Abstract
Of late, decrease in mineral oil supplies has stimulated research on use of biomass as an alternative energy source. Climate change has brought problems such as increased drought and erratic rains. This, together with a rise in land degeneration problems with concomitant loss in soil fertility has inspired the scientific world to look for alternative bio-energy species. Euphorbia tirucalli L., a tree with C3/CAM metabolism in leaves/stem, can be cultivated on marginal, arid land and could be a good alternative source of biofuel.
We analyzed a broad variety of E. tirucalli plants collected from different countries for their genetic diversity using AFLP. Physiological responses to induced drought stress were determined in a number of genotypes by monitoring growth parameters and influence on photosynthesis. For future breeding of economically interesting genotypes, rubber content and biogas production were quantified.
Cluster analysis shows that the studied genotypes are divided into two groups, African and mostly non-African genotypes. Different genotypes respond significantly different to various levels of water. Malate measurement indicates that there is induction of CAM in leaves following drought stress. Rubber content varies strongly between genotypes. An investigation of the biogas production capacities of six E. tirucalli genotypes reveals biogas yields higher than from rapeseed but lower than maize silage.
Introduction
Agriculture faces a range of serious environmental problems such as soil salinisation and depletion of water resources. Additionally, agricultural production and unsustainable human intervention often leave the land under stress, leading to an increase in non-arable land area [1]. The supply of fossil fuel in future will also soon start decreasing. Therefore, efforts are made to find substitute sources of energy. One such source is solar energy, which is unlimited. Plants capture this energy through photosynthesis. Faced with a decrease in arable land and crude oil supply, it is important to find species for growing in marginal, non-arable land. These plants should have high drought and salinity tolerance as well as contain compounds that could be used in phytochemical, pharmaceutical or nutraceutical applications.
Euphorbia tirucalli L. belongs to the dicotyledonous order Euphorbiales, family Euphorbiaceae, subsection tirucalli [2]. The natural distribution of E. tirucalli comprises the Paleotropical region of Madagascar, the Cape region (South Africa), East Africa, and Indochina [3]. This plant is also grown as garden plant in numerous tropical countries, also in America. E. tirucalli seems to have high salinity and drought tolerance [4] and survives in a wide range of habitats even under conditions in which most crops c.q. plants cannot grow. These include tropical arid areas with low rainfall, poor eroded or saline soils and high altitudes but E. tirucalli cannot survive frost [3]. Its high stress tolerance can be explained at least in part by its photosynthetic system. The family of E. tirucalli, the Euphorbiaceae, consists of five subfamilies [5] and its species have C3, C4, intermediate C3–C4 and/or Crassulacean Acid Metabolism (CAM) photosynthetic systems dependent on the ecological conditions [6]. Batanouny et al. [6] reported that Euphorbia species having the C3 photosynthetic pathway grow under conditions of better water resources and lower temperature, whereas CAM and C4 plants grow under high temperature. The photosynthetic system of E. tirucalli stems has been identified to follow CAM [7]. It has been classified based on the C-isotope ratio. The range of values −8 to −18 are characteristic of plants with C4 or CAM [8], while “Kranz” anatomy provides strong evidence of C4 system. Meanwhile Ting et al. [9] described values in the range of −15.4 to −16.2 were classified as CAM plants, whereas −12.6 and −11.3 as C4. Bender [7] showed 13C/12C ratios of E. tirucalli was −15.3. This value indicated that E. tirucalli did not follow C4; this was also supported that there was no Kranz syndrome in E. tirucalli stem [10]. Its photosynthetic system followed C3 in non-succulent leaves and CAM pathway in succulent stems based on gas exchange observations [3]. In CAM plants one can observe an opening/closure of stomata during night/day allowing nightly CO2 uptake accompanied with malate oscillation that follows stomatal opening and closure [11], [12]. Hence, malate presence confirms CAM photosynthetic pathway in E. tirucalli. Under unfavorable conditions, its non-succulent C3 leaves soon die and the plant will then continue its metabolism via the CAM photosynthetic pathway in the stem. The combination of C3 leaves and CAM stems can explain E. tirucalli's fast accumulation of biomass since C3 maximizes growth during favorable conditions and CAM during drought to reduce water loss and maintain photosynthetic integrity [13]. C3 photosynthetic pathway takes place when leaves are present and in combination with CAM stem, whereas CAM stem takes up CO2 when conditions deteriorate. However, to date there is no evidence that there is a change from C3 and CAM at leaf level following drought events, a mechanism that has been evidenced in Mesembryanthemum crystallinum L. [14] and the genus Sedum [15].
E. tirucalli has been reported to present numerous pharmacological activities. The species has been patented for modern drugs such as prostate cancer medicine [16] and has a very high ethnomedicinal value [17]–[20]. E. tirucalli produces and stores abundant amounts of latex in so-called laticifers [21]. E. tirucalli latex contains high amounts of sterols and triterpenes [22] and might be used for rubber fractionation and has been investigated for its diesel oil properties [17], [23]–[27]. Through the hydrocarbons of its latex, the species was documented in 1978 to produce the equivalent to 10–50 barrels oil L ha−1 [24], whereas its biomass can yield 8,250 m3 ha−1 biogas (in the tropical, subhumid conditions of Colombia [28]). Furthermore, E. tirucalli latex has pesticidal properties against such pests as mosquitoes (Aedes aegypti and Culex quinquefasciatus) [29], bacteria (Staphylococcus aureus) [30], molluscs (Lymnaea natalensis) and nematodes such as Haplolaimus indicus, Helicotylenchus indicus and Tylenchus filiformis [31]. E. tirucalli latex can also be used as glue and adhesive [32].
The morphological characteristics of different E. tirucalli accessions do not allow differentiating them amongst themselves, except for one US accession that has yellow tips and has been promoted for ornamental uses. Hence, classification of E. tirucalli based on its genetic characteristic will be more precise than using morphological descriptors. Until now, genetic diversity between E. tirucalli genotypes from different areas has not been investigated. Analysis of genetic diversity among genotypes is also a prerequisite if one wants to start selecting and/or breeding for increased drought tolerance, gain in biomass, rubber content and biogas production. Our final aim is to recommend the best genotypes first for field research experiments and then for initiating commercial E. tirucalli plantations in arid areas for the respective applications.
Materials and Methods
2.1 Plant material, propagation and growth conditions
Mother plants of genotypes Morocco, Senegal, Burundi, Rwanda, Kenya and USA were collected by Van Damme over the last 20 years from wild individuals and grown in greenhouses at Ghent University, Department of Plant Production, Laboratory for Tropical and Subtropical Agriculture and Ethnobotany, Belgium. Genotype India was collected in Ajmer and Jaipur from naturalized plants but genotype Jaipur could not be propagated as it died after delivery. Genotype Indonesia was collected in Yogyakarta from a wild-grown individual, genotype Italy was collected in Calabria from a cultivated ornamental, genotype Togo was collected in Togo from wild plants by Torsten Schmidt (Hannover, Germany), whereas genotype Hannover was an ornamental specimen of unknown origin. No specific permissions were required for collecting on these locations because the plants grow like weed on locations that are not privately-owned or protected in any way and the E. tirucalli species does not belong to endangered or protected species.
Propagation for our experiments was done vegetatively by cuttings taken on no predefined part of the respective mother plants. The 10–15 cm cuttings obtained from healthy plants and planted in pots with volume of 436 cm3 according to the formula of truncated cones that contained a mixture of clay-loam:sand (2∶1). These cuttings were cultivated in the greenhouse of Institute of Botany, Leibniz University Hannover, for six months at 14 h/24°C (day) and 10 h/22°C (night) with a light intensity of 350 µmol m−2 s−1; and watered once every two days. In control conditions fertilizer Wuxal Top N (Aglukon, Düsseldorf, Germany) consisting of 0.6% NPK and 99.4% water was applied once every two days (about 8.6 ml per pot). For the water stress conditions the same concentration of fertilizer was added in a smaller volume of water.
2.2 Molecular analysis through genetic marker
2.2.1 DNA extraction and quantification
DNA was extracted from twelve genotypes of the E. tirucalli collection. DNA isolation procedure using NucleoSpin® Plant II Kit (Macherey & Nagel GmbH & Co. KG, Düren, Germany) was used to extract genomic DNA from 60 mg of young leaf samples. Freshly extracted DNA was quantified photometrically using an Uvikon xs photometer (Biotek Germany, Bad Friedrichshall, Germany). Quantification was done by measuring 2 µl of non-diluted DNA sample at 260 nm wavelength. Extracted DNA was stored at −20°C until use.
2.2.2 Amplified Fragment Length Polymorphism (AFLP)
AFLP analysis was performed essentially as described by Vos et al. [33]. Restriction fragments were produced by digestion of 250 ng genomic DNA for 1 h at 37°C with 0.5 µl EcoRI (10 U/µl) and 0.3 µl MseI (10 U/µl) in a total volume of 25 µl containing 2.5 µl 10×RL Buffer, 100 mM Tris HCl, 100 mM MgAc, 500 mM KAc, 50 mM DTT, pH 7.5, and H2O. The digestion was followed by ligation of specific MseI (50 pmol) and EcoRI (5 pmol) adapters (MWG Biotech Eurofins, Ebersberg, Germany) with 5 µL reaction mix (0.5 µl of EcoRI adapter, 0.5 µl of MseI adapter, 0.6 µl of 10 mM ATP, 0.5 µl 10× RL-Buffer, 0.05 µl of T4-DNA-Ligase (1 U µl−1), and 2.85 µl H2O) which was added to the restricted DNA and incubated for 3.5 h at 37°C.
For the pre-amplification a reaction mix (5 µl of digested and ligated DNA, 1.5 µl EcoRI+0 (5′ GACTGCGTACAA TTC 3′) and MseI+0 (5′ GATGAGTCCTGAGTAA 3′) or EcoRI+A/MseI+A primer combinations (50 ng µl−1), 5 µl dNTPs (2 mM), 5 µl 10×Williams Buffer (100 mM Tris/HCl, pH 8.3; 500 mM KCl; 20 mM MgCl2; 0.01% gelatine; H2O), 1 µl Taq polymerase (5 U µl−1) and 31 µl H2O) was amplified in a thermocycler with 94°C/5 min, then 20 cycles of 94°C/30 s, 60°C/30 s, 72°C/60 s and finally 72°C/10 min. Selective amplifications were performed using primer pairs containing three selective nucleotides. For selective amplification, 2.5 µl of a 20-fold diluted pre-amplification mixture with reaction mix (2.5 µl EcoRI-IRD primer (2 ng µl−1), 0.3 MseI primer (50 ng µl−1), 1 µl dNTPs (2 mM), 0.05 µl Taq polymerase (5 U µl−1), 1 µl 10×Williams Buffer and 2.65 µl H2O) was amplified consisting of 94°C/5 min, one cycle of 94°C/30 s, 65°C/30 s and 72°C/60 s, then lowering the annealing temperature to about 0.7°C reduction per cycle for next 11 cycles, thereafter 24 cycles of 94°C/30 s, 56°C/30 s, 72°C/60 s and lastly 72°C/10 min. IRD 700 labelled EcoRI primers and MseI primers with three selective nucleotides at their 5′ end was used (Table 1). After PCR, an equal volume of sequencing loading buffer (98% formamide, 10 mM EDTA, pararosaniline 0.05%) was added. The mixture was heated to 90°C for 3 min and then cooled on ice.
Table 1. Primer combinations for selective amplification.
| Primer combination | EcoRI 700 | MseI |
| 1 | GACTGCGTACAA TTC ACA | GATGAGTCCTGAG TAA ACT |
| 2 | GACTGCGTACAA TTC ACA | GATGAGTCCTGAG TAA ACT |
| 3 | GACTGCGTACAA TTC ACA | GATGAGTCCTGAG TAA ACA |
| 4 | GACTGCGTACAA TTC ACC | GATGAGTCCTGAG TAA ATTA |
| 5 | GACTGCGTACAA TTC ACC | GATGAGTCCTGAG TAA ATGG |
| 6 | GACTGCGTACAA TTC ACA | GATGAGTCCTGAG TAA ATGG |
| 7 | GACTGCGTACAA TTC ACA | GATGAGTCCTGAG TAA ACAT |
Marked fragments were separated over 6% polyacrylamide gel from Sequa gel X® (16 ml of monomer solution, 4 ml of complete buffer and 160 µl of 10% APS) with 1×TBE buffer. A sizing standard was labeled with IRD 700 at their 5′ end (MWG Biotech Eurofins). Samples were analyzed on a LICOR Gene Reader 4300 automated sequencer (LI-COR Biosciences, Lincoln, USA), at condition 1500 V, 35 A, 40 W, 45°C, slow scan speed and 30 min pre-run.
2.2.3 PCR product detection and phylogenetic analysis
Detection of AFLP products and phylogenetic analysis of DNA AFLP fingerprints was conducted based on the number, frequency and distribution of amplified DNA fragments. AFLP product diversity was determined from the difference in gel migration of PCR products from each individual sample. Based on the presence or absence of AFLP bands, band profiles were translated into binary data. Data were analyzed using fingerprint analysis with missing data 1.0 (FAMD) (program available from http://homepage.univie.ac.at/philipp.maria.schlueter/famd.html) [34]. The tree was generated using Unweighted Pair Group Method with Arithmetic Mean (UPGMA). The tree was visualized using the TreeView program version 1.6.6 [35].
2.3 Investigation of drought tolerance
Investigation of drought effects was conducted based on Jefferies [36] with some modifications. Six month old E. tirucalli plants from Morocco and Senegal with a height of 27–29 cm were selected. This experiment was conducted in a climatic chamber for 8 weeks with condition 24/20°C day (14 h)/night (10 h), at light intensity 155 µmol m−2 s−1 and 60% humidity. Twenty plants from each genotype were grown in clay-loam and sand substrate with four different volumetric water contents (VWC) 25%, 15%, 10% and 5% monitored using Fieldscout® based on time domain reflectometry (TDR) (Spectrum Technologies, Plainfield, USA). Dry set value was 1% below and wet value was 1% above the respective VWCs. According to the manual of this instrument, sandy-clay-loam substrate has water holding capacity of 25% VWC, and a wilting point at 15% VWC. Soil moisture was measured based on water deficit (D) values which indicate the amount of irrigation water necessary to raise the soil water content to the target point. Water was added based on calculation of D values times 8.66 ml for a pot with 7 cm height.
As E. tirucalli grows in semi-arid and arid areas, two VWC points below 15% were investigated for their effect on the species' physiology. Selected VWC points were 10% and 5%. Growth parameters such as plant height, root length, dry matter production, and water content were measured. Plant height and tap root length were measured with a scale. For fresh and dry biomass determination shoots and root of plants were harvested separately and measured after 8 weeks of treatment. Shoots and roots were dried in an incubator at 90°C for 36 h. Investigation on whether there was an effect of drought on photosynthesis during drought application, chlorophyll fluorescence measurements were conducted every week during 8 weeks during drought treatment using the non-invasive method of Imaging PAM (M series, Heinz Walz GmbH, Effeltrich, Germany). Hence, quantum efficiency (Fv/Fm) was measured at leaves having C3 photosynthetic pathway and stems having CAM photosynthetic pathway.
2.4 Investigation of the C3 and CAM photosynthetic pathways: malate determination
Stems and leaves of genotypes Morocco and Senegal were harvested at the end of the dark period (5 am) and the end of the light period (7 pm). The end of the dark period is the phase where malate concentration is highest, whereas the end of the light period is the phase where this value is lowest [37]. Harvested material with 3 replications was put in liquid nitrogen and stored in the freezer at −80°C before malate extraction.
Malate was extracted by putting 60 mg of leaves and stems of each genotype separately in 1.4 ml H2O and vortexing the mixture for 1 min; the mixture as then kept at room temperature for 10 min and mixed again for 1 min. A centrifugation by 13,000 rpm at 4°C for 10 min followed whereupon the supernatant was pipetted into new tubes and centrifuged again at 13,000 rpm for 10 min at 4°C. The supernatant was then pipetted into new tubes and kept at −20°C until measurement by capillary electrophoresis (CE). A P/ACE™ MDQ capillary electrophoresis system (Beckman Coulter, Krefeld, Germany) was used for CE analyses. Separations were performed in a eCAP™ CE-MS capillary (fused silica, 75 µm i.d., 57 cm total length, 50 cm effective length, Beckman Coulter). Before starting the analyses the capillary was equilibrated with the background electrolyte Basic Anion Buffer for HPCE (Agilent Technologies, Waldbronn, Germany) at 14.5 psi for 4 min. Injection was done by applying 0.7 psi for 3.5 s. Separation of the samples was performed by applying 14 kV for 10 min at 22°C. After each run, the capillary was washed with the background electrolyte for 4 min. Buffer was changed after 8 to 10 runs. Samples were detected at 235 nm with a bandwidth of 10 nm. Calibration graphs were generated with 0.313 to 10 mM malic acid. Elaboration of the electropherograms was done using Karat 32 7.0 software (Beckman Coulter).
2.5 Latex analysis
E. tirucalli latex consists of 2.8% to 8.3% rubber and 50.4% to 82.1% resin [38]. Latex of E. tirucalli has attracted a lot of attention because it has an economical potential as source of rubber. Therefore, rubber content was investigated in different genotypes. Rubber content analysis was conducted by LipoFit Analytic GmbH (Regensburg, Germany) using nuclear magnetic resonance (NMR, 600 MHz Bruker Avance+ spectrometer, Bruker Daltonic GmbH, Bremen, Germany). Samples were taken from Burundi, Hannover, Kenya, Morocco, Rwanda, Senegal, Togo and USA genotypes. The input material was 100 to 500 mg fresh weight of stems.
To fresh plant material, 1.5 ml water p.a. (0.03% NaN3) and a sharp aglet were added. By shaking 10 min the material was mechanically milled. The aglet was extracted from the suspension by a magnet. The suspension was centrifuged (20 min; 14,500 rpm; 20°C) to separate cell debris. Sodium phosphate buffer pH 6.8 (final concentration 100 mM), D20 (5%) and sodium trimethyl silyl propionate (0.1 mM) were added to the supernatant. The suspension was then transferred to 5 mm-NMR-tubes.
Relative rubber concentrations refer to the average of the spectra measured in the E. tirucalli samples. The average is calculated out of the integral from all the spectra which are expected to contain rubber signals. The reference for the absolute concentrations was 1,4-polyisoprene with a molar mass of 47,300 g mol−1. The reference was also measured by NMR. In reference to polyisoprene, only the spectra with the same pattern as the reference were calculated.
2.6 Biogas production
Plant material of genotypes Kenya, Morocco, Rwanda, Senegal, Togo, and USA was harvested from the greenhouse (Hannover, Germany), dried, and chopped into 0.5 to 4 cm pieces before being used in biogas batch tests. Biogas yields of the selected genotypes were determined through anaerobic batch digestion tests according to the German Standard Procedure VDI 4630 [39]. The inoculum was biogas slurry from an agricultural biogas plant mainly fed with maize silage. Organic dry matter (ODM), density and chemical oxygen demand (COD) were determined for all samples and the inoculum according to standard methods. Based on results, the weighted samples of the substrates and the inoculum were balanced to obtain a Slurry Loading Rate (SLR; ODMsubstrate to ODMinoculum) of 0.3 as recommended by VDI 4630. Each substrate and one control without the addition of substrate, was incubated in triplicate in gas-tight 1,250 ml dark DURAN glass bottles. Experiments were conducted for 28 days at 38°C in a warming cupboard. Biogas yields (L kg−1 ODM) were calculated based on the pressure in the bottles following biogas production. Rise in pressure was recorded with LabView software connected to the batch plant. After tests were finished, the concentration of CH4 in the biogas produced were analyzed as follows: In each bottle, 20 ml of a 10 molar NaOH solution were injected through the septum with the help of a syringe. The NaOH solution fixes the CO2 in the biogas by reacting to sodium carbonate which precipitates in the liquid phase. As a result, in the bottles a decrease in pressure occurs and on the basis of this data, the methane ratio in the produced biogas can be calculated. H2S in biogas samples of genotypes Morocco, Kenya and USA were quantified using gas chromatography.
2.7 Statistical analysis
All statistical analysis was conducted with Statistix 8 version 2 (Analytical software, Tallahassee, USA). Interaction between means was calculated by the least significant different (LSD) at p<0.05. Graphs were drawn using SigmaPlot Version 12.2 (Systat Software Inc., San Jose, USA).
Results
3.1 Genetic marker analysis
AFLP technique was used as a tool for assessing species relationships within the E. tirucalli collection. Seven primer combinations were selected for AFLP analysis (Table 1). Total number of polymorphic bands was 243 with a mean of 34.7. We were able to derive two main groups from the phylogenetic analysis of the 12 accessions of E. tirucalli cluster analysis using UPGMA with 1000 bootstrap replicates (Fig. 1). Nevertheless, the genotypes tested share a lot of similarities as evidenced from the low bootstrap values. The first group consists of two clades and comprises mainly genotypes from Africa: Burundi, Morocco, Senegal and Togo accessions that are clustered with a bootstrap value of 63. The second group consists of four clades with mainly non-African genotypes (except Kenya and Rwanda): Ajmer (India), Hannover (Germany), Indonesia, Italy, Jaipur (India), Kenya, Rwanda and USA with a bootstrap value of 72. A dendrogram derived from NJ calculation showed the same pattern (data not shown). All genotypes have been propagated by cuttings and cultivated in the greenhouse since a long time or at least for a couple of years. Therefore they should have the same amount of endophytes, if any. In our AFLP analysis the genotypes differ in several hundred bands. In case there are some bands originating from endophytes they would not influence the results significantly.
Figure 1. Dendrogram of twelve E. tirucalli genotypes calculated with UPGMA showing the phenetic relationships within the colletion.
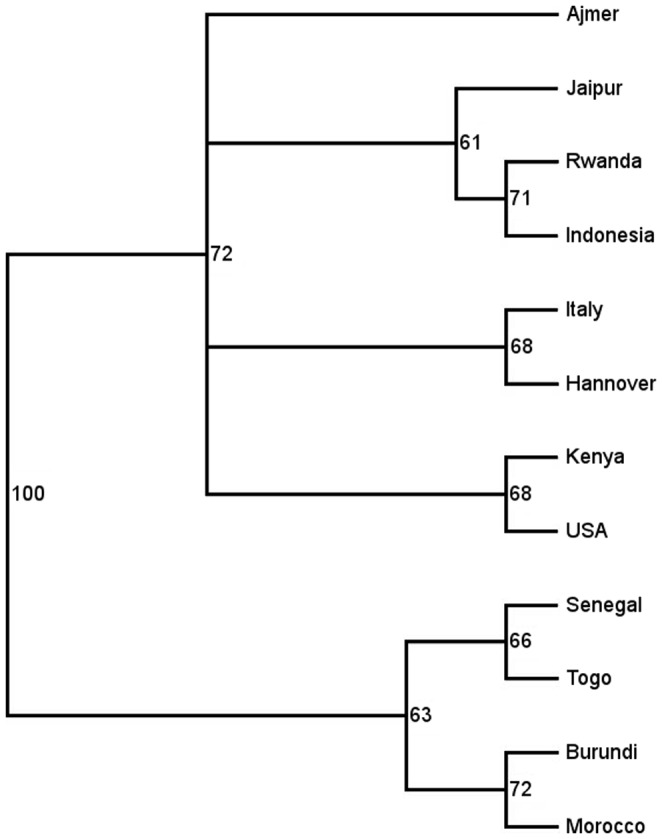
Bootstrap values≥50% are above the branches.
3.2 Stress tolerance
We were interested to analyze physiological differences among members of the genetically quite homogeneous African group. Therefore the response to different soil water contents of E. tirucalli genotypes Morocco and Senegal that were grown on clay-loam:sandy soil type after eight weeks of treatment was evidenced through the measurement of growth parameters.
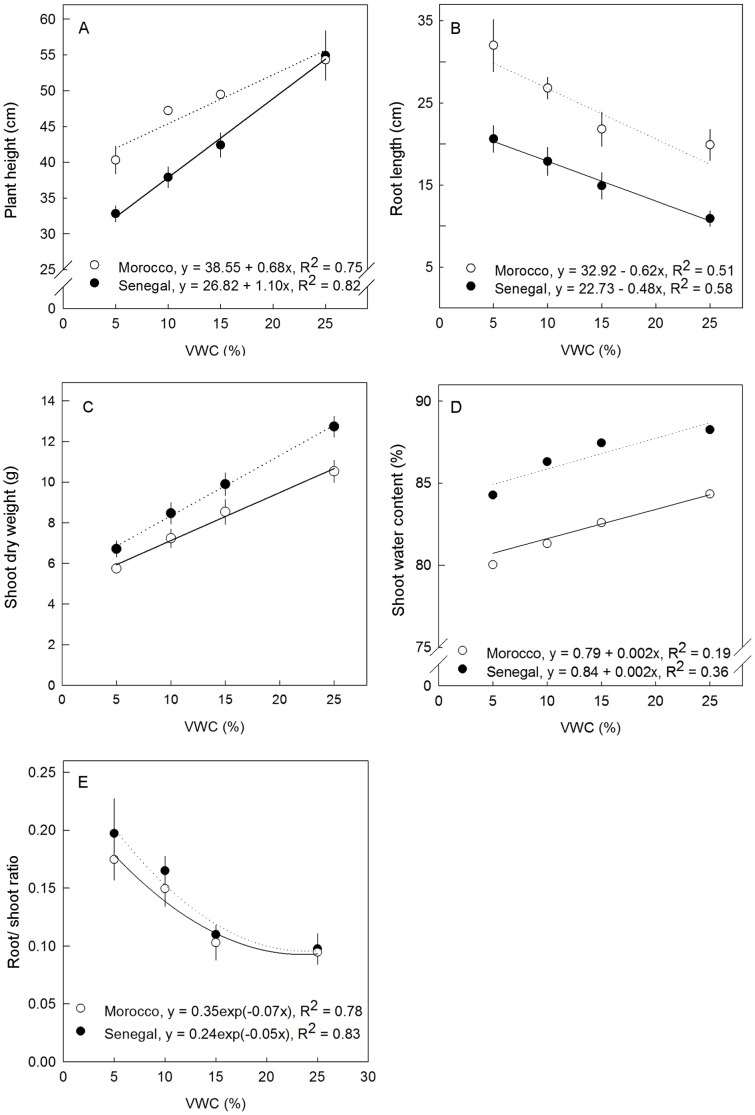
Plant height was significantly reduced by applying drought stress in the experiment (Fig. 2A). It decreased in line with the decrease in VWC (%). Average plant height before treatment was 29.06 cm for Morocco and 27.93 cm for Senegal. After eight weeks the highest height of genotype Morocco was with plants grown in VWC 25% (54.30±1.48 cm) whereas lowest values were obtained in VWC 5% (40.30±1.89 cm). Genotype Senegal had the highest (54.91±3.45 cm) and the lowest (32.80±0.86 cm) heights in the same respective VWCs. Plant height decreased linearly with decrease in water content. Thus, genotype Morocco grew by 86.85% at normal water content and 38.67% at high water limitation. Meanwhile, growth in genotype Senegal was 96.59% at VWC 25% and 17.43% at VWC 5%. Growth percentage showed that genotype Senegal grew faster than genotype Morocco when water was well available, but that drought highly decreased the growth rate.
Figure 2. Effect of water limitation on (A) plant height,(B) root length, (C) shoot dry weight, (D) shoot water content and (E) root/shoot ratio of E. tirucalli genotypes Morocco and Senegal after 8 weeks drought stress treatment.
Vertical error bars denote standard error of mean (SEM), n = 5.
Increased water limitation caused reduction of dry weight (Fig. 2C) and water content (Fig. 2D) in both genotypes. Genotype Senegal had higher biomass accumulation at VWC 25% (12.74±0.51) than genotype Morocco (10.53±0.54). The first genotype also had higher yield at the lowest VWC (6.71±0.39 g) than genotype Morocco (5.74±0.22 g). Decrease in water content percentage was small due to water limitation: genotype Senegal was 88% and Morocco 84% at VWC 25%, and 84% and 79% at VWC 5%, respectively.
Drought stress increased tap root length (Fig. 2B) and root/shoot ratio (Fig. 2E) in both genotypes. Genotype Senegal showed a ratio of 0.09±0.01 at VWC 25% and 0.19±0.03 at VWC 5%, genotype Morocco 0.09±0.01–0.17±0.02 in VWC (%) 25 to 5, respectively. The result implies that both genotypes partitioned photosynthetic products more in root biomass following drought stress. Plant height, dry weight, water content percentage and root/shoot ratio of genotypes Morocco and Senegal showed a significant reduction when plants were subjected to a drought stress of eight weeks. The stress responses of both genotypes differed indicating differences in phenotypic plasticity.
3.3 Chlorophyll fluorescence
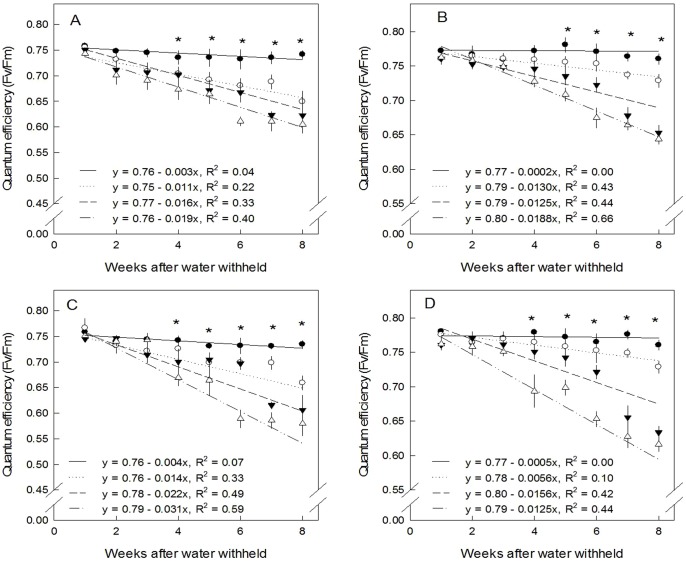
Quantum efficiency of genotypes Morocco and Senegal in the photosystems of leaves and stems over eight weeks decreased linearly with water limitation (Fig. 3). Stems (Fig. 3B, 3D) of both genotypes showed higher quantum efficiency than leaves (Fig. 3A, 3C). Quantum efficiency of Morocco leaves for all VWCs (%) was in a range of 0.757–0.605. These values were higher than those for genotype Senegal (0.758–0.579) at similar VWCs. Genotype Morocco also had higher values at stem level (0.780–0.643) than genotype Senegal (0.780–0.616). In the leaves of both genotypes, there was no significant difference between different VWCs in the first three weeks, but there was a significant difference from week four onwards. When considering stems, however, genotypes performed differently. In genotype Morocco, significant differences between VWCs started to develop in week five, while in genotype Senegal (Fig. 3D) changes started in week four. This shows that genotype Morocco had higher drought tolerance than genotype Senegal.
Figure 3. Effect of water limitation on quantum effciency during 8 weeks drought stress treatment.
n = 5 (A) Morocco leaves, (B) Morocco stem, (C) Senegal leaves (D) Senegal stem, (•) VWC 25%, (○) VWC 15%, (▾) VWC 10% and (Δ) VWC 5%, n = 5. Vertical error bars denote the standard error of mean (SEM). Stars above the point denote significant difference between VWC in each week treatment following the Tukey procedure (p<0.05).
3.4 Malate content
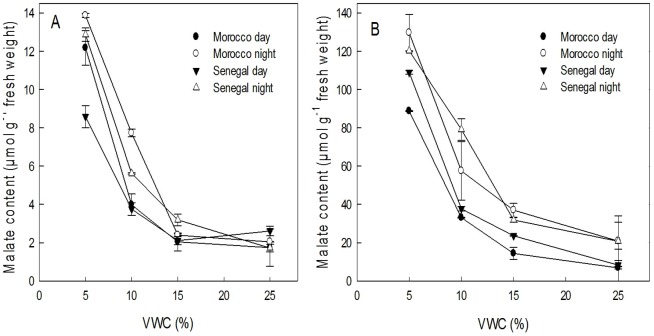
Differences in photosynthetic pathways were ascertained by comparing malate content of leaves and stems before drought stress and after exposure to drought stress. Our results show that before drought exposure, there was malate content oscillation between day and night in both genotypes' stems (Fig. 4). In genotype Morocco, malate content of stems at the end of light period was 58.9% lower than that at the end of dark period. Meanwhile, decrease in genotype Senegal was only 17.4%.
Figure 4. Box plot (n = 3) of malate contents of stems and leaves (µmol g−1 fresh weight) of E. tirucalli genotypes Morroco (Mor) and Senegal (Sen).
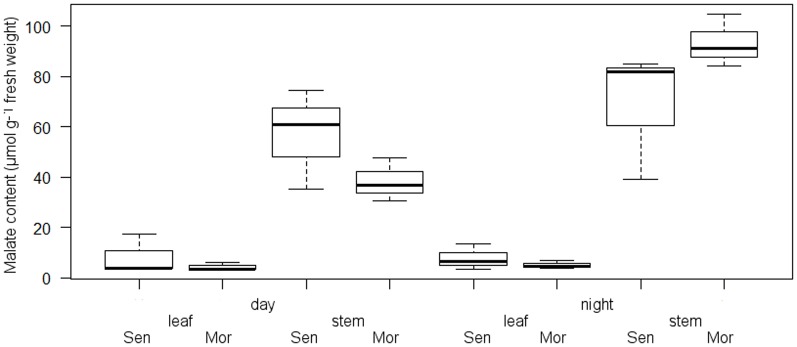
With increasing drought stress, malate content increased in stems of both genotypes (Fig. 5). We noted a significant difference in malate content in stems and leaves of the plants, but there was no significance difference between genotypes. The highest malate oscillation between day and night at stem level for genotype Morocco was 68.75% in VWC 15% whereas for genotype Senegal it was 69.55% at VWC 10%.
Figure 5. Malate content of (A) leaves (B) stem of genotypes Morocco and Senegal at day and night on different VWC after eight weeks of drought stress treatment.
Vertical error bars denote standard error of mean (SEM), n = 3.
In leaves, there were significant differences between day and night malate content at VWCs 10% and 5%. In VWC 10%, malate content was 48.22% and 33.16% lower during the day than during the day for genotypes Morocco and Senegal, respectively. In VWC 5%, we only evidenced a significant different in genotype Senegal. At this VWC, day-time malate content was 50% lower than that at night. These values would indicate that there is CAM induction in leaves following drought stress which strength might be genotype-dependent.
3.5 Rubber content
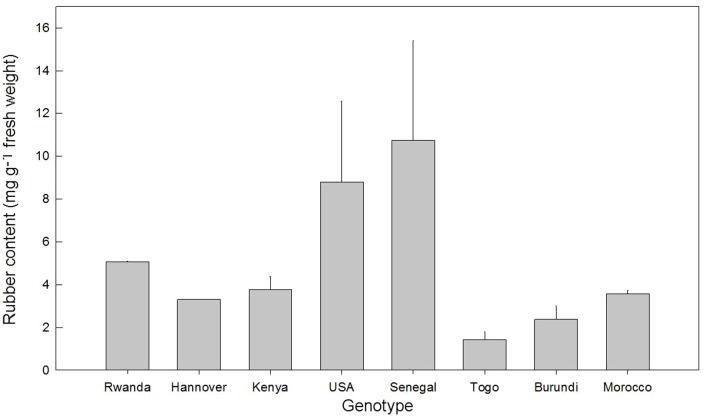
E. tirucalli can be a source of rubber. The rubber content analysis was done by NMR for eight genotypes in our collection, including Morocco and Senegal. The analysis showed strong differences in the concentration of rubber between the genotypes (Fig. 6). Senegal, with 10.74 mg g−1 fresh weight, had the highest amount of rubber among genotypes tested, followed by USA 8.80 mg g−1 fresh weight. The lowest rubber concentration was found in genotype Togo which had 1.42 mg g−1 fresh weight. There is no correlation of rubber content and genotype classification (Fig. 1 and Fig. 6), at least in greenhouse conditions.
Figure 6. Rubber content of eight E. tirucalli genotypes.
Each bar illustrates the mean (n = 3). Vertical error bars denote standard error of mean (SEM).
3.6 Biogas production
The results of the mesophilic anaerobic digestion of dried samples of six different genotypes of E. tirucalli indicate a promising potential with regard to the use of dried biomass of this species as a feedstock for biogas production. Specific biogas production (L biogas kg−1 ODM) was in the range of 114 for genotype Togo and 637 for genotype Kenya. Both genotypes which has been investigated in more detail in the drought stress experiments show values around 440 L biogas kg−1 ODM, about 70% of the highest value. The methane concentrations lie between 43% and 69%, depending on the genotype. These are preliminary results based on two independent experiments. Not for all genotypes data for all the three replicates in each experiment could be obtained due to initial technical problems with our bench-scale biogas plant. Therefore, we are currently not able to calculate any reliable standard deviations. The experiment will be repeated shortly for all genotypes with optimised equipment. Remarkable are the high amounts of H2S which reached up to 1,750 ppm (Table 2).
Table 2. Specific biogas production (L biogas kg−1 ODM) and gas composition in the biogas produced.
| Genotype | Biogas production | CH4(%) | H2S (ppm) |
| Togo | 114 | 69 | n.a. |
| USA | 367 | 44 | ∼1350 |
| Morocco | 435 | 43 | ∼1630 |
| Senegal | 440 | 54 | n.a. |
| Rwanda | 522 | 41 | n.a. |
| Kenya | 637 | 50 | ∼1750 |
n.a., not analyzed. In case standard deviations could be calculated, they were always less than 10%.
Discussion
4.1 Molecular analysis through genetic markers
The division in two groups as presented in Figure 1 is congruent with the geographic division in an African group and a mostly non-African group (except for Kenya and Rwanda). More samples have to be collected for example from Pakistan, Egypt, and Somalia to analyze whether they belong to the non-African group. Analysis of the genotypes from Brazil might help to estimate the phylogenetic position of the USA genotype, if this is domestic species. Genotypes of E. tirucalli are propagated vegetatively since many years in the greenhouse. Therefore the genotype originally collected is not changed since the cultivation due to pollination of flowers. Therefore the genetic drift between generations is low. This vegetative propagation also occurs naturally and/or is conducted by man because this plant seldom produces viable seeds [3]. The dendrogram shows that there is no correlation between morphological characters, as genotype Kenya and USA that have different stem color are clustered as a monophyletic group. Genotype USA has the most distinctive morphological character, i.e. yellow tips. This morphological character is useful for marketing purposes as this accession is sold as an ornamental. The division in two groups within the collection may indicate the breeding potential for different utilizations that can be explored. Generally, genotypes in the African group grow faster and produce more biomass than those in the non-African group (data not shown). It indicates that genotypes in the African group may be suitable as source of biomass and therefore bioenergy, while genotypes in the other group may be suitable for other purposes such as ornamental plant.
4.2 Response of plants to different drought treatments
Variation in drought tolerance within a genotype collection is important for subsequent selection work. Analysis of physiological parameters shows that plant height, dry weight and water content decreased with higher drought stress. Research on other plant species, such as Amaranthus and wheat, showed also that there is reduction in plant height and biomass with increase in drought stress in the soil [40], [41]. In general, decrease in biomass production rate due to stress exposure has been found to be associated with cessation of photosynthesis, metabolic dysfunction and damage of cellular structure [42]. Further, in response to drought stress, E. tirucalli genotypes Morocco and Senegal altered their root dry mass ratio and root length as one of the mechanisms to adapt to drought stress. Root dry mass in drought conditions is higher than in normal condition; this is in accordance with early studies [40], [43] and in line with the theory of functional balance which indicates that plants will respond to low water contents with a relative increase in the flow of assimilates to roots and increased root dry mass [44]. The root grows longer which enables the plant getting to deeper water layers thus escaping from water deficits near the surface [45]. Root elongation reduces shoot dry weight as photosynthesis yield is used for root development at the expense of shoots. Our results of responses to different water content RWC showed that 15% VWC was a critical threshold, below which plants partitioned assimilates to roots which might reduce stem yield.
C3 leaves wither and die quickly after the onset of stress, and also E. tirucalli becomes leafless. CAM stems can proceed with photosynthesis with closed stomata during the day. This provides an ecological advantage of CAM as it allows supplying CO2 [46] through decarboxylation of malate; hence it can prevent photorespiration damage during stress [47]. However, during prolonged drought stress, CO2 release from decarboxylation may be insufficient to protect chloroplast membranes from oxidative stress. This oxidative stress derives from partially reduced forms of atmospheric O2 and influences the repair of PSII during stress [48]. Cessation of photosynthesis is supported by a decline in Fv/Fm along with prolonged drought in both genotypes. The decline of Fv/Fm becomes higher at lower VWCs, whereby VWC 5% shows the highest decline. The decrease of Fv/Fm at high water limitation has been related to a decline in functioning of primary photochemical reactions, primarily involving inhibition of PSII that is located in the thylakoid membrane system [49]. The values between leaves and stems are not significantly different in the three first weeks of the experiments, during which stress symptoms such as leaf senescence did not appear yet. After prolonged stress, values at stems of both genotypes are higher than at leaves. Quantum efficiency values for all VWC values of genotype Morocco at leaf (0.757–0.605) and stem (0.780–0.643) levels were higher than in genotype Senegal for both leaf (0.758–0.579) and stem (0.780–0.616) levels, respectively. This indicates that quantum efficiency difference is also determined genetically. Drought significantly decreases quantum efficiency at week five for stems of genotype Morocco and at week four for stems of genotype Senegal. Lower photosynthetic efficiency under stress is associated with a damaged photosystem due to stress and reflects a certain degree of environmental stress [50]. The CAM photosynthetic pathway in the stem provides an ecological advantage by supplying CO2 through decarboxylation of malate [51]; hence, it can prevent formation of reactive oxygen species (ROS) and limit photorespiration during stress [47]. However, during prolonged drought stress or higher water limitation, the release of CO2 from decarboxylation may be insufficient to protect chloroplast membranes from oxidative stress, which affects the repair of PSII during stress [48].
Stomatal conductance and infrared thermography measurements are suitable for genotype screening towards their drought tolerance. However, due to the cylindrical morphology of the E. tirucalli stem it is impossible to use a regular porometer. We obtained some results using a thermography camera T360 (FLIR Systems, Wilsonville, USA). In several parameters determined we observed differences in drought tolerance among the two genotypes supporting the data shown in Figure 2 to 5. However, due to the E. tirucalli morphology the results could not be exactly calculated and compared. In summary, the genotype Morocco is more tolerant to drought than genotype Senegal.
Water use efficiency, and assimilation rate to transpiration rate ratio increase in CAM is higher than in C3 and C4 [51]. However, biomass accumulation in CAM plants is usually very low, so that growth rate of plants that only rely on CAM is often limited [52]. However, in some species such as M. crystallinum, a plant with facultative CAM, photosynthetic rate is higher than that C3 species due to a high CO2 fixation rate at night which contributes for a great part to biomass production [53].
E. tirucalli genotypes Morocco and Senegal were both shown to tolerate severe drought stress (VWC 5%) without causing any plant death. Thus, our result confirms that the species has very good potential to be grown in arid area. Genotype Morocco had 84% water content and 16% dry weight in VWC 25%; those values decreased down to 79% and 21% in severe drought stress. Meanwhile, genotype Senegal had 88% water content and 12% dry weight, those values decreased down to 84% and 16% at the same VWCs. E. tirucalli water content and dry weight differs between studies: 76.6% water content and 23.4% dry weight [28], 88.33% water content and 11.67% dry weight [54], or 90% water content and 10% dry weight [3]. Different percentages of water content and dry weight might be due to differences in genotypes and growth environment.
4.3 CAM and C3 photosynthetic pathways in E. tirucalli
The analysis of malate content in two genotypes of E. tirucalli shows that there are significant differences in leaves and stem. This clearly indicates that there is a difference in photosynthetic pathways between both parts. This result confirms the findings of Van Damme [55] evidenced by gas exchange experiments that there are two photosynthetic pathways allowing to distinguish C3 leaves from CAM. Malate content before exposure to water limitation shows that the highest content is in nocturnal stems which confirms dark nocturnal CO2 uptake [56]. More gas exchange experiments are needed to quantify the CO2 uptake. We observed open stomata at night and closed stomata during the day. Wax patches appear as a dotted white line along the stem axis in a magnified view and surround the stomata (data not shown). These epicuticular wax patches do not melt in greenhouse conditions to seal or block the stomata. Therefore CO2 influx at night is not hindered by melted wax. Malate content under higher water limitation increases both in stems and leaves, maybe as an indication of CAM induction in the latter. In stems, the highest percentage of malate day–night oscillation of genotype Morocco is at VWC 15% whereas for genotype Senegal we evidenced it at VWC 10%. Malate might be transported from the stem into the leaves. However, so far it was not reported that malate or other water-soluble compounds are transported via the non-articulate laticifers from organ to organ. Phosphoenolpyruvate (PEP) carboxylase enzyme activity and its gene expression could be investigated in stems and leaves to prove our hypothesis that there might be CAM induction in leaves under drought stress.
Photosynthesis in non-succulent leaves of E. tirucalli is reported as C3 and CAM in succulent stems [3]. Having two photosynthetic pathways in two very distinct plant parts is reasonable as it is supported by different anatomy. In genotype Morocco, we evidenced a significant difference in malate content (in µmol g−1 fresh weight) at VWC 10% between 3.9 (day) and 7.7 (night) and at VWC 5% between 13.9 (day) and 12.2 (night) while genotype Senegal shows differences at VWC 10% of 3.7 (day) and 5.5 (night) and at VWC 5% of 8.0 (day) and 12.9 (night). This result, however, reveals that there may be an induction of CAM in leaves due to drought stress as there is oscillation in nocturnal and diurnal malate content. This result which may seem at odds with previous results needs further investigation because anatomically leaves of E. tirucalli are non-succulent, in contrast to the stems. It is thereby tempting to question whether the leaves are really non-succulent. Indeed, CAM is a syndrome that impliesa certain degree of succulence based on the presence of large vacuoles for malate storage [11]. We therefore recommend E. tirucalli leaves would be anatomically investigated for large vacuoles for supporting malate storage. Species such as Tillandsia usneoides L. that perform CAM with non-succulent anatomy still have large vacuoles [57], [58].
Environmental conditions can influence the plasticity of photosynthetic pathways. Strong stress leads to conversion of C3 to CAM photosynthetic pathway, for example in the genus Clusia [59]. Change of C3 to CAM has been documented in other, succulent, species such as M. crystallinum [14], genus Sedum [15], and some species of Peperomia and Clusia [60], [61]. CAM induction during stress positively influences the activities of enzymes involved in malate metabolism [14], [62], [63]. These enzymes are nicotinamine adenine dinucleotide-dependent malic enzyme (NAD-ME) [64], nicotinamide adenine dinucleotide phosphate dependent malic enzyme (NADP-ME) [14], and PEP carboxylase [65].
With two photosynthetic pathways present at leaf and stem levels, and certain plasticity in switching between C3/CAM metabolism in E. tirucalli, it is not surprising that this plant is recommended as source of biomass for biofuel production that can be grown in marginal conditions. Loke et al. [28] mentioned the prospect of planting E. tirucalli; they are already monitoring plantations in Colombia, and are planning to have more in Somalia and other dry African countries. The species can yield 22–25 t dry weight biomass ha−1 y−1 under optimal conditions whereby optimal planting density is estimated at 14,000 plants ha−1. However, the data presented by the latter authors are not complemented by detailed information on cropping conditions such as irrigation, planting density, and genotypes used. In addition, Van Damme (unpublished data) was able to show that a 3 years' old plantation in Kenya was able to fetch around 500 t ha−1 of fresh material.
4.4 Potential use as source of rubber and biogas
Our results indicate that rubber content varies between genotypes, independently of the affiliation to one AFLP group. This result is supported by a study with several other genotypes: rubber content was different in each genotype depending on soil, climate and year [66], whereas it is not clear whether this is due only to genetic determinants or whether there are also some environmental influences that intervene. Akpan et al. [67], who analysed latex yield of Hevea brasiliensis L. found that rubber yield was influenced by clone and soil type. The authors revealed that when soil fertility was better, rubber (latex) yield was also higher. We evidenced the highest rubber content in genotype Senegal. This result supports Van Damme [55] who mentioned that the Senegal genotype was promising as a source of rubber.
Latex of E. tirucalli has drawn a lot of attention because it contains high levels of rubber. It has been used as such since the early 20th century [68]. The type of rubber of E. tirucalli is a mixture of long chain ketones and cis-1,4 polyisoprene, and is slightly soluble in hot alcohol [66], [69]. Beside rubber, the latex of this plant also consists of a resin which prevents long-term stability of latex [54]. Although the rubber has lower quality than that of H. brasiliensis, its properties should be further explored in order to fully exploit its potential as a naturally occurring polymer. The detailed composition of sterols and triterpenoids in greenhouse-grown plants and field-grown plants has to be analyzed by GC-MS in the future. Also the expression of the rate limiting enzyme of the mevalonate pathway, 3-hydroxy-3-methylglutaryl-CoA reductase, should be analyzed for its expression in different E. tirucalli genotypes to analyze the genetic dependency of the biosynthesis of latex components.
The use of E. tirucalli as a source of energy is promising because it grows fast whilst having at the same time low water requirements and a low demand for nutrients [3]. It was stated that this species could be used for biofuel production due to its high latex content [24]. Our results indicate that the biogas production in our batch tests varies among genotypes (Table 2). The results also show that E. tirucalli definitely has potential to serve as a feedstock for the production of biogas.
To date only a few experimental results concerning the biogas production potential of E. tirucalli have been published. Sow et al. [70] reported a potential annual methane production of around 3,000 m3 ha−1 per year based on research carried out in Kenya with a stand density of 80,000 plants per hectare and a biomass yield of 20 t ha−1 y−1 (DM). In field experiments in Colombia, 30 t ha−1 y−1 (DM) of E. tirucalli biomass brought about 8,250 m3 ha−1 biogas [28]. Assuming a methane content of approx. 50% (Table 2), the methane yield of E. tirucalli seems to be smaller compared to the yields of maize silage (5,800 m3 ha−1 y−1) and forage beet plus leaves (5,800 m3 ha−1y−1); however, its yield exceeds that of wheat (2,960 m3 ha−1 y−1) and rapeseed (1,190 m3 ha−1 y−1) [71].
In the results presented here it is remarkable that the H2S concentrations are the comparatively high in the E. tirucalli-derived biogas. H2S contents are indeed lower than those from the fermentation of manure, biowaste and food waste which are in the range of 2,000–6,000 ppm due to a high content of sulfur-containing proteins [72], but higher than those of maize silage-derived biogas with approx. 500 ppm. H2S can impair the utilization of biogas, as it has the ability to corrode the metal parts of the fermenting installation and can cause health problems in high doses and long exposures [73]. To decrease H2S content during processing, different techniques are available, such as biofilters consisting of phototrophic (Cholorobium limicola) or chemotrophic bacteria (Thiobacillus spp.) [74]. In order to improve the reliability of the method, further biogas batch tests with E. tirucalli should comprise a systematical variation of the following parameters: age of plant material (because the older the plant, the higher the lignin content), particle size of the substrate in order to investigate its influence on biodegradability of feedstock, optimization of choice and pre-treatment of the inoculum [75], and last but not least genotype-dependent differences.
The presented data are based on lab-scale experiments. Further field experiments will be necessary before a specific E. tirucalli genotype can be proposed for practical application. Among the genotypes tested, Kenya has the highest yield in biogas per organic dry matter and should be further analyzed for its biomass gain during drought stress conditions in the greenhouse and in the field. Senegal is promising as a source of biomass and biogas as well. When water availability is limited, using genotype Morocco with higher drought tolerance as a source of bio-energy is recommended, because biogas production using genotype Morocco is as high as with genotype Senegal. Genotype USA is promising as an ornamental plant and source of biogas, but its drought tolerance is not yet known. Combining these valuable characteristics through breeding may bring more benefit. Stocked genotypes could be distributed to interested farmers and researchers in arid areas for performing field experiments and challenge the greenhouse results by natural conditions.
Conclusion
E. tirucalli has a high potential as drought-tolerant crop plant because of its unique combination of photosynthetic pathways and as source of biofuel, rubber and maybe even phytochemicals. The genetic relationship within the collection was analyzed by AFLP. There may be induction of CAM in leaves due to stress. Despite these substantial results, several questions remain to be addressed. The confirmation of E. tirucalli photosynthetic pathways' plasticity at leaf level, that may play an important role to survive during drought stress, needs to be investigated in more detail. Thus, it will be interesting to analyze how enzymes influence metabolic adjustment to stress conditions in leaves and stem. To explore the use of E. tirucalli, determination of rubber composition in different genotypes, and quality and technical optimization of fermentation processes for the production of biogas need to be performed. The characterized genotypes from our greenhouse should be used in field experiments in tropical regions to verify and extend the data obtained in greenhouse conditions.
Acknowledgments
Samples from India were kindly provided by Dr. Vijendra Shekhawat, University of Mumbai, India. We would like to thank the gardeners for growing plants and Pamela von Trzebiatowski for malate analysis. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Leibniz Universität Hannover.
Funding Statement
B.R. Hastilestari was supported by Katholischer Akademischer Ausländer-Dienst (KAAD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dai A (2012) Increasing drought under global warming in observations and models. Nature Clim Change doi:10.1038/nclimate1633
- 2. Bruyns PV, Mapaya RJ, Hedderson T (2006) A new subgeneric classification for Euphorbia (Euphorbiaceae) in southern Africa based on ITS and psbA-trnH sequence data. Taxon 55: 397–420. [Google Scholar]
- 3.Van Damme PLJ (2001) Euphorbia tirucalli for high biomass production. In: Schlissel A, Pasternak D, editors. Combating desertification with plants, Kluwer Academic Pub. pp. 169–187. [Google Scholar]
- 4. Janssens MJ, Keutgen N, Pohlan J (2009) The role of bio-productivity on bio-energy yield. J Agr Rural Dev Trop 110: 39–47. [Google Scholar]
- 5. Webster GL (1975) Conspectus of a new classification of the Euphorbiaceae. Taxon 24: 593–601. [Google Scholar]
- 6. Batanouny KH, Stichler W, Ziegler H (1991) Photosynthetic pathways and ecological distribution of Euphorbia species in Egypt. Oecologia 87: 565–569. [DOI] [PubMed] [Google Scholar]
- 7. Bender MM (1971) Variation in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10: 1239–1244. [Google Scholar]
- 8. Pearcy RW (1975) C4 photosynthesis in form Euphorbia species from Hawaiian rainforest sites. Plant Physiol 55: 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ting IP, Bates L, Sternberg LO, Denior MJ (1985) Physiological and isotopic aspects of photosynthesis in peperomia. Plant Physiol 78: 246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BN (1982) General characteristics of terrestrial plants (agronomic and forests)-C3, C4 and Crassulacean Acid Metabolism plants. CRC Handbook of biosolar resources 1 (2): , 99–113. [Google Scholar]
- 11. Nuernbergk EL (1961) Endogener Rhythmus und CO2 Stoffwechsel bei Pflanzen mit diurnalem Säurerhythmus. Planta 56: 28–70. [Google Scholar]
- 12. Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Biol 29: 379–414. [Google Scholar]
- 13. Cushman JC, Borland AM (2002) Induction of Crassulacean acid metabolism by water limitation. Plant Cell Environ 25: 295–310. [DOI] [PubMed] [Google Scholar]
- 14. Holtum JAM, Winter K (1982) Activity of enzymes of carbon metabolism during the induction of Crassulacean acid metabolism in Mesembryanthemum crystallinum L. Planta. 155: 8–16. [DOI] [PubMed] [Google Scholar]
- 15. Gravatt DA, Martin CE (1992) Comparative ecophysiology of five species of Sedum (Crassulaceae) under well watered and drought stressed conditions. Oecologia 92: 532–541. [DOI] [PubMed] [Google Scholar]
- 16.Aylward JH, Parsons PG (2008) Treatment of prostate cancer. Peplin Research May, 27 2008: US 7378445 Available: http://appft1.uspto.gov/. Accessed 2010 Dec 28.
- 17.Duke J (1983) Euphorbia tirucalli L., handbook of energy crops. Purdue University centre for new crops and plant products. www.hort.purdue.edu. Accessed 5 December 2010. [Google Scholar]
- 18.Kumar A (1999) Some potential plants for medicine from India, Ayurvedic medicines, University of Rajasthan, Rajasthan. pp. 1–12. [Google Scholar]
- 19.Schmelzer GH, Gurib-Fakim A (2008) Medicinal plants. Plant Resources of Tropical Africa. pp. 412–415. [Google Scholar]
- 20. Van Damme PLJ (1989) Het traditioneel gebruik van Euphorbia tirucalli . African Focus 5: 176–193. [Google Scholar]
- 21. Uchida H, Yamashita H, Kajikawa M, Ohyama K, Nakayachi O, et al. (2009) Cloning and characterization of a squalene synthase gene from a pretroleum plant, Euphorbia tirucalli L. Planta. 229: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen PE, Nishimura H, Liang Y, Calvin M (1979) Steroids from Euphorbia and other latex-bearing plants. Phytochemistry 18: 103–104. [Google Scholar]
- 23. Furstenberger G, Hecker E (1977) New highly irritant euphorbia factors from latex of Euphorbia tirucalli L. Experentia. 33: 986–988. [DOI] [PubMed] [Google Scholar]
- 24. Calvin M (1978) Chemistry, population, resources. Pure Appl Chem 50: 407–425. [Google Scholar]
- 25. Calvin M (1980) Hydrocarbons from plants: Analytical methods and observations. Naturwissenschaften 67: 525–533. [Google Scholar]
- 26. Kalita D (2008) Hydrocarbon plant - New source of energy for future. Renew Sust Energ Rev 12: 455–471. [Google Scholar]
- 27. Mwine J, Van Damme P (2011) Euphorbia tirucalli L. (Euphorbiaceae) – The miracle tree: Current status of available knowledge. Sci Res Essay 6: 4905–4914. [Google Scholar]
- 28.Loke J, Mesa LA, Franken JY (2011) Euphorbia tirucalli biology manual: Feedstock production, bioenergy conversion, application, economics Version 2. FACT. [Google Scholar]
- 29. Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 102: 867–873. [DOI] [PubMed] [Google Scholar]
- 30. Lirio LG, Hermano ML, Fontanilla MQ (1998) Antibacterial activity of medicinal plants from the Philippines. Pharm Biol 36: 357–359. [Google Scholar]
- 31. Vassiliades G (1984) Note on the molluscidal properties of two Euphorbiaceae plants – Euphorbia tirucalli and Jatropha curcas . Rev Elev Med Vet Pays Trop 37: 32–34. [Google Scholar]
- 32.Murali R, Mwangi JG (1998) Euphorbia tirucalli resin: potential adhesive for wood-based industries, in: F. d. FAO corporate document repository (Ed.), International conference on domestication and commercialization of non-timber forest products in Agrosystems. FAO. Rome. [Google Scholar]
- 33. Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, et al. (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlüter PM, Harris SA (2006) Analysis of multilocus fingerprinting data sets containing missing data. Mol Ecol Notes 6: 569–572. [Google Scholar]
- 35. Page RDM (1996) TREEVIEW: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358 Available: http://taxonomy.zoology.gla.ac.uk/rod/treeview.html Accessed 2011 Mar 25. [DOI] [PubMed] [Google Scholar]
- 36. Jefferies RA (1994) Drought and chlorophyll fluorescence in field-grown potato (Solanum tuberosum). Physiol Plant 90: 93–97. [Google Scholar]
- 37. Kluge M (1971) Veränderliche Markierungsmuster bei 14CO2-Fütterung yon Bryophyllum tubiflorum zu verschiedenen Zeitpunkten der Hell-Dunkelperiode II. Beziehungen zwischen dem Malatgehalt des Gewebes und dem Markierungsmuster nach 14CO2-Lichtfixierung. Planta 98: 20–30. [DOI] [PubMed] [Google Scholar]
- 38.Duke J (1983) Euphorbia tirucalli L., handbook of energy crops. Purdue University centre for new crops and plant products. www.hort.purdue.educ. Accessed on 5 December 2010. [Google Scholar]
- 39.VDI 4630 (2006) Fermentation of organic materials, Characterisation of the substrate, sampling, collection of material data, fermentation tests. Beuth Verlag. Berlin, Germany. 92 p. [Google Scholar]
- 40. Liu F, Stützel H (2004) Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci Hortic 15: 15–27. [Google Scholar]
- 41. Zhang J, Hao C, Ren Q, Chang X, Liu G, et al. (2011) Association mapping of dynamic developmental plant height in common wheat. Planta 234: 891–902. [DOI] [PubMed] [Google Scholar]
- 42. Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 64: 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dias PC, Araujo WL, Moraes GABK, Barros RS, DaMatta FM (2007) Morphological and physiological responses of two coffee progenies to soil water availability. J Plant Physiol 164: 1639–1647. [DOI] [PubMed] [Google Scholar]
- 44.Brouwer R (1963) Some aspects of the equilibrium between overground and underground plant parts. In: Jaarboek IBS, Wageningen. pp. 31–39. [Google Scholar]
- 45. Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90: 80–494. [Google Scholar]
- 46. Martin CE, Jackson JL (1986) Photosynthetic pathways in a midwestern rock outcrop succulent, Sedum nuttallianum Raf. (Crassulaceae). Photosyn Res 8: 17–29. [DOI] [PubMed] [Google Scholar]
- 47. Borland A, Elliot S, Patterson S, Taybi T, Cushman J, et al. (2006) Are the metabolic components of Crassulacean acid metabolism up-regulated in response to an increase in oxidative burden? J Exp Bot 57: 319–328. [DOI] [PubMed] [Google Scholar]
- 48. Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757: 742–749. [DOI] [PubMed] [Google Scholar]
- 49. Souza RP, Machado EC, Silva JAB, Lagôa AMMA, Silveira JAG (2003) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot 51: 45–56. [Google Scholar]
- 50. Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51: 659–668. [DOI] [PubMed] [Google Scholar]
- 51. Herrera A (2008) Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for? Ann Bot 103: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heldt HW, Piechulla B (2011) Plant Biochemistry. 4th edition, Elsevier, London, UK. 656 p. [Google Scholar]
- 53. Bloom AJ, Troughton JH (1979) High productivity and photosynthetic flexibility in a CAM plant. Oecologia 38: 35–43. [DOI] [PubMed] [Google Scholar]
- 54.Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A (2009) Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. Available: http://www.worldagroforestry.org/af/treedb/. Accessed 2010 Dec 20.
- 55. Van Damme PLJ (1990) Gebruik van Euphorbia tirucalli als rubberleverancier en energiewas. African Focus 6: 19–44. [Google Scholar]
- 56. Kluge M, Heinigner B (1973) Untersuchungen über den Efflux yon Malat aus den Vacuolen der assimilierenden Zellen von Bryophyllum und mögliche Einflüsse dieses Vorganges auf den CAM. Planta 113: 333–343. [DOI] [PubMed] [Google Scholar]
- 57. Kluge M, Lange OL, Eichmann V, Schmid R (1973) Diurnaler Säurerhythmus bei Tillandsia usneoides: Untersuchungen über den Weg des Kohlenstoffs sowie die Abhängigkeit des CO2–Gaswechsels von Lichtintensität, Temperatur und Wassergehalt der Pflanze. Planta 112: 357–372. [DOI] [PubMed] [Google Scholar]
- 58. Loeschen VS, Martin CE, Smith M, Eder SL (1993) Leaf anatomy and CO2 recycling during Crassulacean acid metabolism in twelve epiphytic species of Tillandsia (Bromeliaceae). Int J Plant Sci 154: 100–106. [Google Scholar]
- 59. Taybi T, Nimmo HG, Borland AM (2004) Expression of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxylase kinase genes. Implications for genotypic capacity and phenotypic plasticity in the expression of Crassulacean acid metabolism. Plant Physiol 135: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ting IP, Hann J, Sipes DL, Patel A, Walling LL (1993) Expression of p-enolpyruvate carboxylase and other aspects of CAM during the development of Peperomia camptotricha leaves. Bot Acta 106: 313–319. [Google Scholar]
- 61. Borland AM, Tecsi LI, Leegood RC, Walker RP (1998) Inducibility of crassulacean acid metabolism (CAM) in Clusia species: physiological/biochemical characterisation and intercellular localization of carboxylation and decarboxylation processes in three species which exhibit degress of CAM. Planta 205: 342–351. [Google Scholar]
- 62. Ostrem JA, Vernon DM, Bohnert HJ (1990) Increased expression of a gene coding for NAD-glyceraldehyde-3-phosphate dehydrogenase during the transition from C3 photosynthesis to Crassulacean acid metabolism in Mesembryanthemum crystallinum . J Biol Chem 256: 3497–3502. [PubMed] [Google Scholar]
- 63. Cushmann JC (1992) Characterization and expression of a NADP-malic enzyme cDNA induced by salt stress from the facultative crassulacean acid metabolism plant, Mesembryanthemum crystallinum . Eur J Biochem 208: 259–266. [DOI] [PubMed] [Google Scholar]
- 64. Dittrich P, Campbell WH, Black CC Jr (1973) Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean acid metabolism. Plant Physiol 52: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ting IP (1968) CO Metabolism in Corn Roots. III. Inhibition of p-enolpyruvate carboxylase by L-malate. Plant Physiol 43: 1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uzabakiliho B, Largeau C, Casadevall E (1987) Latex constituents of Euphorbia candelabrum, E. grantii, E. tirucalli and Synadenium grantii . Phytochemistry 26: 3041–3045. [Google Scholar]
- 67. Akpan AU, Edem SO, Ndaeyo NU (2007) Latex yield of rubber (Hevea brasiliensis Muell Argo) as influenced by clone planted and locations with varying fertility status. J Agricul Soc Sci 3: 1813–2235. [Google Scholar]
- 68. Scasselati-Sforzolini G (1916) L'Euphorbia tirucalli. Istituto Agricolo Coloniale Italiano S. 25: 40. [Google Scholar]
- 69.Blaschek W, Hänsel R, Keller K, Reichling J, Rimpler H, et al. (1998) Hagers Handbuch der Pharmazeutischen Praxis, Drogen A-K. Berlin, Heidelberg, Springer Verlag. 909 p. [Google Scholar]
- 70. Sow D, Ollivier B, Viaud P, Garcia JL (1989) Mesophillic and thermophilic methane fermentation of Euphorbia tirucalli. . Mircen J Appl Microb 5: 547–550. [Google Scholar]
- 71. Weiland P (2003) Production and energetic use of biogas from energy crops and wastes in Germany. Appl Biochem Biotechnol 109: 263–274. [DOI] [PubMed] [Google Scholar]
- 72. Schieder D, Quicker P, Schneider R, Winter H, Prechtl S, et al. (2003) Microbiological removal of hydrogen sulfide from biogas by means of a separate biofilter system: experience with technical operation. Water Sci Technol 48: 209–212. [PubMed] [Google Scholar]
- 73.Binder R, Deninger A, Grous-Göldner A, Huter E, Jungwirth M et al.. (2009) Gefahrenpotential von Schwefelwasserstoff beim Betrieb von Biogasanlagen. Available: http://www.lea.at/download/Biogas/H2S_Leitfaden%20Biogasanlagen_2009.pdf. Accessed 2012 Sep 8.
- 74. Syed M, Soreanu G, Falletta P, Béland M (2006) Removal of hydrogen sulfide from gas streams using biological processes–A review. Can Biosyst Eng 48: 1–14. [Google Scholar]
- 75.Tomala F (2012) Entwicklung einer Methodik zur Ermittlung der Biogas und Methanausbeuten verschiedener Herkünfte von Euphorbia tirucalli als vielversprechende Energiepflanze. Bachelor thesis. Hannover, University of Applied Science Hannover. [Google Scholar]