2009b). That is, administration of SB-334867 prior to a single cocaine-cue conditioning session had no effect on the subsequent ability of those cues to elicit reinstatement of extinguished cocaine seeking (Smith et al., 2009b). Orexin may therefore help ascribe motivational significance to drug cues under certain circumstances, but is not likely to be required for Pavlovian learning about the value or significance of cocaine cues per se.
In summary, orexin plays multiple roles in models of stimulant addiction. Orexin is required for stimulant locomotor sensitization, expression of cocaine CPP, and instrumental cocaine seeking when it is driven by highly motivated states, or external stimuli like cues and stressors. In contrast, orexin is not necessary for the primary reinforcing or priming effects of cocaine. This dissociation is important, in that orexin may specifically promote seeking of stimulant drugs, but leave intact the ability of these drugs to act on motivational or reward-related circuits themselves.
Nicotine
Orexin has also been evaluated for its role in nicotine addiction, which is one of the largest preventable causes of death in humans (CDCP, 2011). Despite the typical classification of nicotine as a stimulant, the involvement of orexin in nicotine reward is different than in cocaine or amphetamine reward. In particular, orexin appears to play a role in the reinforcing aspects of nicotine during self-administration, as well as in the aversive effects of nicotine, such as withdrawal and anxiety.
Somatic signs of antagonist-precipitated nicotine withdrawal were attenuated in orexin knockout mice and mice pretreated with the OX1R antagonist SB-334867, but not the OX2R antagonist TCSOX229 (Plaza-Zabala et al., 2011). This effect may be mediated via orexin actions in the paraventricular nucleus of the hypothalamus (PVN), as local administration of SB-334867 to this area also reduced nicotine withdrawal (Plaza-Zabala et al., 2011). Fos expression was increased in lateral and medial orexin neurons following acute nicotine administration or nicotine withdrawal, indicating that orexin neurons are activated by both nicotine exposure and subsequent withdrawal (Pasumarthi et al., 2006; Plaza-Zabala et al., 2011). Following chronic nicotine administration, orexin peptide and receptor expression was upregulated; however, there appears to be a concomitant decrease in the binding affinity of the receptor (Kane et al., 2000, 2001). This may explain, in part, why a study in human smokers found that orexin plasma levels (transported from the brain to blood for metabolism) were inversely correlated to nicotine craving during early withdrawal (von der Goltz et al., 2010).
Orexin also seems to be involved in anxiogenic effects of acute high-dose nicotine (0.8 mg/kg, s.c.). When an elevated plus maze was used to measure anxiety, knockout mice or mice pretreated with SB-334867 lacked an anxiogenic response to high-dose nicotine (Plaza-Zabala et al., 2010). This dose of nicotine increased Fos in orexin neurons in PFA and DMH, but not LH, as well as in PVN neurons. Nicotine-induced Fos in PVN was absent in orexin knockouts and mice pretreated with SB-334867 (Plaza-Zabala et al., 2010). These findings again implicate PVN orexin signaling in the aversive effects of nicotine, and indicate that medial orexin neurons might be particularly important in anxiogenic effects of nicotine.
A role for orexin in the reinforcing properties of nicotine has also been demonstrated with FR-5 or PR schedules of self-administration, where SB-334867 significantly decreased nicotine intake with no effects on food responding in food-restricted control rats (Hollander et al., 2008; LeSage et al., 2010). The OX1R/OX2R antagonist almorexant also reduced nicotine self-administration, but only at doses that also reduced food responding (LeSage et al., 2010). SB-334867 also blocked nicotine-induced reductions in ICSS threshold, while SB-334867 alone had no effect (Hollander et al., 2008). To identify a local site of action for orexin in nicotine reward, investigators examined the insular cortex, which has been implicated in cigarette smoking in humans (Naqvi et al., 2007; Wang et al., 2007). Local administration of SB-334867 to insular cortex decreased nicotine self-administration, confirming the importance of orexin in this site for nicotine reward (Hollander et al., 2008).
Finally, orexin also plays a role in reinstatement of nicotine seeking. ICV administration of orexin reinstated extinguished nicotine seeking in mice, an effect that was blocked by SB-334867 but not by the CRF1 receptor antagonist antalarmin (Plaza-Zabala et al., 2010). In contrast, footshock-induced reinstatement of nicotine seeking was reduced by antalarmin, but not by SB-334867 (Plaza-Zabala et al., 2010). This is different from what was observed for cocaine, where SB-334867 blocked footshock-induced reinstatement and a CRF antagonist blocked orexin-induced reinstatement (described above) (Boutrel et al., 2005), further emphasizing the point that orexin appears to play unique roles in addiction depending upon the type of drug.
In sum, orexin seems to play a role in both the reinforcing and aversive properties of nicotine, as well as in the aversive properties of nicotine withdrawal (Fig. 1). The PVN and insula may be particularly important for these effects. On the other hand, orexin seems to play a lesser role in stress-induced reinstatement of nicotine seeking.
Opiates
As with nicotine, orexin is involved in both self-administration of, and withdrawal from, opiates. Somatic signs of precipitated morphine withdrawal were reduced in orexin knockout mice, as well as in wild-type mice given SB-334867 (Georgescu et al., 2003; Sharf et al., 2008). This may not be surprising in light of the fact that approximately 50% of orexin neurons express the μ opioid receptor (Georgescu et al., 2003). Morphine withdrawal in mice caused an increase in cyclic AMP response element and c-fos 12th page in PDF mRNA in orexin neurons, but not in the interspersed melanin-concentrating hormone (MCH) neurons, as well as an increase in orexin mRNA expression in rats (Georgescu et al., 2003; Zhou et al., 2006). Further mouse studies found that withdrawal-associated Fos protein was present in DMH and PFA, but not LH orexin cells (Sharf et al., 2008). Systemic SB-334867 administration in mice reduced withdrawal-induced Fos expression in NAc shell, but not in locus coeruleus (LC) or VTA (Sharf et al., 2008). However, infusions of SB-334867 into LC reduced somatic signs of morphine withdrawal in rats (Azizi et al., 2010). Interestingly, administration of an OX2R antagonist, TCSOX229, into the paraventricular nucleus of the thalamus (PVT; not to be confused with PVN discussed above) reduced the expression, but not acquisition, of a conditioned place aversion for morphine withdrawal, whereas SB-334867 was ineffective (Li et al., 2011). This indicates that orexin actions in different brain areas may be involved in different aspects of the withdrawal process, such as physical symptoms versus aversiveness of withdrawal.
The first study linking the orexin system and drug reward found that Fos expression in LH orexin cells, and not those in DMH/PFA, was correlated with the degree of CPP for morphine, cocaine, or food in rats (Harris et al., 2005). Systemic administration of SB-334867 reduced the expression of morphine CPP in rats and mice (Harris et al., 2005; Sharf et al., 2010a). Orexin knockout mice showed a lack of morphine CPP and reduced morphine-induced hyperlocomotion (Narita et al., 2006), although neither of these effects was replicated in a subsequent report (Sharf et al., 2010a). VTA appears to be an important site of action for orexin’s role in opiate reward, as reinstatement of extinguished morphine CPP was triggered by either local administration of orexin in VTA or pharmacologic activation of LH orexin neurons (Harris et al., 2005); the latter was blocked by administration of SB-334867. Recent studies from our laboratory further characterized the pathway between orexin cells and VTA in the expression of morphine CPP (Richardson and Aston-Jones, 2012). In naïve rats, CPP behavior was correlated with Fos expression in LH orexin cells that project to rostral VTA (i.e., those containing retrograde tracer from rostral VTA). However, in rats with a history of morphine dependence and protracted abstinence, CPP behavior was instead correlated with Fos expression in LH orexin cells projecting to caudal VTA, indicating that the function of orexin projections to distinct VTA subregions differs based upon an animal’s state of opiate dependence/withdrawal (Richardson and Aston-Jones, 2012). These results also indicate a potentially important role of the orexin system in the behavioral and addictive properties of opiate dependence, extending earlier studies linking these cells to opiate dependence (Georgescu et al., 2003).
Further investigations found that LH orexin neurons were Fos activated following morphine administration in a novel context, but not when morphine was given in the home cage (Harris et al., 2007). When combined with previous findings that chronic morphine administration caused no change in either c-fos or orexin mRNA levels in orexin cells of mice when administered in a familiar place, these results indicate that orexin may play a special role in learning associations between contexts and morphine, rather than in mediating the intrinsic rewarding properties of morphine itself (Georgescu et al., 2003; Harris et al., 2007; Zhou et al., 2006). Accordingly, acquisition of morphine CPP was blocked in mice given intra-VTA SB-334867, rats with bilateral LH lesions, or rats with unilateral LH lesion combined with contralateral intra-VTA SB-334867 injected on CPP conditioning days (Harris et al., 2007; Narita et al., 2006).
Recently, we also investigated the role of orexin in heroin self-administration and reinstatement. We found that SB-334867 reduced, but did not block, self-administration of heroin on either FR-1 or PR schedules (Smith and Aston-Jones, 2012). Under both schedules of reinforcement, SB-334867 increased the time between self-administered heroin infusions, such that FR-1 responding was reduced throughout the 2-h session and PR responding was reduced primarily in the first 2 h of the session. This contrasts with what was observed for cocaine PR self-administration, where SB-334867 did not affect low-effort responding in PR, but only reduced high-effort responding required later in the session (and did not affect FR-1 responding at all) (Borgland et al., 2009; Espana et al., 2010). In addition, SB-334867 reduced cue-induced reinstatement of extinguished heroin seeking, but did not attenuate heroin-primed reinstatement, similar to what has been observed for cocaine (Mahler et al., 2012; Smith and Aston-Jones, 2012; Smith et al., 2009b).
Therefore, orexin appears to play different roles in stimulant and heroin reinforcement, adding to the known differences between the neural substrates underlying opiate versus stimulant reward (Badiani et al., 2011). However, orexin’s involvement in reinstatement seems to be similar for opiates and cocaine, such that orexin is required for cue-induced reinstatement but not drug-induced reinstatement for both drug types. In sum, orexin is required for cues to reinstate opiate seeking, and for the reinforcing and learning-related effects of acute opiates, but not for their priming effects.
Ethanol
A growing number of studies have demonstrated that the orexin system plays a significant role in ethanol consumption and abuse (Lawrence, 2010). However, heterogeneity across studies, both in methods and in outcomes, indicates that the role of orexin in ethanol seeking may be complex, as are the pharmacological actions of ethanol itself.
Perhaps the most direct evidence of the role of the orexin system in ethanol seeking is the relationship to ethanol drinking behavior. Orexin microinjections into LH and PVN increased voluntary ethanol, but not food, consumption in Sprague Dawley rats, whereas injection into NAc produced the opposite effect, arguing for circuit specificity in the influence of orexin in ethanol drinking (Schneider et al., 2007). Our laboratory has shown that systemic administration of SB-334867 decreased ethanol consumption and preference in Sprague Dawley rats using a two-bottle choice paradigm (Moorman and Aston-Jones, 2009). This effect was primarily expressed in rats that naturally showed a high predisposition for ethanol consumption/preference, indicating that orexin may be involved in the propensity to abuse ethanol. Chronic ethanol exposure causes alterations to orexin mRNA expression, but the direction of these changes is opposite in different studies. For example, Lawrence and colleagues found that chronic ethanol drinking increased orexin mRNA in the LH, but not DMH/PFA, orexin field in ethanol-preferring rats (Lawrence et al., 2006). On the other hand, Morganstern and colleagues reported that chronic ethanol drinking in Sprague–Dawley rats led to reduced orexin mRNA expression in the entirety of the orexin field, whereas acute ethanol administered through gavage increased orexin mRNA and peptide selectively in LH, but not DMH/PFA (Morganstern et al., 2010). These studies indicate that there may be differential roles played by orexin in ethanol reward, depending upon whether ethanol is self- or experimenter administered, and whether it is administered acutely or chronically.
The role of orexin in ethanol reward has also been examined using CPP models. Two groups found little effect of the OX1R antagonists SB-334867 or SB-408142 on the expression of ethanol CPP (Shoblock et al., 2011; Voorhees and Cunningham, 2011). Voorhees and Cunningham (2011) reported decreased CPP following SB-334867; however, neither vehicle- nor SB-334867-treated animals showed a strong ethanol preference in this study, and the authors failed to replicate the effect in animals expressing stronger CPPs. SB-334867 reduced ethanol-induced hyperlocomotion, with no effect on baseline locomotion (Voorhees and Cunningham, 2011), indicating a dissociation between the role of orexin in the rewarding and locomotor-stimulating effects of ethanol (Chester and Cunningham, 1999; Risinger et al., 1992). In contrast to the lack of OX1R antagonist effect on CPP expression, the OX2R antagonist JNJ-10397049 decreased acquisition, expression, and reinstatement of CPP in mice (Shoblock et al., 2011), raising the intriguing possibility that OX2R, but not OX1R, may be uniquely involved in the rewarding effects of ethanol in the CPP paradigm. Future studies need to investigate this issue (which may reflect differences in species, strains, or experimental design), as there appears to be a significant role for OX1R in ethanol preference and consumption in other models (as reviewed here).
Orexin is also linked to ethanol seeking in operant self-administration models. Systemic administration of SB-334867 decreased self-administration of ethanol in both ethanol-preferring rats and outbred Long-Evans rats, under FR-3 and PR self-administration schedules (Jupp et al., 2011a; Lawrence et al., 2006; Richards et al., 2008). However, a different study found that the OX2R antagonist JNJ-10397049, but not the OX1R antagonist SB-408124, decreased FR-3 ethanol self-administration in Wistar rats, again indicating that the OX2R could play a prominent role in ethanol reward (Shoblock et al., 2011). These authors suggest that the concentration of OX1R antagonists used in previous studies was high enough (20–30 mg/kg) to also have nonspecific actions at the OX2R. However, lower doses of SB-334867 (5–10 mg/kg) produced marked decreases in ethanol self-administration in another study, arguing that both OX1R and OX2R may be involved in instrumental ethanol self-administration (Jupp et al., 2011a).
As with other drugs, orexin seems to be involved in ethanol seeking in reinstatement models. Systemic SB-334867 reduced ethanol reinstatement driven by olfactory and/or light cues (Jupp et al., 2011a; Lawrence et al., 2006), yohimbine (Richards et al., 2008), or intra-LH infusion of neuropeptide S (Cannella et al., 2009). Orexin neurons were also activated during beer seeking, as evidenced by an increase in Fos expression following DS or context-induced reinstatement behavior (Dayas et al., 2008; Hamlin et al., 2007). Fos activation of orexin neurons was also positively correlated with the level of context-induced reinstatement of beer seeking, either only in LH (Hamlin et al., 2007) or in both LH and DMH/PFA (Millan et al., 2010). Interestingly, orexin may also be involved in reinstatement-related Fos activation of prelimbic and orbitofrontal PFC and NAc core. Following short-term and protracted abstinence from ethanol self-administration, ethanol cues induced reinstatement behavior as well as Fos in these structures, and systemic SB-334867 reduced both of these effects (Jupp et al., 2011b).
In another study, Dhaher et al. (2010) examined the role of orexin in ethanol seeking and self-administration following a period of abstinence. In female ethanol-preferring rats that underwent ethanol self-administration, extinction, and 2 additional weeks of abstinence, SB-334867 failed to affect the strong abstinence/context-induced ethanol seeking under extinction conditions (no alcohol upon lever pressing). However, when ethanol was available when levers were pressed in this final session, SB-334867 blocked the abstinence-induced increases in ethanol seeking seen in vehicle-treated animals. These results suggest that orexin may not be required for contextual reinstatement of ethanol seeking after abstinence in females, except when ethanol itself is present on this test session (paralleling the lack of blockade of discrete cue-induced reinstatement of cocaine seeking in female rats) (Zhou et al., 2011). This said, the bulk of the evidence supports a role for OX1R in driving stimulus-driven ethanol seeking either in the presence or in the absence of an ethanol reinforcer in most paradigms.
Finally, two groups recently reported an association between orexin blood levels and withdrawal symptoms in human alcoholics. Bayerlein et al. (2011) reported that orexin A mRNA levels in blood cells were lower in “recovered” (90+ days abstinent) alcoholics thanin those undergoing acute withdrawal in a clinic. However, orexin A expression was negatively correlated with the severity of physical ethanol withdrawal symptoms experienced by subjects. In another study, von der Goltz et al. (2011) reported that blood plasma levels of orexins (but not the stress-related hormones adrenocorticotropic hormone or cortisol) were positively correlated with psychological distress experienced by alcoholics acutely withdrawing in a clinic. While these preliminary clinical findings are intriguing, the relationship between blood and brain levels of orexin is far from clear, so future clinical studies examining the role of orexin in human alcoholism are required.
In summary, there is a clear role of orexin in ethanol seeking, although the specific nature of the involvement is dependent upon experimental methodologies. Like for other drugs, orexin is required for cue- and stress-induced ethanol seeking, and like for opiates and nicotine, OX1R activation is required for ethanol self-administration on both PR and FR schedules. One group has also found a potential selective role for OX2Rs in ethanol reward, a surprising result that merits follow-up study (Shoblock et al., 2011). Many additional questions remain regarding orexin’s role in ethanol seeking and reward—whether oral consumption of ethanol is required, whether orexin is selectively involved in high (as opposed to moderate) levels of drinking, and whether there are differences across species (mice vs. rats vs. humans). However, given the prominent role of the orexin system in reward seeking for drugs as well as food (see below and other reviews in this issue), it is not surprising that orexin is also important for consumption and seeking of the ingested, caloric drug ethanol.
Food
Obesity in humans can result from overeating that is akin in some ways to drug addiction. Feeding can be influenced by learned habits and preferences, and addictive drugs likely co-opt motivational brain circuits that evolved to regulate motivation for natural rewards like food (Kelley and Berridge, 2002; Nesse and Berridge, 1997; Volkow and Wise, 2005). Therefore, it seems pertinent to briefly review the literature on the role of orexin in feeding, particularly evidence that orexin may play an especially strong role in “reward-based feeding,” or food seeking and/or consumption beyond caloric or nutritive needs.
One of the original reports of the discovery of orexins showed that acute central administration of orexin A induced feeding behavior, leading the authors to coin the name “orexin,” after the Greek word for “appetite,” orexis (Sakurai et al., 1998). Since then, it has been shown that blocking OX1Rs with SB-334867 can also reduce food intake under certain circumstances (Haynes et al., 2000; Ishii et al., 2005; Rodgers et al., 2001). However, orexin-induced feeding is influenced by several factors including the sleep–wake cycle, hunger states, and palatability of the consumed food—indicating that orexin does more than simply increase food intake indiscriminately (Clegg et al., 2002; Haynes et al., 1999; Yamanaka et al., 1999). One common theme of orexin’s involvement in food seeking and consumption seems to be in mediating reward-based feeding, which is influenced by several factors including palatability and conditioned stimuli. We use the (admittedly imprecise) term “reward-based feeding” here, because the preponderance of evidence supports a role for orexin primarily in food seeking and consumption that involves particularly palatable and/or salient foods. This concept has also been referred to as overconsumption, hedonic feeding, or non-homeostatic feeding (Berthoud, 2011; Choi et al., 2010; Kelley et al., 2005; Pandit et al., 2011; Saper et al., 2002; Welch et al., 1996). Some evidence also supports a role for orexin in food seeking driven by conditioned cues (Cason et al., 2010; Harris et al., 2005; Petrovich and Reppucci, 2011). In these ways, orexin’s role in food seeking seems to parallel its role in drug seeking.
When food-restricted rats were trained to self-administer regular chow, there was no effect of acute administration of SB-334867 on FR-5 responding or PR responding, indicating that orexin is not necessary for all types of food self-administration and seeking (Borgland et al., 2009; Hollander et al., 2008; LeSage et al., 2010). However, chronic administration of SB-334867 or RNAi-mediated knockdown of the orexin gene reduced FR and PR self-administration of chow in food-restricted mice, indicating that chronically reduced orexin may result in more pronounced effects on food consumption, at least in mice (Sharf et al., 2010b). In addition, Petrovich and Reppucci (2011) reported that orexin neurons were Fos activated by a discrete, chow-predictive cue that induces feeding in sated rats, suggesting that orexin can play a role in chow intake under certain circumstances, such as when feeding is triggered by a conditioned cue.
In contrast to regular chow, when rats are trained to self-administer a high-fat food, SB-334867 reduced FR-1 responding in food-restricted animals, as well as PR responding in food-restricted and food-sated rats (Borgland et al., 2009; Choi et al., 2010; Nair et al., 2008). SB-334867 also reduced the high-fat diet overconsumption that occurs when rats sated on food chow are given access to high-fat food (Choi et al., 2010). Additionally, expectation of chocolate or daily chow also increased Fos expression in orexin neurons of PFA but not LH (Choi et al., 2010). Surprisingly, however, SB-334867 did not reduce reinstatement of high-fat food seeking elicited by orexin A, a food prime, or yohimbine (Nair et al., 2008). These high-fat food findings indicate that orexin plays a role in feeding when it is reward based, such as when self-administration or consumption involves highly palatable foods.
The role of orexin in reward-based feeding is not limited to high-fat foods, however. LH orexin neurons are Fos activated in relation to CPP expression for a sweet cereal reward in food-sated rats (Harris et al., 2005). Further, self-administration of sucrose pellets under FR-1 or PR schedules is reduced by SB-334867 in both food-sated and food-restricted rats, although results differ across studies (Angie Cason and Gary Aston-Jones, submitted; Cason et al., 2010; Espana et al., 2010). In addition, preliminary results from our laboratory show that SB-334867 reduced cue-induced reinstatement of sucrose seeking in food-restricted rats, but not significantly in food-sated rats, perhaps reflecting the greater incentive salience of sucrose cues when animals are hungry (Cason et al., 2010; Cason et al, submitted.). Again, this resembles the role for orexin in responding to cues associated with drug availability.
The hypothesis that orexin preferentially affects reward-based feeding is also supported by the finding that an ICV dose of orexin A that did not change chow intake in free-feeding rats caused an increased breakpoint for sucrose on a PR schedule of self-administration in rats (Choi et al., 2010). However, a higher dose of ICV orexin A was capable of inducing chow feeding in ad libitum fed animals (Choi et al., 2010; Sakurai et al.,1998). In addition, we have preliminary findings that SB-334867 decreased FR-1 self-administration of the non-nutritive but highly palatable sweetener saccharin and also reduced cue-induced reinstatement of saccharin seeking (Angie Cason and Gary Aston-Jones, unpublished findings).
For self-administration of liquid sucrose, reported effects of orexin blockade are somewhat variable. Richards et al. (2008) found that SB-334867 had no effect on FR-3 sucrose responding in food-sated rats, whereas Jupp et al. (2011a) reported that SB-334867 reduced FR-3 sucrose responding in ethanol-preferring, food-sated rats without affecting breakpoint under a PR schedule. Unlike for high-fat food, SB-334867 also blocked yohimbine-induced reinstatement of extinguished sucrose seeking (Richards et al., 2008). In contrast, SB-334867 had no effect on water self-administration in either rats or mice (Jupp et al., 2011a; Lawrence et al., 2006; Plaza-Zabala et al., 2010). Despite these discrepancies, the most consistent theme emerging from the literature is an involvement of orexin primarily in reward-based feeding and food seeking.
Further support for a role of orexin in reward-based feeding comes from studies examining interactions of orexin with other brain systems involved in reward-based feeding. For example, microinjections of the μ opioid agonist DAMGO into NAc preferentially induce intake of palatable, and especially fatty, foods (Baldo and Kelley, 2007; Kelley et al., 2002). High-fat feeding induced by intra-NAc DAMGO in food-sated rats was blocked by SB-334867 injected ICV or intra-VTA, and intra-NAc DAMGO failed to increase high-fat liquid (corn oil) intake in orexin knockout mice (Zheng et al., 2007). Further, intra-NAc DAMGO induced Fos in orexin neurons (particularly medial populations) that receive direct projections from the same sites in NAc where DAMGO was injected. However, injection of muscimol into NAc shell (which induces food intake) also induced Fos in LH, but not DMH/PFA, orexin neurons (Baldo et al., 2004), though this manipulation is thought to drive non-palatability-related aspects of feeding (Stratford and Kelley, 1997; Zhang et al., 2003). Finally, orexin may also modulate hedonic aspects of food consumption itself (which has been termed “liking”), as opposed to simply mediating motivated pursuit of food (termed “wanting”) (Berridge and Robinson, 2003). Microinjections of orexin A into the caudal ventral pallidum, a brain region thought to be particularly linked to hedonic “liking” of sucrose tastes (Smith and Berridge, 2005; Smith et al., 2009c), increased orofacial hedonic reactions to intraoral sucrose infusions in the taste reactivity paradigm (Berridge et al., 2010b).
Although several lines of evidence suggest that orexin may specifically promote reward-based (non-homeostatic) feeding, some groups have pointed out that orexin neurons also respond to homeostatic signals of food deprivation status, including glucose, leptin, and ghrelin, as well as dietary amino acids (Adamantidis and de Lecea, 2009; Cai et al., 1999; Griffond et al., 1999; Karnani et al., 2011; Moriguchi et al., 1999; Sakurai, 2002; Sutcliffe and de Lecea, 2000; Yamanaka et al., 2003a). In addition, neuropeptide Y stimulates feeding in an orexin-dependent manner, and this interaction may be modulated by leptin signaling (Niimi et al., 2001). However, it is worth noting that hunger and satiety signals do not simply induce homeostatic feeding but also modulate food hedonic palatability, as well as the salience of food and food-associated cues (Berridge et al., 2010b; Berthoud, 2011; Cabanac, 1971; Kelley et al., 2005; Kringelbach et al., 2003; O’Doherty et al., 2002).
Altogether, these findings indicate that orexin plays a role in food pursuit by promoting “reward-based feeding,” perhaps especially when motivation is high (e.g., food deprivation), foods are palatable, or conditioned cues are present. This may give insight into the involvement of orexin in drug addiction, particularly because orexin seems to play a role when strong rewards and their conditioned stimuli override normal homeostatic drives.
Summary of orexin’s roles in drug seeking
The previous sections have reviewed our understanding of orexin’s roles in drug reinforcement and drug seeking, and how these roles vary by drug (Fig. 1). For cocaine and amphetamine, orexin seems to play a role in sensitization and drug-seeking motivation, especially when triggered by external stimuli, such as discrete drug-paired cues, contexts, or stressors. However, orexin does not appear to be involved in the reinforcing or priming properties of cocaine (Fig. 2). For nicotine, orexin is involved in both primary reinforcement and withdrawal, but apparently not stress-induced reinstatement of seeking. For opiates, orexin again seems to play a role in drug seeking driven by cues but may also modulate the rewarding or reinforcing properties of opiates themselves. Orexin also mediates somatic and affective withdrawal from opiates via actions in several brain structures. For ethanol, most evidence suggests that orexin again mediates stimulus-driven drug seeking, and likely self-administration of ethanol as well. For food, orexin seems to promote “reward-based feeding” in particular, as occurs when the food reward is highly palatable.
Some common themes that emerge from these diverse studies are that orexin modulates some types of high-motivated reward seeking, especially when this seeking is triggered by external stimuli. However, the role of orexin in the reinforcing properties of drugs varies somewhat by the drug in question. One reason for this variation could have to do with the mechanisms by which drugs increase forebrain dopamine release, which is widely believed to modulate motivated reward seeking, especially when driven by conditioned stimuli (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999; Salamone et al., 2007; Satoh et al., 2003; Wanat et al., 2009). Nicotine, opiates, and ethanol increase dopamine by acting within the VTA, where they modulate glutamate and/or GABA inputs and cause increased dopamine cell firing (Cami and Farre, 2003). In contrast, cocaine and amphetamines increase dopamine primarily via actions at the terminal level to increase synaptic levels of the transmitter (Aston-Jones et al., 2010; Cami and Farre, 2003). Therefore, the neural circuitry in which orexin is embedded becomes very important for understanding the role of orexin in drug seeking and reward. In the next section, we review this circuitry, highlight inputs and outputs to orexin neurons, and discuss the role of these connections in addiction.
Brain circuits underlying orexin modulation of drug seeking
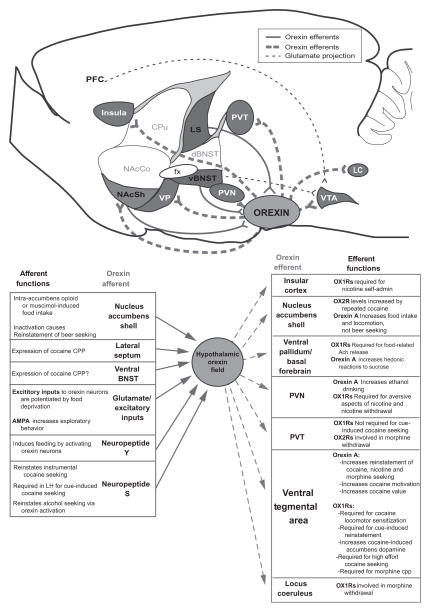
LH orexin neurons are anatomically located in an ideal position for reward processing, as they are interconnected with several reward-related macro-systems including mesocorticolimbic dopamine, extended amygdala, and intrahypothalamic circuits. Many studies have focused on the projection of orexin neurons to VTA, which plays a crucial role in reward- and addiction-related behaviors. However, less is known about functional roles played by other orexin targets, or by the numerous afferents to orexin neurons. Here, we review the current state of knowledge for these orexin connections (known reward-related functions of orexin afferents and efferents are summarized in Fig. 3) and highlight recent studies from our laboratory which indicate that the orexin afferents from lateral septum play a role in cocaine seeking. Finally, we review evidence indicating that VTA is a key site of action for orexin’s role in reward processing and that interactions between orexin and glutamate within VTA may be a particularly important mechanism for this role.
Fig. 3.
Summary of reward-related behavioral functions of orexin afferents and efferents. At top, a circuitry diagram shows the relative anatomical positions of orexin afferents (red/dark grey structures and solid lines) and efferents (blue/light grey structures and dashed lines) that may modulate reward-related behaviors via interactions with orexin. NAcSh is colored purple as both afferents and efferents of this structure interact with orexin to modulate reward. Glutamate inputs to VTA thought to interact with VTA orexin to modulate reward are represented with black dashed lines. At bottom, known reward functions of various orexin afferents and efferents are summarized. For further details and references, see text. ACh, acetylcholine; CPu, caudate/putamen; dBNST, dorsal bed nucleus of the stria terminalis; fx, fornix; insula, insular cortex; LC, locus coeruleus; LS, lateral septum; NAcCo, nucleus accumbens core; NAcSh, nucleus accumbens shell; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular thalamus; vBNST, ventral bed nucleus of the stria terminalis; VP, ventral pallidum; VTA, ventral tegmental area. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
Orexin afferents
Two studies using retro- and anterograde tracers in rats (Yoshida et al., 2006), or a genetically encoded retrograde tracer in mice (Sakurai et al., 2005), described the major afferents to the orexin field. Sakurai and colleagues showed strong inputs from medial PFC (mPFC), NAc shell, BNST, basal fore-brain, basolateral and medial amygdala, preoptic area, arcuate nucleus, periventricular hypothalamus, and medial raphe. Yoshida and colleagues further showed that the LH orexin field, in particular, receives strong inputs from NAc shell, dorsolateral septum, BNST, ventral pallidum, central amygdala, VTA, and dorsal raphe, whereas PFA/DMH orexin neurons receive relatively stronger inputs from the subiculum, preoptic area, ventromedial and anterior hypothalamus, and arcuate nucleus.
In our laboratory, we sought to determine which inputs to orexin neurons modulate their roles in reward processing, by examining neuronal activation of these afferents during expression of cocaine CPP. Using injections of the retrograde tracer cholera toxin B into either lateral or medial orexin neuron fields, we confirmed the variety of forebrain regions (summarized above) that project to the orexin field. We quantified activation in these afferents associated with the expression of cocaine CPP using double immunohistochemical labeling for the tracer and Fos. Results showed that neurons projecting to the LH orexin cell field from the rostral lateral septum and ventral BNST (vBNST) showed unique activation during cocaine CPP, and the degree of Fos activation of these afferents correlated with the degree of CPP expression (Sartor and Aston-Jones, 2012). We describe those results below and discuss other orexin inputs that may be important for reward and drug seeking.
Lateral septum has long been implicated in reward and addiction in humans and rodents and is considered to be a key node in brain circuits underlying pleasure (Heath, 1996; Olds and Milner, 1954) (though see Smith et al., 2009a for an argument that these studies may have been generating incentive motivation rather than pleasure). For the past several decades, lateral septum received relatively little attention as a brain region involved in reward, although some reports showed that exposure to cocaine or cocaine-paired environments increased Fos expression and electrophysiological activity there (Brown et al., 1992; Franklin and Druhan, 2000; Lesse and Harper, 1985; Shoji et al., 1998; Simms and Gallagher, 1996). Our laboratory also recently revealed an important connection between caudal lateral septum and VTA in context-induced reinstatement of cocaine-seeking behaviors (Luo et al., 2011).
Given our finding that rostral lateral septum afferents to the LH orexin field were Fos activated during expression of cocaine CPP, we asked whether this projection is necessary for cocaine CPP. We found that inactivation of rostral lateral septum with the GABA agonists baclofen/muscimol attenuated the expression of cocaine CPP, as well as Fos activation in LH, but not DMH/PFA, orexin neurons. Next, we used a bilateral disconnection technique in which unilateral temporary inactivation (with baclofen/muscimol) of lateral septum was combined with contralateral knockdown of orexin expression in LH neurons via an orexin antisense morpholino, an oligomer that specifically inhibits production of orexin but spares orexin and interspersed MCH neurons themselves (Reissner et al., 2012). Again, we found that septal inputs to LH orexin neurons were essential for cocaine CPP expression (Sartor and Aston-Jones, 2012). Interestingly, rostral lateral septum receives strong projections from the dorsal hippocampus (Risold and Swanson, 1997a,b), a region highly implicated in processing of contextual cues (Luo et al., 2011; Vorel et al., 2001; Winocur and Gilbert, 1984). Based on the current studies and previous anatomical reports, we hypothesize that rostral lateral septum acts as a relay between contextual information from the dorsal hippocampus and reward processing in LH orexin neurons.
BNST also plays an important role in drug-seeking behaviors, likely due in part to its involvement in stress and anxiety (Buffalari and See, 2011; Dumont et al., 2005; Leri et al., 2002). As described above, we recently found that vBNST neurons that project to LH were activated during cocaine CPP. Accordingly, we also found that bilateral inactivation of vBNST blocked cocaine CPP, showing that BNST is necessary for the expression of conditioned cocaine seeking. However, vBNST inactivation increased Fos in LH orexin and non-orexin neurons (Sartor and Aston-Jones, 2010), which is surprising given that we previously found LH orexin neurons to be Fos activated in proportion to cocaine preferences (Harris et al., 2005). It is presently unclear why vBNST inactivation results in both reduced CPP and increased Fos in LH orexin neurons. One possibility is that Fos activation of LH in this study reflects not increased firing of LH orexin neurons but instead reflects inhibition of these neurons. Another possibility is that inhibiting vBNST GABA projection neurons disinhibits LH orexin and non-orexin cells, but that activation of these LH neurons (as measured with Fos) does not cause cocaine CPP, and is instead only correlated with the expression of CPP, caused by other neuronal substrates. For example, it may be that vBNST projections to LH orexin neurons are not required for cocaine CPP, but rather that vBSNT projections to other brain regions are required (e.g., glutamatergic projections to VTA) (Georges and Aston-Jones, 2001, 2002). These findings leave several tantalizing questions about the neurochemical phenotypes and mechanisms involved in vBNST regulation of orexin, requiring additional study to resolve.
We did not find that NAc shell projections to the orexin field were Fos activated in relation to cocaine CPP; however, other reports indicate that this pathway modulates reward. Tract-tracing studies from our laboratory and others revealed that NAc shell strongly projects to the LH orexin area in rats (Hamlin et al., 2008; Marchant et al., 2009; Yoshida et al., 2006; Zheng et al., 2007), though one study in mice reported that the NAc projection is primarily to anterior LH, rostral of the orexin field (Sano and Yokoi, 2007). Functionally, injections of the GABAA agonist muscimol into NAc shell strongly enhance feeding behavior and Fos expression in LH, but not DMH/PFA, orexin neurons (Baldo et al., 2004). Similarly, temporary inactivation of NAc shell with baclofen/muscimol triggered reinstatement of beer seeking and Fos activation of LH orexin neurons (Millan et al., 2010). In the same study, concurrent inactivation of LH blocked reinstatement produced by inactivation of NAc shell, indicating that connections between NAc shell and LH orexin neurons are involved in this type of reward seeking.
Several reports indicate that glutamate has a major role in regulating orexin neuronal activity and release. Orexin neurons are strongly depolarized by glutamate, and glutamatergic axons outnumber all other inputs on orexin cells (Horvath and Gao, 2005; Li and van den Pol, 2005), though GABA terminals also contact a substantial number of orexin neurons (Henny and Jones, 2006). Furthermore, food deprivation potentiates excitatory currents on orexin cells, suggesting that glutamatergic input to orexin cells may be important in driving motivated behaviors, at least for food reward (Horvath and Gao, 2005). Other neurotransmitters and peptides that have been shown to influence orexin activity and that are implicated in a variety of reward-seeking behaviors include acetylcholine, cannabinoids, CRF, dopamine, dynorphin, GABA, ghrelin, leptin, μ opioids, neuropeptides Y and S, neurotensin, norepinephrine, serotonin, and vasopressin (Bayer et al., 2005; Cannella et al., 2009; Fu et al., 2004; Hakansson et al., 1999; Huang et al., 2007; Kallupi et al., 2010; Li and van den Pol, 2005, 2006, 2008; Liu et al., 2002; Muraki et al., 2004; Ohno et al., 2008; Taslimi et al., 2011; Tsujino et al., 2005; Winsky-Sommerer et al., 2005; Yamanaka et al., 2003b).
Orexin efferents
Orexin neurons send extensive projections throughout the brain (Baldo et al., 2003; Date et al., 1999; de Lecea et al., 1998; Fronczek et al., 2005; Nambu et al., 1999; Peyron et al., 1998; Sakurai et al., 1998; Thannickal et al., 2000; van den Pol, 1999), and OX1Rs and OX2Rs are widely expressed in both unique and overlapping patterns (Cluderay et al., 2002; Hervieu et al., 2001; Marcus et al., 2001; Suzuki et al., 2002; Trivedi et al., 1998). OX1Rs are densely expressed in prelimbic, infralimbic, and insular cortices, BNST, laterodorsal tegmental nucleus, and LC. In contrast, OX2Rs are more densely expressed in NAc shell, CA3 of dorsal hippocampus, LH, and PVT. The dorsal raphe and VTA have moderate levels of both receptors (Marcus et al., 2001). Functionally, most studies have focused on orexin projections to VTA, due to the known importance of this region in reward processing. These studies are elaborated upon in the following section, followed by a summary of the few studies examining functional roles of other orexin efferents throughout the brain.
Ventral tegmental area orexin
The VTA is a brain region where orexin appears to play a prominent role in drug and natural reward seeking. Although OX1R and OX2R are expressed in VTA (Marcus et al., 2001; Narita et al., 2006), there are reported to be few orexin-containing synapses there (Balcita-Pedicino and Sesack, 2007), suggesting that the majority of orexin input is via nonsynaptic release by en passant fibers, or nonsynapsing terminals. Nonetheless, a large number of behavioral experiments have shown that VTA orexin is necessary and sufficient for several types of reward-seeking behavior, demonstrating that this is a crucial site for the reward-related effects of orexin.
In general, VTA orexin signaling appears to be particularly important for high-effort motivation, and reward seeking driven by external stimuli. Intra-VTA orexin increased cocaine self-administration under PR and DT reinforcement, but not under simple FR-1 reinforcement (Espana et al., 2011). Intra-VTA orexin also reinstated extinguished drug-seeking behavior in both cocaine self-administration and morphine CPP paradigms (Harris et al., 2005; Wang et al., 2009). Conversely, intra-VTA SB-334867 reduced the acquisition of cocaine sensitization and morphine CPP (Borgland et al., 2006; Narita et al., 2006), and LH orexin projections to VTA are necessary for learning morphine CPP (Harris et al., 2007). Intra-VTA SB-334867 also reduced the amount of effort expended for cocaine in PR, DT, and decreasing-dose (behavioral economics) self-administration paradigms, but not at FR-1 reinforcement schedules (Espana et al., 2010). Intra-VTA SB-334867 also reduced reinstatement of cocaine seeking elicited by cues, but not by cocaine prime or footshock (Mahler et al., 2012; Wang et al., 2009). These findings strongly point to VTA as a key site in which orexin acts to facilitate seeking of a variety of drugs.
One of the key ways in which VTA orexin may act to increase conditioned reward seeking is by modulating forebrain dopamine neurotransmission, which has been tied to reward seeking and approach efforts (Berridge and Robinson, 1998; Cheer et al., 2007; Fiorillo et al., 2003; Ikemoto and Panksepp, 1999; Salamone et al., 2007; Satoh et al., 2003). In support of this, intra-VTA administration of orexin A increased dopamine release in NAc and PFC as measured by microdialysis or voltammetry (Espana et al., 2011; Vittoz et al., 2008). Intra-VTA infusion of orexin A also increased local glutamate and dopamine release, which was attenuated by coadministration of the AMPA/NMDA antagonist kynurenic acid, but not by a CRF antagonist (Wang et al., 2009). Conversely, intra-VTA SB-334867 reduced cocaine-induced dopamine overflow in NAc but failed to block footshock-induced glutamate or dopamine release (Wang et al., 2009).
One mechanism by which orexin elicits its influence on VTA dopamine cell firing is via direct depolarization of dopamine neurons, which occurs especially with high orexin concentrations (Korotkova et al., 2003). However, orexin also affects dopamine cell activity via its interactions with several other neurotransmitters within VTA, and these interactions may be critical for understanding orexin’s role in reward. In particular, orexin interactions with glutamate in VTA appear to be an important mechanism by which orexin acts during reward processing (Borgland et al., 2006; Moorman and Aston-Jones, 2010), as discussed in further detail in the following section. Another possible interaction is with endo-cannabinoid receptors, which form heterodimers with OX1Rs in some cases (Ellis et al., 2006; Milligan and Smith, 2007). Additionally, activating LH orexin neurons with the cholinergic agonist carbachol induces a CPP, which is blocked by intra-VTA administration of the cannabinoid 1 receptor antagonist AM251 (Taslimi et al., 2011). Orexin/cannabinoid interactions are intriguing but still poorly understood. In contrast, interactions of orexin with glutamate in VTA have been studied more extensively and appear to be critical for conditioned drug seeking.
Interactions of orexin and glutamate in ventral tegmental area
Glutamate inputs control both tonic and burst firing of VTA dopamine neurons, and these inputs are very likely involved in the capacity of dopamine neurons to perform their roles in motivation and reward prediction (Hyman et al., 2006; Wise and Morales, 2010; Zellner and Ranaldi, 2010). However, VTA glutamate also interacts with other inputs to activate dopamine cells, including orexin. As previously mentioned, orexins A and B can directly excite VTA dopamine neurons (Korotkova et al., 2003; Moorman and Aston-Jones, 2010; Muschamp et al., 2007), but orexin also has a profound influence on dopamine neurons by affecting glutamatergic plasticity, as described in detail elsewhere in this issue (Baimel and Borgland, 2012). Briefly, orexin A application to VTA slices enhanced glutamatergic synaptic strength on dopamine neurons primarily via trafficking of NMDA and AMPA receptors to the synapse (Borgland et al., 2006). Similar excitatory plasticity was produced by cocaine exposure, and this effect was blocked by SB-334867. In addition, orexin B influences VTA dopamine neuron synaptic plasticity via actions at OX2R (Borgland et al., 2008), although the mechanisms of this pathway are less clear.
We speculated that PFC input to VTA might be one important source of glutamatergic influence on dopamine neurons that is regulated by orexin (Aston-Jones et al., 2010). mPFC in the rat has a robust glutamatergic projection to VTA (Geisler et al., 2007), and mPFC activity is associated with reward seeking and other goal-driven behaviors (Grabenhorst and Rolls, 2011; Grace et al., 2007; Kalivas et al., 2005; Peters et al., 2005; Phillips et al., 2008; Zavala et al., 2003). To test this interaction, we recorded dopamine neurons of anesthetized rats while stimulating mPFC [specifically the prelimbic–infralimbic border region, where the strongest glutamatergic projection originates (Geisler et al., 2007)] and simultaneously applied either orexin A or SB-334867 to the recorded neuron using a double-barreled pipette (Moorman and Aston-Jones, 2010). As demonstrated by other groups (Korotkova et al., 2003; Muschamp et al., 2007), we observed increases in dopamine neuron activity when orexin was applied alone. However, orexin also enhanced mPFC-evoked responses in VTA dopamine neurons, in some cases, revealing evoked responses that were not observed with mPFC stimulation alone (Fig. 4a). Accordingly, application of SB-334867 to dopamine neurons decreased mPFC-evoked responses. These results indicate that the orexin system plays an important role in regulating PFC control over dopamine neuron activation.
Fig. 4.
Interactions of orexin and glutamate in ventral tegmental area. (a) Orexin potentiates glutamatergic control of VTA dopamine neurons. Top panels: Example of short-latency mPFC-evoked excitatory responses in a dopamine neuron before (left) and after microinfusion onto the recorded neuron (right) of orexin-A (ORX-A, 60 nl, 1.4 μM). Short-latency-evoked responses were enhanced both during and following ORX-A administration. Time 0.00 demarcates the time of mPFC stimulation (50 pulses, 0.5 Hz). Bottom panels: Example of long-latency-evoked responses before (left) and during microinfusion (stim+infusion; right) of ORX-A onto a recorded DA neuron. Long-latency-evoked responses were enhanced by ORX-A administration. Modified with permission from Moorman and Aston-Jones (2010). (b) Enhancing AMPA neurotransmission rescues cue-induced reinstatement blocked by OX1R antagonism. The allosteric modulator PEPA was used to facilitate effects of endogenously released glutamate at AMPA receptors in VTA. Intra-VTA PEPA alone failed to cause or potentiate reinstatement of cocaine seeking. VTA microinjections of SB-334867 blocked cue-induced reinstatement, which was reversed by coadministration of PEPA into VTA (see Mahler et al., 2012) for more details. (c) Hypothesized orexin–glutamate interaction in VTA leads to reinstatement. Upper panel: In VTA dopamine cells, orexin potentiates AMPA and NMDA responses to VTA glutamate inputs (Borgland et al., 2006). During reinstatement, orexin facilitates VTA AMPA responses to cue-related glutamate inputs, allowing cues to promote renewed drug-seeking behavior and relapse. Lower panel: When OX1Rs are blocked in VTA with local microinjections of SB-334867, AMPA signaling is not potentiated, reducing the motivational impact of cue-related glutamate inputs, and attenuating cocaine-seeking behavior. (For color version of this figure, the reader is referred to the online version of this chapter.)
A role for VTA orexin in facilitating mPFC control of dopamine neuronal activity may be one reason why previous reports found that mPFC stimulation failed to evoke direct responses in dopamine neurons (Tong et al., 1996), despite the strong mPFC projection to VTA (Carr and Sesack, 2000; Geisler et al., 2007). From a systems/behavioral neuroscience perspective, the interaction between orexin- and reward-related mPFC inputs in VTA may have functional importance. Orexin neurons are activated in circumstances of heightened arousal and motivation (Siegel, 2004), and in response to reward-associated stimuli (Aston-Jones et al., 2010; Harris et al., 2005). mPFC is also known to modulate behavioral responses to salient stimuli and to coordinate goal-directed behaviors (Balleine and O’Doherty, 2010; Miller and Cohen, 2001). The simultaneous activation of PFC inputs and orexin release in VTA could be involved in the detection of important environmental stimuli and generation of appropriate stimulus salience and goal-directed motivated behaviors (Berridge, 2007; Bromberg-Martin et al., 2010; Salamone et al., 2007; Schultz, 2007).
Given previous findings that orexin increases responses of VTA dopamine neurons to glutamate in brain slices, and our recent demonstration that orexin facilitates control of dopamine neurons by PFC glutamate inputs in anesthetized rats, we next asked whether orexin also potentiates VTA glutamate signals that influence drug-seeking behaviors in awake, behaving animals. Previously, Wang et al. (2009) found that intra-VTA orexin A caused reinstatement of cocaine seeking, and increased local extrasynaptic glutamate levels. Both of these effects were attenuated by coadministration of the AMPA/NMDA antagonist kynurenic acid, supporting a link between VTA orexin and glutamate in reinstatement. We therefore asked whether endogenous VTA orexin also potentiates glutamate transmission in a manner that is required for cue-induced reinstatement (Mahler et al., 2012).
We first determined that bilateral intra-VTA injections of either the SB-334867 or the AMPA antagonist CNQX robustly attenuated cue-induced reinstatement of cocaine seeking. We also found that simultaneous VTA blockade of orexin in one hemisphere and glutamate in the contralateral hemisphere similarly attenuated cue-induced reinstatement, indicating that both VTA orexin and AMPA signaling are concurrently needed for cues to elicit cocaine seeking. Based on work by Borgland et al. (2006), we further hypothesized that orexin facilitates cue-induced glutamate inputs by promoting plasticity in dopamine neurons. To address this possibility, we next asked whether potentiating AMPA transmission via a non-orexin-dependent mechanism would rescue reinstatement reduced by intra-VTA SB-334867. We employed the positive AMPA allosteric modulator PEPA, which increases cellular responses to endogenously released glutamate without direct AMPA agonist effects (Kessler and Arai, 2006; LaLumiere et al., 2010; Sekiguchi et al., 1997; Zushida et al., 2007), and found that intra-VTA PEPA completely reversed deficits in cue-induced reinstatement induced by intra-VTA SB-334867 (Fig. 4b). Importantly, PEPA alone did not induce reinstatement (presumably because cue-related glutamate inputs were not active in the absence of cues, and therefore this allosteric modulator was inactive), nor did it potentiate reinstatement in the presence of intact orexin inputs (presumably because orexin had already facilitated AMPA transmission maximally; Fig. 4b). These findings support the idea that orexin normally facilitates the ability of cue-related VTA glutamate inputs to control dopamine cells and to elicit reinstatement of cocaine-seeking behavior.
In this same set of studies, we found no effect of the NMDA antagonist AP-5 on cue-induced reinstatement when administered in VTA, despite the previously described action of orexin to rapidly facilitate responses on VTA dopamine neurons via NMDA receptors (Borgland et al., 2006). Presumably, orexin-induced enhancement of NMDA receptor function is related to functions of VTA that are not involved in the expression of reinstatement behavior, such as appetitive learning (Ranaldi et al., 2011; Zellner et al., 2009). These data provide the first behavioral evidence that orexin may act in VTA to facilitate cue-related glutamate transmission at AMPA receptors on dopamine cells, thereby transforming them into real-time triggers of cocaine seeking (Fig. 4c).
Functions of other orexin efferents
Although VTA is the orexin efferent for which a role in reward seeking is best characterized, it is not the only efferent involved in reward-related behaviors. One potentially important target of the orexin system is nucleus accumbens, where OX2R protein expression is upregulated in a long-lasting manner following repeated cocaine administration (Zhang et al., 2007). NAc has moderate to high levels of OX2Rs in particular (Marcus et al., 2001; Trivedi et al., 1998), and orexins A and B both alter neuronal activity in NAc (Mori et al., 2011; Mukai et al., 2009). Administration of orexin A into NAc stimulates food intake, but not ethanol intake (Schneider et al., 2007; Thorpe and Kotz, 2005). However, a retrograde tracing study by Sharf and colleagues reported that orexin neurons send only few projections into NAc core or dorsal NAc shell (Sharf et al., 2008). It is clear, then, that orexin indirectly influences NAc by facilitating dopamine release there via midbrain projections (Espana et al., 2011; Narita et al., 2006; Quarta et al., 2010).
There are only a few brain areas outside VTA and NAc where the reward-related functions of orexin have been studied in any detail. For example, there is evidence that orexin has a unique influence in nicotine addiction via projections to insular cortex, as OX1R antagonism there attenuated nicotine self-administration (Hollander et al., 2008). Additionally, strong orexin inputs to basal forebrain may promote cortical acetylcholine release related to palatable food presentation (Frederick-Duus et al., 2007). In hypothalamus, injections of orexin A into LH or PVN stimulate ethanol intake in rats (Schneider et al., 2007). OX1R signaling in LC and OX2R signaling in PVT are also involved in nalox-one-precipitated morphine withdrawal (Azizi et al., 2010; Li et al., 2011), potentially reflecting a role for orexin in these areas in modulating stress, anxiety, or arousal. On the other hand, SB-334867 micro-injections into vBNST had no effect on cocaine CPP(SartorandAston-Jones,2010), and SB-334867 injections into PVT had no effect on cue-induced reinstatement of cocaine seeking (James et al., 2011). The small number of studies investigating functions of orexin outside the mesocorticolimbic dopamine system limits our understanding of orexin’s role in wider motivation-related brain circuits. Additional research is needed to elucidate the likely roles played by the orexin system in its multiple targets throughout the brain.
Behavioral pharmacology of orexin
Most of the experimental studies described in this review have used pharmacological methods to explore the role of orexin in drug reward and addiction. However, all pharmacological studies are inherently limited by the drugs available to manipulate neurochemical systems. Here, we will briefly discuss the pharmacological agents that have been used for manipulating orexin in addiction research (for more complete reviews of the available orexin antagonists, see Roecker and Coleman, 2008; Scammell and Winrow, 2011; Gotter et al., 2012).
For studies aimed at determining the effects of orexin receptor agonism, the peptides orexin A or B have been used, as there is no synthetic orexin agonist currently available. Like most other peptides, the orexins have the limitation of very poor permeability of the blood–brain barrier (Fujiki et al., 2003) and, therefore, need to be either administered into the cerebral ventricles or microinjected into brain regions of interest [though intranasal administration may also be centrally effective (Deadwyler et al., 2007; Dhuria et al., 2009)]. Intracranial administration requires that animals undergo surgery to implant chronic cannulae, which is invasive and time consuming. In addition, orexin A binds with equal affinity to both OX1R and OX2R, whereas orexin B binds relatively specifically to OX2R (Sakurai et al., 1998). These factors should be kept in mind for studies positing receptor-specific effects of direct injections of orexin peptides.
The majority of studies have investigated antagonism of orexin receptors, and the most commonly used compound in behavioral studies is the OX1R antagonist SB-334867. This agent has 50-fold selectivity for OX1Rs over OX2Rs and 100-fold selectivity for OX1Rs over approximately 50 other receptor targets (Porter et al., 2001; Smart et al., 2001). A similar compound SB-408124 has been used in some studies and possesses comparable selectivity (Langmead et al., 2004; Wang et al., 2009). However, these SB compounds have several practical limitations. First, SB-334867 has poor solubility in aqueous solutions at concentrations over 5 mg/ml (often used for systemic and intracranial studies). Therefore, vehicle preparations typically consist of various concentrations of the solvent DMSO and/or the hydrophilic caging compound cyclodextrin. At high concentrations, this forms a suspension that can be injected with a 23-g needle (i.p. or s.c.). If lower concentrations are used for intracranial administration (e.g., 1 mM), we found that the SB compounds will generally suspend in artificial cerebrospinal fluid after sonication and agitation. A second limitation is that, in our experience, SB-334867 is a relatively unstable compound, and the molecule can degrade in the presence of water (Anita H. Lewin, RTI, personal communication)—therefore requiring daily preparation of the compound for injection. Finally, there is a great deal of variability for SB-334867 among different suppliers and batches, which results in differences in molecular weight, color, solubility, and dose–response properties (which might account for some of the variability in dosing and vehicles used by different laboratories). Additional OX1R antagonists are being developed in an attempt to improve binding specificity and address other issues present with SB-334867 (Di Fabio et al., 2011; Gozzi et al., 2011; McDonald, 2011).
Although one study (Shoblock et al., 2011) showed that the OX2R may play a role in ethanol CPP and self-administration, most experiments have shown that this receptor plays a larger role in arousal than in reward. For example, in our laboratory, we tested the OX2R antagonist 4-PT (also called TCS OX229), which has 250-fold selectivity over OX1R and 50-fold over other targets (Hirose et al., 2003). We found no effect of 4-PT on cocaine self-administration or cue-induced reinstatement, but this compound substantially reduced locomotor activity (Smith et al., 2009c). This is consistent with previous reports that signaling at the OX2R, rather than OX1R, has been implicated in the modulation of arousal (Akanmu and Honda, 2005; Marcus et al., 2001; Willie et al., 2003). Increased measures of sleep were observed in rats following administration of the OX2R antagonist JNJ-10397049, but not the OX1R antagonists SB-408124 or GSK-1059865 (Dugovic et al., 2009; Gozzi et al., 2011). Moreover, dogs lacking the OX2R show narcoleptic symptoms (Lin et al., 1999). In a locomotor chamber, the OX2R antagonists EMPA or 4-PT both reduced locomotion in rats (Malherbe et al., 2009; Smith et al., 2009b). On the other hand, SB-334867 has been shown repeatedly to have little or no effect on locomotor activity in rats and mice at moderate to high doses, either systemically or intra-VTA (James et al., 2011; Richards et al., 2008; Sartor and Aston-Jones, 2012; Sharf et al., 2010b; Smith et al., 2009b; Voorhees and Cunningham, 2011). Therefore, OX2R seems in most cases to be involved in arousal rather than reward, at least for drugs other than ethanol.
Finally, a few studies have tested dual OX1R and OX2R antagonists on addiction behaviors. The dual orexin receptor antagonist DORA reduced the development of amphetamine sensitization, and the dual antagonist almorexant (ACT-078573 or SB-649868) reduced nicotine self-administration (LeSage et al., 2010; Winrow et al., 2010). However, nicotine self-administration was only affected at doses that also reduced food self-administration, whereas SB-334867 affected nicotine taking without affecting food self-administration (LeSage et al., 2010). This again indicates that OX2R signaling may be importantly involved in regulating arousal. Dual antagonists such as DORA and almorexant, as well as the compound MK-4305, are currently being explored clinically for their sleep-promoting effects (Brisbare-Roch et al., 2007; Coleman et al., 2010; Malherbe et al., 2009; Scammell and Winrow, 2011; Whitman et al., 2009; Winrow et al., 2010).
Reconciling orexin’s roles in appetitive motivation versus stress
Clearly, orexin plays a complex role in addiction that varies based on the aspect of addiction being studied (e.g., appetitive motivation for drugs vs. the rewarding effects of drugs themselves), as well as the addictive drug in question. Further, orexin itself acts like a reinforcer in certain experiments. For example, administration of either orexin A or B into VTA caused CPP in rats (Narita et al., 2007), as did chemical activation of LH with the cholinergic agonist carbachol, an effect that was blocked by pretreatment with intra-VTA SB-334867 (Taslimi et al., 2011). Like other reinforcers, intra-VTA orexin A or B administration increases dopamine and its metabolites in NAc and PFC (Espana et al., 2011; Narita et al., 2006, 2007; Vittoz and Berridge, 2006). These findings point to a role for orexins in potentiating reward circuits, and the above sections lay out extensive evidence that orexin is necessary for the seeking of various classes of addictive drugs.
However, there is also evidence that orexin activation of stress pathways may be involved in addiction-related behaviors. Orexin A administered either ICV or intra-VTA-increased ICSS thresholds, and this effect was blocked by coinfusion of a CRF antagonist (Boutrel et al., 2005; Hata et al., 2011). In addition, footshock-induced reinstatement of cocaine seeking was blocked by ICV (but not intra-VTA) SB-334867 (Boutrel et al., 2005; Wang et al., 2009), indicating that orexins can have effects that involve brain stress pathways (Johnson et al., 2012).
What could be the reason for orexin seemingly playing a role in both reward-appetitive behaviors as well as stress-related behaviors? One possibility, mentioned above, is that orexin neurons in medial versus lateral hypothalamic areas have different functional roles in behavior (Harris and Aston-Jones, 2006). We have previously hypothesized that LH orexin populations play a specific role in reward-related behavior, including CPPs for opiates and cocaine, and stimulant sensitization. In contrast, the DMH/PFA orexin neurons may play a greater role in arousal- and/or stress-related behaviors (Aston-Jones et al., 2010).
A second, overlapping possibility is that orexin might function as a modulator of gain for brain circuits engaged in guiding motivated behaviors, regardless of affective valence. For example, orexin could enhance either appetitive or aversive salience, depending on the context and the predominant neurocircuitry underlying the motivated behavior. In other words, activation of orexin neurons could potentiate an animal’s approach to rewards or avoidance of stressors, depending on the situation at hand. Orexin might therefore increase an animal’s responsivity to important cues and situations, facilitating adaptive behavioral responses at moments of either opportunity or danger. Future studies need to be conducted to test these and other theories concerning the mechanism by which orexin plays a role in addiction.
Future directions and clinical usefulness of modulating orexin
The studies reviewed above clearly show that orexin neurons in LH play important roles in reward processing, motivated behavior, and drug abuse. However, many questions remain for future studies to better understand the role of this neuro-peptide system in these behavioral processes. One issue concerns the general relationship between the roles of orexin in arousal and reward. Are these two functions separately and independently mediated by different groups of orexin neurons, or are they somehow functionally linked in a broader functional context that incorporates both of these activities?
A second important research goal is to conduct a thorough input–output analysis of orexin neurons from functional perspectives. We have begun this analysis as reviewed above, discovering that the lateral septum and vBNST are likely to be important inputs to LH orexin neurons that regulate their activity in accordance with cocaine preference. However, what afferents drive orexin neurons that are involved in arousal and stress functions? Our results, as well as studies by others, have shown that VTA is an important target of orexin neurons for reward effects. A smaller number of studies have shown roles of orexin in NAc, PVN, and insular cortex in reward-related processes as well. However, orexin projections are very wide throughout the central nervous system, and it seems quite surprising that only a few targets such as these could be responsible for so many reward-related functions. This raises the question: What roles do the myriad other targets of orexin play in the functioning of this system?
A third important set of questions concerns the functional attributes of the two orexin receptors. Several studies appear to coalesce around the idea that reward functions are conveyed primarily by OX1R and arousal/stress functions by OX2R. However, the distributions of these receptors are different, so through which targets does each receptor mediate its dominant behavioral effects?
Finally, considerable evidence shows that orexin is involved in plasticity of neural responses in VTA dopamine neurons and elsewhere. This implies a possible role in learning, and some studies (reviewed above) confirm that the LH orexin to VTA projection is critical for learning stimulus–reward associations, at least for morphine CPP. Future research should address several vital questions about orexin’s role in learning. For example, in what types of learning is orexin involved? How general is its role in learning? Is it involved in more than reward-based learning, and, if so, does orexin modulate plasticity in other of its targets to modulate other types of learning as well?
Exciting new virus-mediated approaches to regulation of these neurons using optogenetics (e.g., channelrhodopsin-2) or pharmacogenetics (e.g., designer receptors) also offer great potential for developing specific and powerful ways to manipulate neural systems, both experimentally and clinically (Bernstein and Boyden, 2011; Fenno et al., 2011; Rogan and Roth, 2011). Promoter sequences have been identified for the orexin gene that have allowed generation of plasmids that direct expression of component genes in orexin neurons, but not in non-orexin neurons, after local viral vector microinjections into hypothalamus in vivo (Adamantidis et al., 2007; Carter et al., 2009). If orexin neurons primarily play a role in driving conditioned motivational responses to reward-associated stimuli (as proposed here), specific modulation of these neurons’ activities via these methods may offer a powerful means of selectively treating at least some types of motivational disorders including drug abuse and feeding disorders leading to obesity. Moreover, the ability to virally transduce orexin neurons in LH versus more medial hypothalamic sites affords the possibility of modulating reward functions of LH orexin neurons without having undesirable effects on arousal and stress by affecting more medial orexin cells. Therefore, the specificity offered by these new approaches promises treatments for complex motivational disorders not previously possible.
Most studies of orexin have focused on reward or arousal. Although it is clear that this system plays an important role in positively motivated behavior, there has been little examination of a possible role also in aversively motivated behaviors or learning as related to addiction. This seems especially important for future studies, in view of the roles of the medial orexin neurons in stress and anxiety responses (Berridge et al., 2010a; Boutrel and de Lecea, 2008; Martin-Fardon et al., 2010; von der Goltz et al., 2011). In addition, while early studies found that orexin is important in behavioral responses to opiate withdrawal (Georgescu et al., 2003), the implications of this for a full range of behavioral functions have not been explored (e.g., negative reinforcing effects of drugs when administered during a period of withdrawal/abstinence).
The above studies indicate that orexin neurons and receptors represent an important new target for clinical interventions in a variety of disorders. Drugs to interfere with orexin receptors are being developed to treat sleep and addictive disorders, but such drugs may well also prove useful for mood disorders, given the role of orexin in reward, hedonics, and motivation (Lutter et al., 2008; Scott et al., 2011). Additionally, orexinergic drugs could also have potential for treating deficits in learning, and other cognitive disorders such as dementia. It is clear that additional research in the future will yield ever increasing insights into functions of the orexin neuropeptide system, and ways in which modulating this system can be applied for treating a host of mental and behavioral disorders.
Acknowledgments
NIH Grants R37-DA006214, P50-AA010761, P50-DA015369, F32-DA026692, F32-DA031519, and R21-DA032005.
Abbreviations
- BNST
bed nucleus of the stria terminalis
- CPP
conditioned place preference
- CR
conditioned reinforcement
- CRF
corticotropin-releasing factor
- DMH
dorsomedial hypothalamus
- DS
discriminative stimulus
- DT
discrete trials
- FR
fixed ratio
- ICSS
intracranial self-stimulation
- ICV
intracerebroventricular injection
- LC
locus coeruleus
- LH
lateral hypothalamus
- MCH
melanin-concentrating hormone
- NAc
nucleus accumbens
- OX1/2R
orexin 1/2 receptor
- PFA
perifornical area
- PFC
prefrontal cortex
- Pmax
maximum price (value) of a reward in behavioral economics
- PR
progressive ratio
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular thalamus
- VTA
ventral tegmental area
References
- Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. The Journal of Physiology. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanmu MA, Honda K. Selective stimulation of orexin receptor type 2 promotes wakefulness in freely behaving rats. Brain Research. 2005;1048:138–145. doi: 10.1016/j.brainres.2005.04.064. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Research. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi H, Mirnajafi-Zadeh J, Rohampour K, Semnanian S. Antagonism of orexin type 1 receptors in the locus coeruleus attenuates signs of naloxone-precipitated morphine withdrawal in rats. Neuroscience Letters. 2010;482:255–259. doi: 10.1016/j.neulet.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: The differences do matter. Nature Reviews. Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Borgland SL. Hypocretin modulation of drug-induced synaptic plasticity. Progress in Brain Research. 2012;198 doi: 10.1016/B978-0-444-59489-1.00008-2. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. The Journal of Comparative Neurology. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. The Journal of Comparative Neurology. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. The European Journal of Neuroscience. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: Insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, et al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Bayerlein K, Kraus T, Leinonen I, Pilniok D, Rotter A, Hofner B, et al. Orexin A expression and promoter methylation in patients with alcohol dependence comparing acute and protracted withdrawal. Alcohol. 2011;45:541–547. doi: 10.1016/j.alcohol.2011.02.306. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends in Cognitive Science. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Research. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Current Opinion in Neurobiology. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Mueller ET, Jones BA, Christensen DR. The behavioral economics of drug dependence: Towards the consilience of economics and behavioral neuroscience. Current Topics in Behavioral Neurosciences. 2010;3:319–341. doi: 10.1007/7854_2009_22. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. The European Journal of Neuroscience. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology. Animal Behavior Processes. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Boutrel B, de Lecea L. Addiction and arousal: The hypocretin connection. Physiology and Behavior. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nature Medicine. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: Role of forebrain limbic structures. The Journal of Neuroscience. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: Effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, et al. Hypothalamic orexin expression: Modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M. Drug addiction. The New England Journal of Medicine. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, et al. Persistent increase of alcohol-seeking evoked by neuropeptide S: An effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason A, Aston-Jones G. Role of orexin / hypocretin in conditioned sucrose-seeking submitted. [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: Implications for obesity. Physiology and Behavior. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDCP. Tobacco-related mortality. Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacology, Biochemistry, and Behavior. 1999;63:325–331. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Amphetamine-induced place preference in humans. Biological Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D(1) antagonists. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. The effects of nicotine on locomotor activity in nontolerant and tolerant rats. British Journal of Pharmacology. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regulatory Peptides. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Schreier JD, Roecker AJ, Mercer SP, McGaughey GB, Cox CD, et al. Discovery of 3,9-diazabicyclo[4.2.1]nonanes as potent dual orexin receptor antagonists with sleep-promoting activity in the rat. Bioorganic & Medicinal Chemistry Letters. 2010;20:4201–4205. doi: 10.1016/j.bmcl.2010.05.047. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: A review. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biological Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Progress in Brain Research. 2012;198 doi: 10.1016/B978-0-444-59489-1.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology. 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, et al. The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in alcohol-preferring (P) rats. Journal of Addiction Medicine. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. Journal of Pharmaceutical Sciences. 2009;98:2501–2515. doi: 10.1002/jps.21604. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Facilitated acquisition but not persistence of responding for a cocaine-paired conditioned reinforcer following sensitization with cocaine. Neuropsychopharmacology. 2008;33:1426–1431. doi: 10.1038/sj.npp.1301542. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. European Journal of Pharmacology. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Di Fabio R, Pellacani A, Faedo S, Roth A, Piccoli L, Gerrard P, et al. Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2 receptor antagonist useful for treatment of sleep disorders. Bioorganic & Medicinal Chemistry Letters. 2011;21:5562–5567. doi: 10.1016/j.bmcl.2011.06.086. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano AR, Wilson-Perez HE, Lehman MN, Coolen LM. Lesions of orexin neurons block conditioned place preference for sexual behavior in male rats. Hormones and Behavior. 2011;59:1–8. doi: 10.1016/j.yhbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano AR, Yong-Yow S, Wagner L, Lehman MN, Coolen LM. Orexin mediates initiation of sexual behavior in sexually naive male rats, but is not critical for sexual performance. Hormones and Behavior. 2010;58:397–404. doi: 10.1016/j.yhbeh.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs AW, Eddy NB. The effect of repeated doses of cocaine on the rat. The Journal of Pharmacology and Experimental Therapeutics. 1932;46:199–202. [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. The Journal of Pharmacology and Experimental Therapeutics. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nature Neuroscience. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzung Le A, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. The Journal of Biological Chemistry. 2006;281:38812–38824. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. The European Journal of Neuroscience. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: An overview. British Journal of Pharmacology. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annual Review of Neuroscience. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. The European Journal of Neuroscience. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Lammers GJ, Balesar R, Unmehopa UA, Swaab DF. The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader-Willi syndrome. The Journal of Clinical Endocrinology and Metabolism. 2005;90:5466–5470. doi: 10.1210/jc.2005-0296. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: Tonic depression of the hypothalamic arousal system. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: A critical role for the dorsolateral caudate-putamen. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–959. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. The Journal of Neuroscience. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. The Journal of Neuroscience. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: A novel excitatory amino acid input to midbrain dopamine neurons. The Journal of Neuroscience. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Aston-Jones G. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Research. 2008;1224:43–52. doi: 10.1016/j.brainres.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ. Orexin receptors as therapeutic drug targets. Progress in Brain Research. 2012;198 doi: 10.1016/B978-0-444-59489-1.00010-0. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS One. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Science. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Griffond B, Risold PY, Jacquemard C, Colard C, Fellmann D. Insulin-induced hypoglycemia increases preprohypocretin (orexin) mRNA in the rat lateral hypothalamic area. Neuroscience Letters. 1999;262:77–80. doi: 10.1016/s0304-3940(98)00976-8. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. Journal of Neuroendocrinology. 1999;11:653–663. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: Evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends in Neurosciences. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behavioural Brain Research. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural Brain Research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T, Chen J, Ebihara K, Date Y, Ishida Y, Nakahara D. Intra-ventral tegmental area or intra-cerebroventricular orexin-A increases the intra-cranial self-stimulation threshold via activation of the corticotropin-releasing factor system in rats. The European Journal of Neuroscience. 2011;34:816–826. doi: 10.1111/j.1460-9568.2011.07808.x. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regulatory Peptides. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Heath RG. Exploring the mind-brain relationship. Baton-Rouge, LA: Moran Printing, Inc; 1996. [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. The Journal of Comparative Neurology. 2006;499:645–661. doi: 10.1002/cne.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: Modification by Pavlovian conditioning. Pharmacology, Biochemistry, and Behavior. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hirose M, Egashira S, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, et al. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: The first orexin-2 receptor selective non-peptidic antagonist. Bioorganic & Medicinal Chemistry Letters. 2003;13:4497–4499. doi: 10.1016/j.bmcl.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: Possible clues to obesity’s association with insomnia. Cell Metabolism. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Huang H, Acuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: Implications for cannabinoid actions on food intake and cognitive arousal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:4870–4881. doi: 10.1523/JNEUROSCI.0732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behavioural Pharmacology. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behavioural Brain Research. 2005;157:331–341. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Long-term gene expression in the nucleus accumbens following heroin administration is subregion-specific and depends on the nature of drug administration. Addiction Biology. 2005;10:91–100. doi: 10.1080/13556210412331284748. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, et al. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. The International Journal of Neuropsychopharmacology. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh AI, Fitz SD, Truitt WA, Shekhar A. Orexin, stress and anxiety states. Progress in Brain Research. 2012;198 doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Research. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: Pattern of neural activation and involvement of orexin receptors. British Journal of Pharmacology. 2011;162:880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research. Brain Research Reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Li MD. Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neuroscience Letters. 2001;298:1–4. doi: 10.1016/s0304-3940(00)01730-4. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology and Behavior. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiology and Behavior. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Arai AC. Use of [3H]fluorowillardiine to study properties of AMPA receptor allosteric modulators. Brain Research. 2006;1076:25–41. doi: 10.1016/j.brainres.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Annals of the New York Academy of Sciences. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of addiction. Amsterdam: Academic Press; 2006. [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. The Journal of Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning and Memory. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. British Journal of Pharmacology. 2004;141:340–346. doi: 10.1038/sj.bjp.0705610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Research. 2010;1314:124–129. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. The Journal of Neuroscience. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: Effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology. 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse H, Harper RK. Frequency-related, bidirectional limbic responses to cocaine: Comparisons with amphetamine and lidocaine. Brain Research. 1985;335:21–31. doi: 10.1016/0006-8993(85)90272-0. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, et al. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiology and Behavior. 2011;102:42–50. doi: 10.1016/j.physbeh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2681-5. No page numbers or volume as this is an epub ahead of print. (PMID: 22411428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, et al. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. British Journal of Pharmacology. 2009;156:1326–1341. doi: 10.1111/j.1476-5381.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. The Journal of Comparative Neurology. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dys-regulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Research. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P SRIMS Center. PubChem, editor. Summary of the probe development effort to identify antagonists of the orexin 1 receptor (OX1R; HCRTR1) [Summary] 2011. [Google Scholar]
- McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: Differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CS, Featherby T, Krstew E, Lawrence AJ. Quantification of phosphorylated cAMP-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: Activation of hypothalamic orexin A-containing neurons. The Journal of Pharmacology and Experimental Therapeutics. 2007;323:805–812. doi: 10.1124/jpet.107.125732. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: An animal model of relapse. Behavioural Pharmacology. 1996;7:754–763. [PubMed] [Google Scholar]
- Mieda M, Sakurai T. Overview of orexin/hypocretin system. Progress in Brain Research. 2012;198 doi: 10.1016/B978-0-444-59489-1.00002-1. [DOI] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: Emerging therapeutic targets for sleep disorders. Nature Neuroscience. 2002;5(Suppl):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. The Journal of Neuroscience. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT (1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends in Pharmacological Sciences. 2007;28:615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Sprague–Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: Diurnal influences. The Journal of Neuroscience. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcoholism, Clinical and Experimental Research. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kim J, Sasaki K. Electrophysiological effects of orexin-B and dopamine on rat nucleus accumbens shell neurons in vitro. Peptides. 2011;32:246–252. doi: 10.1016/j.peptides.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neuroscience Letters. 1999;264:101–104. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- Mukai K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: An in vitro study. Peptides. 2009;30:1487–1496. doi: 10.1016/j.peptides.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Deurveilher S, Semba K. Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience. 2003;121:269–275. doi: 10.1016/s0306-4522(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. The Journal of Neuroscience. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Pickens CL, Shaham Y. Role of dopamine receptors, but not a2 adrenoceptors, in yohimbine-induced reinstatement of high-fat food seeking. San Diego, CA: Society for Neuroscience. Society for Neuroscience; 2009. [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. British Journal of Pharmacology. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Research. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. The Journal of Neuroscience. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. The European Journal of Neuroscience. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- NIDA; DoHaHS National Institutes of Health. NIH, editor. 2009 Principals of Drug Addiction Treatment: A Research-Based Guide. 2009. [Google Scholar]
- Niimi M, Sato M, Taminato T. Neuropeptide Y in central control of feeding and interactions with orexin and leptin. Endocrine. 2001;14:269–273. doi: 10.1385/ENDO:14:2:269. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Annals of the New York Academy of Sciences. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neuroscience and Biobehavioral Reviews. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ohno K, Hondo M, Sakurai T. Cholinergic regulation of orexin/hypocretin neurons through M(3) muscarinic receptor in mice. Journal of Pharmacological Sciences. 2008;106:485–491. doi: 10.1254/jphs.fp0071986. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, de Jong JW, Vanderschuren LJ, Adan RA. Neurobiology of overeating and obesity: The role of melanocortins and beyond. European Journal of Pharmacology. 2011;660:28–42. doi: 10.1016/j.ejphar.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. European Journal of Pharmacology. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Reppucci C. Selective activation of the hypothalamic peptide orexin/hypocretin, but not melanin-concentrating hormone by a learned cue that stimulates feeding in sated rats. Program No. 285.23 2011 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2011. Online. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacology, Biochemistry, and Behavior. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Research. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behavioural Pharmacology. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Characteristics of stimulant reinforcement. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. New York: Appleton-Century-Crofts; 1971. [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: The neural basis of ingrained behaviors in cocaine addiction. Neuroscience and Biobehavioral Reviews. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biological Psychiatry. 2011;71:214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. The Journal of Neuroscience. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, et al. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorganic & Medicinal Chemistry Letters. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochemistry International. 2010;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Kest K, Zellner MR, Lubelski D, Muller J, Cruz Y, et al. The effects of VTA NMDA receptor antagonism on reward-related learning and associated c-fos expression in forebrain. Behavioural Brain Research. 2011;216:424–432. doi: 10.1016/j.bbr.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Sartor GC, Vazey EM, Dunn TE, Aston-Jones G, Kalivas PW. Use of vivo-morpholinos for control of protein expression in the adult rat brain. Journal of Neuroscience Methods. 2011;203:354–360. doi: 10.1016/j.jneumeth.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to VTA are differentially activated with morphine preference. The Journal of Neuroscience. 2012;32:3809–3817. doi: 10.1523/JNEUROSCI.3917-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Riday TT, Fish EW, Robinson JE, Jarrett TM, McGuigan MM, Malanga CJ. Orexin-1 receptor antagonism does not reduce the rewarding potency of cocaine in Swiss-Webster mice. Brain Research. 2011;1431:53–61. doi: 10.1016/j.brainres.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Dickinson SD, Cunningham CL. Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology. 1992;107:453–456. doi: 10.1007/BF02245175. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Research. Brain Research Reviews. 1997a;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Research. Brain Research Reviews. 1997b;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Research. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neuroscience and Biobehavioral Reviews. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, et al. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. The European Journal of Neuroscience. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Roecker AJ, Coleman PJ. Orexin receptor antagonists: Medicinal chemistry and therapeutic potential. Current Topics in Medicinal Chemistry. 2008;8:977–987. doi: 10.2174/156802608784936746. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacological Reviews. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Barker DJ, Ma S, Pawlak AP, West MO. Evidence for habitual and goal-directed behavior following devaluation of cocaine: A multifaceted interpretation of relapse. PLoS One. 2009;4:e7170. doi: 10.1371/journal.pone.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexins in the regulation of feeding and arousal. Sleep Medicine. 2002;3(Suppl 2):S3–S9. doi: 10.1016/s1389-9457(02)00156-9. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Lateral hypothalamic orexin/hypocretin afferents that are necessary for the expression of cocaine conditioned place preference. San Diego, CA: Society for Neuroscience; 2010. [Google Scholar]
- Sartor GC, Aston-Jones G. A septal-hypothalamic pathway drives orexin neurons which is necessary for conditioned cocaine preference. The Journal of Neuroscience. 32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. The Journal of Neuroscience. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Winrow CJ. Orexin receptors: Pharmacology and therapeutic opportunities. Annual Review of Pharmacology and Toxicology. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcoholism, Clinical and Experimental Research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in Neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, et al. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behavioural Brain Research. 2011;222:289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Waters RP. Pharmacologically-induced stress: A cross-species probe for translational research in drug addiction and relapse. American Journal of Translational Research. 2010;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Geyer MA, Schuckit MA. Stimulant-induced psychosis: An evaluation of animal methods. Essays in Neurochemistry and Neuropharmacology. 1981;5:95–129. [PubMed] [Google Scholar]
- Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, et al. A novel allosteric potentiator of AMPA receptors: 4–2-(phe-nylsulfonylamino)ethylthio–2,6-difluoro-phenoxyacetamide. The Journal of Neuroscience. 1997;17:5760–5771. doi: 10.1523/JNEUROSCI.17-15-05760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Research. Brain Research Reviews. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Research. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Research. 2010;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biological Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biological Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, et al. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology. 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- Shoji S, Simms D, Yamada K, Gallagher JP. Cocaine administered in vitro to brain slices from rats treated with cocaine chronically in vivo results in a gamma-aminobutyric acid receptor-mediated hyperpolarization recorded from the dorsolateral septum. The Journal of Pharmacology and Experimental Therapeutics. 1998;286:509–518. [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): Role in normal behavior and neuropathology. Annual Review of Psychology. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms D, Gallagher JP. Modification of serotonin responses in rat dorsolateral septal nucleus neurons by acute and chronic cocaine. The Journal of Pharmacology and Experimental Therapeutics. 1996;276:1292–1303. [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: The first selective orexin-1 receptor antagonist. British Journal of Pharmacology. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biological Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. The European Journal of Neuroscience. 35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: Generating sensory pleasure in the brain. In: Kringelback ML, Berridge KC, editors. Pleasures of the brain. Oxford, UK: Oxford University Press; 2009a. pp. 27–49. [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European Journal of Neuroscience. 2009b;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009c;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behavioural Pharmacology. 1991;2:65–71. [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: Excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. Journal of Neuroscience Research. 2000;62:161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Shimojima H, Funahashi H, Nakajo S, Yamada S, Guan JL, et al. Orexin-1 receptor immunoreactivity in chemically identified target neurons in the rat hypothalamus. Neuroscience Letters. 2002;324:5–8. doi: 10.1016/s0304-3940(02)00140-4. [DOI] [PubMed] [Google Scholar]
- Taslimi Z, Haghparast A, Hassanpour-Ezatti M, Safari MS. Chemical stimulation of the lateral hypothalamus induces conditioned place preference in rats: Involvement of OX1 and CB1 receptors in the ventral tegmental area. Behavioural Brain Research. 2011;217:41–46. doi: 10.1016/j.bbr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Research. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. The Journal of Neuroscience. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. The Journal of Neuroscience. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Research. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: Involvement of the ventral tegmental area. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. The European Journal of Neuroscience. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, et al. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Hormones and Behavior. 2011;60:644–650. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Rockenbach C, Grosshans M, et al. Orexin and leptin are associated with nicotine craving: A link between smoking, appetite and reward. Psychoneuroendocrinology. 2010;35:570–577. doi: 10.1016/j.psyneuen.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology. 2011;214:805–818. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hip-pocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Current Drug Abuse Reviews. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: Independence from the local corticotropin-releasing factor network. Biological Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch CC, Kim EM, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic dynorphin peptide and mRNA levels. Brain Research. 1996;721:126–131. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- Whitman DB, Cox CD, Breslin MJ, Brashear KM, Schreier JD, Bogusky MJ, et al. Discovery of a potent, CNS-penetrant orexin receptor antagonist based on an n, n-disubstituted-1,4-diazepane scaffold that promotes sleep in rats. Chem Med Chem. 2009;4:1069–1074. doi: 10.1002/cmdc.200900069. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: Molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Winocur G, Gilbert M. The hippocampus, context, and information processing. Behavioral and Neural Biology. 1984;40:27–43. doi: 10.1016/s0163-1047(84)90146-8. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, et al. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology. 2010;58:185–194. doi: 10.1016/j.neuropharm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: The corticotrophin-releasing factor/hypocretin circuitry. Molecular Neurobiology. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Wise RA. Intravenous drug self-administration: A special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 117–141. [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annual Review of Neuroscience. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug self-administration viewed as ingestive behaviour. Appetite. 1997;28:1–5. doi: 10.1006/appe.1996.0059. [DOI] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Research. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochemical and Biophysical Research Communications. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Research. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. Principles of brain stimulation. New York: Oxford University Press; 1990. [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. The European Journal of Neuroscience. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. The Journal of Comparative Neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuro-science. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Research. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zellner MR, Kest K, Ranaldi R. NMDA receptor antagonism in the ventral tegmental area impairs acquisition of reward-related learning. Behavioural Brain Research. 2009;197:442–449. doi: 10.1016/j.bbr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Zellner MR, Ranaldi R. How conditioned stimuli acquire the ability to activate VTA dopamine cells: A proposed neurobiological component of reward-related learning. Neuroscience and Biobehavioral Reviews. 2010;34:769–780. doi: 10.1016/j.neubiorev.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: Contrasting effects revealed by a progressive ratio study in the rat. Behavioral Neuroscience. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. Journal of Neurochemistry. 2007;103:400–407. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. The Journal of Endocrinology. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, et al. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, et al. Orexin-1 receptor mediation of cocaine-seeking in male and female rats. The Journal of Pharmacology and Experimental Therapeutics. 2012;340(3):801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: A potentiator of AMPA receptors. The Journal of Neuroscience. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]