Abstract
Dopamine modulates the synaptic plasticity in the primary motor cortex (M1). To evaluate whether the functioning of the cortico-striatal circuit is necessary for this modulation, we applied a paired associative stimulation (PAS) protocol that comprised an electric stimulus to the right median nerve at the wrist and subsequent transcranial magnetic stimulation of the left M1, to 10 patients with Parkinson's disease (PD) and 10 with multiple system atrophy of the parkinsonian type (MSA-P) with and without dopamine replacement therapy (-on/off). To investigate the M1 function, motor-evoked potentials (MEPs) were measured before and after the PAS. In both patient groups without medication, the PAS protocol failed to increase the averaged amplitude of MEPs. The dopamine replacement therapy in PD, but not in MSA-P effectively restored the PAS-induced MEP increase. This suggests that not the existence of dopamine itself but the activation of cortico-striatal circuit might play an important role for cortical plasticity in the human M1.
Introduction
Dopamine modulates the induction of synaptic plasticity in the striatum and primary motor cortex (M1). Animal models have shown that the dopaminergic signal projecting from the substantia nigra (SN) is essential for inducing cortico-striatal synaptic plasticity in the striatum [1], [2], [3], [4], [5]. Regional and training-specific changes in excitatory synaptic transmission in the striatum have been recorded in brain slices of trained mice [5], while the elimination of dopamine receptors and dopaminergic terminals from the M1 itself specifically impairs the induction of synaptic plasticity and motor skill acquisition [6]. Recently we showed that striatal dopamine release is essential for motor skill learning in humans [7].
Paired associative stimulation (PAS), which combines cortical stimulation by transcranial magnetic stimulation (TMS; through the intra-cortical fibers) and peripheral nerve electrical stimulation (through the thalamo-cortical pathway) with a specific interstimulus interval (ISI), appears to be a powerful method of inducing and evaluating plasticity in the M1 [8], [9], [10]. The effect is thought to involve a similar physiological mechanism to that of associative long-term potentiation (LTP) in animal models [11], [12]. Striatal dopamine depletion caused by degeneration of the nigrostriatal dopaminergic neuron leads to motor disturbances in patients with Parkinson's disease (PD), including symptoms of rigidity, tremor and hypokinesia as well as impairments of motor learning [13], [14], [15], [16]. By applying this method to patients with PD, it was found that M1 cortical plasticity is reduced during off-medication and restored during on-medication [17], [18]. Although M1 motor plasticity is also modulated by dopamine in humans, it is not clear whether this modulation is direct or secondary via the cortico-striatal circuit.
To evaluate this, we applied PAS to patients with multiple system atrophy with predominant parkinsonism (MSA-P) both with and without dopamine-replacement therapy. Recent diagnostic criteria characterize probable MSA-P as a sporadic, progressive, adult-onset disorder including rigorously defined autonomic failure and poorly levodopa-responsive parkinsonism [19]. This poor levodopa response of MSA-P compared with PD is caused by neuropathological differences, involving neuronal loss and gliosis in the substantia nigra as well as the striatum [20], [21].
Patients and Methods
Patients
Ten PD patients and 10 MSA-P patients were recruited from Kyoto University Hospital, Kyoto, Japan and Nagoya City University Hospital, Nagoya, Japan. All patients gave their informed written consent to participate, and the study was conducted according to the Declaration of Helsinki and approved by the local ethics committees of the Nagoya city university and Kyoto university institutions, respectively. The TMS exclusive criteria such as use of a pacemaker and medical interference in a patient's body were checked in writing. No patients were receiving antidepressant or neuropsychological medication.
The PD patients were not tremor-dominant and had been responsive to L-dopa therapy for more than two years. MSA-P patients fulfilled established clinical diagnostic criteria after extended clinical follow up at least 3 years. All MSA-P patients were eventually diagnosed as probable MSA of parkinsonian type according to Gilman's criteria [19]. The mean age ± SD of PD patients was 66±7.7 years, while that of MSA-P patients was 59.5±11.2 years. Parkinsonian symptoms in both diseases were assessed using the motor subscale (item 19–31) of the Unified Parkinson's Disease Rating Scale (UPDRS) to provide comparable parameters of motor symptom severity between the two groups. In MSA-P patients, cerebellar dysfunction was examined by gait ataxia, cerebellar dysarthria, limb ataxia, and cerebellar oculomotor dysfunction. Autonomic failure was diagnosed if there was at least one feature of postural hypotension and urinary incontinence.
Paired Associative Stimulation (PAS)
The optimal motor point for the right abductor pollicis brevis (APB) muscle was identified by connecting a focal TMS (figure-of-eight coil) to the Magstim 200 magnetic nerve stimulator. Surface electromyography (EMG) was recorded from the APB muscle (bandpass, 5–2000 Hz) and the optimal motor point for eliciting the best motor response was established over the M1 45° to the mid-sagittal line. The resting motor threshold (RMT) was defined, in accordance with a previous study [22], as the lowest stimulus intensity required to elicit a motor-evoked potential (MEP) with a peak-to-peak amplitude of >50 µV in the right APB muscle in at least five out of 10 trials. The intensity of the TMS test for following PAS was adjusted to produce an MEP of ∼1 mV from the APB muscle (SI1 mV).
The PAS consisted of a single electrical stimulus delivered to the right median nerve at the wrist (110% of the motor threshold) and a subsequent TMS (with an intensity of SI1 mV) over the left M1. Two hundred and forty pairs of stimuli were delivered at 0.2 Hz for 20 min with an interstimulus interval of 25 ms. To measure the mean peak-to-peak MEP amplitudes, 20 stimuli were delivered over the left M1, both before and immediately after PAS using a stimulus intensity of SI1 mV.
Experimental Design
All patients participated twice in the PAS study. They were examined in the practically defined off state after the withdrawal of L-dopa/carbidopa and selegiline for at least 12 h and dopamine agonists for at least 24 h (off-medication). To investigate the effect of dopaminergic medication, we repeated the same experiment 2 h after administration of the antiparkinsonian drugs (on-medication) subsequent to at least three days after the off-medication. Peak-to-peak MEP amplitudes for each experiment were measured to determine the motor cortical plasticity (PD-on, PD-off and MSA-P-on, MSA-P-off). To evaluate the somatosensory system, we recorded somatosensory evoked potentials (SEP) from the scalp (Fz and CPc) after right median nerve stimulation (MNS) only during on-medication. The peak latency of N20 was also measured.
Statistical Analysis
To evaluate the effect of PAS on motor cortex excitability in both patients group, the changes in MEP amplitudes were evaluated using a three-factor analyses of variance (ANOVA), treatment (off or on), time (pre- or post-) and disease (PD or MSA) as the main factors. If necessary, the Greenhouse–Geisser correction was used to adjust for sphericity, changing the degrees of freedom using a correction coefficient epsilon. The Bonferroni correction for multiple comparisons was used for the post hoc t-test. The threshold of significance was set at P<0.05.
Results
Patient demographic and clinical details are summarized in Table 1. There was no significant difference among duration of symptoms (2.7±0.7 years vs. 2.7±0.9 years, respectively, P = 1.0) at the time of PAS experiment and levodopa-equivalent daily dose between PD and MSA-P patients (230±79 mg vs. 260±70 mg, respectively, P = 0.3). There were also no differences in the UPDRS motor scores during the off-medication state (18.4±8.9 vs. 24.5±10.8, respectively, P = 0.24). The PD patients showed a higher UPDRS motor score in the off-medication condition than the on-medication condition (18.4±8.9 and 7.8±4.6, respectively, *P = 0.0004 according to t-test). The MSA-P patients showed a slightly higher UPDRS motor score in the off-medication condition than in the on-medication condition (24.5±10.8 and 21.9±11.6, respectively, P = 0.05). No patients presented with levodopa-induced dyskinesia. Although MSA3 had not yet fulfill the clinical diagnostic criteria at the time of PAS experiment, after 2 years-clinical follow up, this patient presented autonomic failure, cerebellar ataxia and putaminal signal changes in MRI and was eventually diagnosed as probable MSA.
Table 1. Patient demographic and clinical details.
| Patient | Age | Gender | Duration of | Parkinsonian symptoms | Autonomic | Cerebellar | Babinski sign | MRI singal change or atrophy | Medication | |||
| symptoms | UPDRS (part 3) | failure | dysfucntion | with hyperreflexia | Putamen | Brainstem | Cerebellum | |||||
| (years) | (years) | On | Off | (mg/day) | ||||||||
| PD1 | 63 | F | 2 | 3 | 10 | - | - | - | - | - | - | 100 |
| PD2 | 61 | F | 3 | 4 | 14 | - | - | - | - | - | - | 200 |
| PD3 | 67 | M | 2 | 8 | 14 | - | - | - | - | - | - | 100 |
| PD4 | 68 | F | 3 | 6 | 28 | - | - | - | - | - | - | 300 |
| PD5 | 65 | F | 3 | 5 | 17 | - | - | - | - | - | - | 250 |
| PD6 | 58 | M | 4 | 18 | 35 | + | - | - | - | - | - | 300 |
| PD7 | 75 | F | 2 | 6 | 14 | - | - | - | - | - | - | 200 |
| PD8 | 62 | M | 3 | 5 | 11 | - | - | - | - | - | - | 300 |
| PD9 | 57 | F | 3 | 13 | 29 | - | - | - | - | - | - | 250 |
| PD10 | 62 | M | 2 | 10 | 12 | + | - | - | - | - | - | 300 |
| MSA1 | 54 | F | 3 | 29 | 29 | + | - | - | + | + | + | 300 |
| MSA2 | 68 | F | 3 | 39 | 39 | + | - | - | + | - | - | 300 |
| MSA3 | 42 | M | 2 | 7 | 19 | - | - | - | - | - | - | 100 |
| MSA4 | 48 | F | 2 | 15 | 17 | + | - | - | + | - | - | 300 |
| MSA5 | 68 | M | 2 | 11 | 13 | + | - | - | + | - | - | 300 |
| MSA6 | 75 | F | 3 | 13 | 13 | + | - | - | + | + | + | 200 |
| MSA7 | 64 | M | 3 | 28 | 31 | + | - | - | + | + | + | 300 |
| MSA8 | 42 | F | 2 | 32 | 34 | + | + | - | + | + | + | 200 |
| MSA9 | 76 | F | 5 | 34 | 38 | + | + | - | + | + | + | 300 |
| MSA10 | 62 | M | 2 | 11 | 12 | + | - | - | + | - | - | 300 |
During off-medication, resting motor thresholds of the maximal stimulator output were 52.9±12.6 % for PD and 57.8±11.0% for MSA patients. During on-medication, these values were 52.9±12.6% and 56.5±11.1%, respectively. There were no significant differences between the two groups, either in on- (P = 0.58) or off-medication (P = 0.51). SI1 mV off-medication values were 64.2±13.7 % for PD and 68.1±12.6% for MSA, and for on-medication these were 62.9±13.6 % and 68.1±12.6 %, respectively. There were no significant differences between the two groups, either in on- (P = 0.43) or off-medication (P = 0.46). The strength of MNS at the right wrist was 14.5±1.7 mA for PD and 13.7±1.4 mA for MSA patients during off-medication, and 14.5±2.2 mA and 13.7±1.3 mA, respectively, during on-medication. The onset of N20-P20 did not differ significantly between the two groups for on-medication (19.1±1.0 ms for PD and 19.0±1.2 ms for MSA-P, P = 1.0).
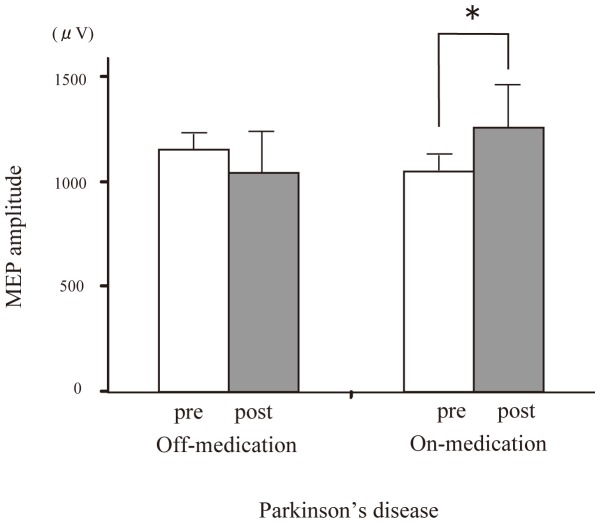
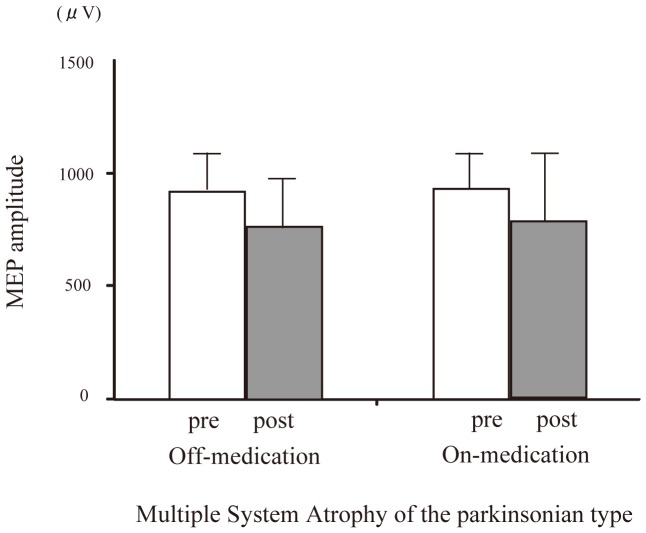
Looking at the between-subjects effects, the three-factor ANOVA showed no significant main effects for disease (P = 0.13), treatment (P = 0.20) and time (P = 0.36). There were significant time × treatment × disease (*P = 0.006), time × treatment (*P = 0.032), time × disease (*P<0.001) interactions, but not significant disease × treatment interaction (P = 0.11). Post hoc t-tests revealed the significant increase of MEP amplitude after PAS only for the PD patients with medication (*P = 0.03) (Figure 1). In contrast, there were no significant change of MEP amplitude after PAS in PD patients without medication (P = 0.86) and in MSA patients both with (P = 0.2) and without medication (P = 0.86) (Figure 2).
Figure 1. Effect of dopaminergic medication on PAS-induced modulation of the MEP amplitude with PD-on and-off.
In PD patients, the average MEP amplitude in the right APB was significantly elevated after dopaminergic medication (*P<0.05).
Figure 2. Effect of dopaminergic medication on PAS-induced modulation of the MEP amplitude with MSA-P-on and-off.
In MSA-P patients, the average MEP amplitude in the right APB was unchanged by dopaminergic medication.
Discussion
The main finding of the present study is that the effect of dopamine on cortical plasticity in the M1 differs between PD and MSA-P patients. Although the PAS-induced cortical plasticity in the M1 was decreased in both patients during off-medication, dopamine re-established the plasticity in patients with PD, but not in patients with MSA-P.
The physiological mechanism underlying the reduced M1 cortical plasticity in PD and MSA-P during off-medication appears to be related to degeneration of the nigrostriatal dopamine pathway. In animal studies, the dopaminergic signal projecting from the substantia nigra is essential for inducing the cortico-striatal synaptic plasticity in the striatum [1], [2], [3], [4], [5]. To induce the plastic changes of the cortico-striatal synapse, it is critical to activate the dopamine and NMDA receptors that are situated on the membrane of spiny neurons in the striatum [23], [24], [25]. This co-activation causes the intracellular signal transduction of a common intracellular integrator for inducing the synaptic plasticity in the striatum [3]. Moreover, recent models of the basal ganglia suggest that abnormal pattern of oscillations within the basal ganglia leads to the abnormal motor cortical plasticity via basal ganglia-motor cortical feedback loop [26], [27]. In PD, by applying PAS protocol, it was found that M1 cortical plasticity is reduced during off-medication [17], [18]. Therefore, it is possible that dopamine controls the dynamic circuitry of the cortical plasticity in the M1 through an indirect nigrostriatal pathway via the cortico-striatal circuit in PD and MSA-P.
The administration of dopamine restored the M1 cortical plasticity in PD, whereas cortical plasticity was not restored in MSA-P. These differences in the response to dopamine might be caused by neuropathological differences, possibly involving neuronal loss including medium spiny neuron and gliosis in the striatum [20], [21]. With regard to dopaminergic receptors, by measuring the binding potential of the striatal D2/D3-receptor with 11C-raclopride (RAC) positron-emission tomography (PET), loss of D2/D3-receptor binding in the putamen especially the posterior putamen is prominent in MSA-P compared with PD even in the early stage [28] [29]. In the present study, nine out of 10 patients showed putaminal signal changes in MRI indicative of neuronal loss and gliosis in the putamen. Together, these differences in the response to dopamine in PD and MSA-P seem to be caused by dopamine receptors in the putamen. Thus, the striatal dopamine and its receptors are essential for inducing cortical plasticity in the human M1 via the basal ganglia-motor cortical feedback loop.
In the present study, there was no significant difference in disease duration at the time of PAS and levodopa-equivalent daily dose between PD and MSA-P groups. Raclopride-PET study suggested relative and absolute increases in the number of dopamine D2/D3-receptors in the putamen contralateral to the predominant symptoms in the early stage of PD compared with age-matched healthy controls, related to the reduction of presynaptic dopaminergic nerve terminals [30]. However, previous reports showed that the motor cortical plasticity by PAS was not different between PD during on-medication and age-matched healthy controls [17], [18]. Therefore, upregulation of dopamine receptor in the early stage of PD may less modify the MEP facilitation effect by PAS in PD during on-medication.
In animal studies, the background dopamine concentration dependently facilitates LTP in the rat prefrontal cortex through postsynaptic D1 and/or D2receptor stimulation [31] [32] [33]. LTP induction depends on the level of tonic dopamine stimulation through dopamine receptors, and follows an inverted-U shape curve where both too-low and too-high levels induce LTD rather than LTP [34]. In humans, the modulation of D2 receptor activity produces a similar inverted-U shape curve on motor cortical plasticity by using the PAS and transcranial direct current stimulation (tDCS) techniques as a model of bidirectional or non-linear dopamine dose-dependent responses of synaptic plasticity [35], [36], [37], [38]. In the present study, MEP amplitudes tended to be reduced by PAS in on-medications in MSA-P, although this was not statistically significant. Based on these, the differences in dopaminergic modulation of motor cortical plasticity between PD and MSA-P might reflect another model of bidirectional dopamine dose-dependent effects on motor cortical plasticity [38].
To be effective, PAS requires a synchronized TMS pulse and a peripheral sensory input over the M1. A previous SEP study reported that the central sensory conduction time was progressively prolonged in parallel with disease duration in MSA [39]. In this study, since the peak N20 latencies were not prolonged in either PD or MSA-P, the timing of TMS over the M1 appears to be appropriate for inducing cortical plasticity in the M1. Although we did not record the cortical silent period, previous report clearly suggested that the cortical silent period was shortened by PAS in PD during off-medication and prolonged by levodopa treatment in non-dyskinetic group of PD but not in dyskinetic group [17]. Morgante et al. suggested that lack of LTP-like plasticity in the M1 and prolonged cortical silent period by PAS in dyskinetic patients both on-and off-medication contributed to the underlying mechanism of Levodopa-induced dyskinesias [17]. Therefore, the abnormal modification of cortical inhibitory system in M1 by PAS might be also seen in MSA-P.
Taking a clinical point of view, the distinction between PD and MSA-P is sometimes difficult, especially in the early stages of the disease and despite the use of diagnostic criteria [19], [40], [41]. Although neuroradiological methods such as magnetic resonance imaging (MRI) and PET are useful in detecting pathological changes [28], [40], [42], [43], [44], it is still difficult to accurately establish a diagnosis. With regard to TMS, some studies have focused on the motor cortical disinhibition assessed by intracortical inhibition and the cortical silent period and the impaired cortico-spinal tract assessed by the triple stimulation technique [45], [46], [47], whereas others showed that abnormal motor cortical excitability was not correlated with clinical features in MSA [48], [49], [50], [51], [52]. In the present study, one MSA-P patient showed no abnormal putaminal findings at the time of the PAS experiment, though the cortical plasticity in the M1 was already reduced (MSA-P 3 in Table 1). These differences in the cortical plasticity responsiveness to dopamine may provide supportive neurophysiological information in differentiating MSA-P from PD, in addition to neuroimaging findings. However, it would be more important to test motor cortical plasticity in other atypical parkinsonian syndromes such as Progressive Supranuclear Palsy and Cortoco-Basal Degeneration, because it is likely that motor cortical plasticity is altered in these patients. Further study is needed to demonstrate this based on larger patient populations of atypical parkinsonian syndromes and a combination of other quantitative modalities such as MRI and dopamine receptor imaging.
Funding Statement
This work was supported by grants from Takeda Pharmaceutical Company and the Ministry of Education, Culture, Sports, Science and Technology to Y.U. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Charpier S, Deniau JM (1997) In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci U S A 94: 7036–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P (2001) Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci 13: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 3. Picconi B, Gardoni F, Centonze D, Mauceri D, Cenci A, et al. (2004) Abnormal Ca2+-Calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J Neurosci 24(23): 5283–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, et al. (2003) Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci 23: 8506–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, et al. (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, et al. (2009) Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 4: e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawashima S, Ueki Y, Kato T, Matsukawa N, Mima T, et al. (2012) Changes in striatal dopamine release associated with human motor-skill acquisition. PLoS One 7: e31728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123 Pt 3: 572–584. [DOI] [PubMed] [Google Scholar]
- 9. Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J (2002) Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, et al. (2003) A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol 89: 2339–2345. [DOI] [PubMed] [Google Scholar]
- 11. Markram H, Lubke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275: 213–215. [DOI] [PubMed] [Google Scholar]
- 12. Dan Y, Poo MM (2004) Spike timing-dependent plasticity of neural circuits. Neuron 44: 23–30. [DOI] [PubMed] [Google Scholar]
- 13. DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trend Neurosci 13: 281–285. [DOI] [PubMed] [Google Scholar]
- 14. Smiley-Oyen AL, Worringham CJ, Cross CL (2003) Motor learning processes in a movement-scaling task in olivopontocerebellar atrophy and Parkinson's disease. Exp Brain Res 152: 453–465. [DOI] [PubMed] [Google Scholar]
- 15. Frith CD, Bloxham CA, Carpenter KN (1986) Impairments in the learning and performance of a new manual skill in patines with Parkinson's disease. J Neurol Neurosurg Psychiatry 49: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schnider A, Butbrod K, Hess CW (1995) Motion imagery in Parkinson's disease. Brain 118: 485–493. [DOI] [PubMed] [Google Scholar]
- 17. Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R (2006) Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain 129: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 18. Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, et al. (2006) Altered plasticity of the human motor cortex in Parkinson's disease. Ann Neurol 59: 60–71. [DOI] [PubMed] [Google Scholar]
- 19. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oppenheimer DR, Esiri MM (1992) Diseases of the basal ganglia, cerebellum and motor neurons. Greenfield's neuropathology Hume Adams J, Duchen LW, eds 5th ed.: 988–1045. [Google Scholar]
- 21. Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94: 79–100. [DOI] [PubMed] [Google Scholar]
- 22. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, et al. (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92. [DOI] [PubMed] [Google Scholar]
- 23. Kita H (1996) Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience 70: 925–940. [DOI] [PubMed] [Google Scholar]
- 24. Cherubini E, Herrling PL, Lanfumey L, Stanzione P (1988) Excitatory amino acids in synaptic excitation of rat striatal neurones in vitro. J Physiol 400: 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herrling PL (1985) Pharmacology of the corticocaudate excitatory postsynaptic potential in the cat: evidence for its mediation by quisqualate- or kainate-receptors. Neuroscience 14: 417–426. [DOI] [PubMed] [Google Scholar]
- 26. Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord 18: 357–363. [DOI] [PubMed] [Google Scholar]
- 27. Brown P, Marsden CD (1998) What do the basal ganglia do? Lancet 351: 1801–1804. [DOI] [PubMed] [Google Scholar]
- 28. Van Laere K, Clerinx K, D'Hondt E, de Groot T, Vandenberghe W (2010) Combined striatal binding and cerebral influx analysis of dynamic 11C-raclopride PET improves early differentiation between multiple-system atrophy and Parkinson disease. J Nucl Med 51: 588–595. [DOI] [PubMed] [Google Scholar]
- 29. Brooks DJ, Seppi K (2009) Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord 24: 949–964. [DOI] [PubMed] [Google Scholar]
- 30. Rinne JO, Laihinen A, Ruottinen H, Ruotsalainen U, Nagren K, et al. (1995) Increased density of dopamine D2 receptors in the putamen, but not in the caudate nucleus in early Parkinson's disease: a PET study with [11C]raclopride. J Neurol Sci 132: 156–161. [DOI] [PubMed] [Google Scholar]
- 31. Huang YY, Simpson E, Kellendonk C, Kandel ER (2004) Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci U S A 101: 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsuda Y, Marzo A, Otani S (2006) The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci 26: 4803–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolomiets B, Marzo A, Caboche J, Vanhoutte P, Otani S (2009) Background dopamine concentration dependently facilitates long-term potentiation in rat prefrontal cortex through postsynaptic activation of extracellular signal-regulated kinases. Cereb Cortex 19: 2708–2718. [DOI] [PubMed] [Google Scholar]
- 34. Goto Y, Yang CR, Otani S (2010) Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry 67: 199–207. [DOI] [PubMed] [Google Scholar]
- 35. Nitsche MA, Kuo MF, Grosch J, Bergner C, Monte-Silva K, et al. (2009) D1-receptor impact on neuroplasticity in humans. J Neurosci 29: 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nitsche MA, Monte-Silva K, Kuo MF, Paulus W (2010) Dopaminergic impact on cortical excitability in humans. Rev Neurosci 21: 289–298. [DOI] [PubMed] [Google Scholar]
- 37. Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA (2010) Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol 588: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS (2011) Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain 134: 2312–2320. [DOI] [PubMed] [Google Scholar]
- 39. Miyatake S, Mochizuki H, Naka T, Ugawa Y, Tanabe H, et al. (2010) Brain volume analyses and somatosensory evoked potentials in multiple system atrophy. J Neurol 257: 419–425. [DOI] [PubMed] [Google Scholar]
- 40. Gilman S, Koeppe RA, Junck L, Little R, Kluin KJ, et al. (1999) Decreased striatal monoaminergic terminals in multiple system atrophy detected with positron emission tomography. Ann Neurol 45: 769–777. [DOI] [PubMed] [Google Scholar]
- 41. Osaki Y, Wenning GK, Daniel SE, Hughes A, Lees AJ, et al. (2002) Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology 59: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 42. Sakurai K, Kawaguchi T, Kawai T, Ogino H, Hara M, et al. (2010) Usefulness of 3D-PRESTO imaging in evaluating putaminal abnormality in parkinsonian variant of multiple system atrophy. Neuroradiology 52: 809–814. [DOI] [PubMed] [Google Scholar]
- 43. Ito S, Shirai W, Hattori T (2009) Putaminal hyperintensity on T1-weighted MR imaging in patients with the Parkinson variant of multiple system atrophy. AJNR Am J Neuroradiol 30: 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rolland Y, Verin M, Payan CA, Duchesne S, Kraft E, et al. (2011) A new MRI rating scale for progressive supranuclear palsy and multiple system atrophy: validity and reliability. J Neurol Neurosurg Psychiatry 82: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abbruzzese G, Marchese R, Trompetto C (1997) Sensory and motor evoked potentials in multiple system atrophy: a comparative study with Parkinson's disease. Mov Disord 12: 315–321. [DOI] [PubMed] [Google Scholar]
- 46. Eusebio A, Azulay JP, Witjas T, Rico A, Attarian S (2007) Assessment of cortico-spinal tract impairment in multiple system atrophy using transcranial magnetic stimulation. Clin Neurophysiol 118: 815–823. [DOI] [PubMed] [Google Scholar]
- 47. Kuhn AA, Grosse P, Holtz K, Brown P, Meyer BU, et al. (2004) Patterns of abnormal motor cortex excitability in atypical parkinsonian syndromes. Clin Neurophysiol 115: 1786–1795. [DOI] [PubMed] [Google Scholar]
- 48. Cantello R, Tarletti R, Varrasi C, Cecchin M, Monaco F (2007) Cortical inhibition in Parkinson's disease: new insights from early, untreated patients. Neuroscience 150: 64–71. [DOI] [PubMed] [Google Scholar]
- 49. Rothwell J (2007) Transcranial magnetic stimulation as a method for investigating the plasticity of the brain in Parkinson's disease and dystonia. Parkinsonism Relat Disord 13 Suppl 3S417–420. [DOI] [PubMed] [Google Scholar]
- 50. Wu AD, Petzinger GM, Lin CH, Kung M, Fisher B (2007) Asymmetric corticomotor excitability correlations in early Parkinson's disease. Mov Disord 22: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 51. Lefaucheur JP (2005) Motor cortex dysfunction revealed by cortical excitability studies in Parkinson's disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol 116: 244–253. [DOI] [PubMed] [Google Scholar]
- 52. Marchese R, Trompetto C, Buccolieri A, Abbruzzese G (2000) Abnormalities of motor cortical excitability are not correlated with clinical features in atypical parkinsonism. Mov Disord 15: 1210–1214. [DOI] [PubMed] [Google Scholar]