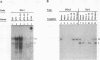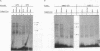Abstract
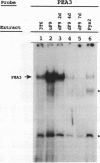
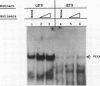
Proteins binding to the PEA3 enhancer motif (AGGAAG) activate the polyomavirus early promoter and help comprise the viral late mRNA initiator element (W. Yoo, M. E. Martin, and W. R. Folk, J. Virol. 65:5391-5400, 1991). Because many developmentally regulated cellular genes have PEA3 motifs near their promoter sequences, and because Ets family gene products activate the PEA3 motif, we have studied the expression of PEA3-binding proteins and Ets-related proteins during differentiation of F9 embryonal carcinoma cells. An approximately 91-kDa protein (PEA3-91) was identified in F9 cell nuclear extracts by UV cross-linking to a radiolabeled PEA3 oligonucleotide probe, and expression of PEA3-91 was down-regulated after differentiation of F9 cells to parietal endoderm. The c-ets-1 gene product binds to a sequence in the murine sarcoma virus long terminal repeat that is similar to the PEA3 motif (cGGAAG), but PEA3-91 was not cross-linked to this Ets-1-binding motif, nor did antiserum which recognizes murine c-ets-1 and c-ets-2 proteins have any effect on PEA3-binding activity in mobility shift assays. Furthermore, c-ets-1 mRNA was not detected in undifferentiated or differentiated F9 cells, and c-ets-2 mRNA levels remained high after differentiation. Antiserum against the Drosophila Ets-related E74A protein, however, recognized an approximately 92-kDa protein in F9 cells whose expression during differentiation varied in a manner identical to that of PEA3-91. These data suggest that PEA3-91 is not the product of the ets-1 or ets-2 genes but is likely to be the product of a murine homolog of the Drosophila E74 gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Begue A., Galibert F., Gesquière J. C., Stéhelin D., Ghysdael J. Identification in chickens of an evolutionarily conserved cellular ets-2 gene (c-ets-2) encoding nuclear proteins related to the products of the c-ets proto-oncogene. EMBO J. 1988 Mar;7(3):697–705. doi: 10.1002/j.1460-2075.1988.tb02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K. C., Thummel C. S., Jones C. W., Karim F. D., Hogness D. S. The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell. 1990 Apr 6;61(1):85–99. doi: 10.1016/0092-8674(90)90217-3. [DOI] [PubMed] [Google Scholar]
- Chen J. H. Cloning, sequencing, and expression of mouse c-ets-1 cDNA in baculovirus expression system. Oncogene Res. 1990;5(4):277–285. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Clevers H. C., Dunlap S., Wileman T. E., Terhorst C. Human CD3-epsilon gene contains three miniexons and is transcribed from a non-TATA promoter. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8156–8160. doi: 10.1073/pnas.85.21.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Cremisi C., Babinet C. Negative regulation of early polyomavirus expression in mouse embryonal carcinoma cells. J Virol. 1986 Sep;59(3):761–763. doi: 10.1128/jvi.59.3.761-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandolo L., Blangy D., Kamen R. Regulation of polyoma virus transcription in murine embryonal carcinoma cells. J Virol. 1983 Jul;47(1):55–64. doi: 10.1128/jvi.47.1.55-64.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Silbert P. E., Eckhart W., Linney E. Polyoma virus infection of retinoic acid-induced differentiated teratocarcinoma cells. J Virol. 1981 Jul;39(1):306–312. doi: 10.1128/jvi.39.1.306-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Fisher R. J., Bhat N. K., Diaz de la Espina S. M., Papas T. S. A short-lived nuclear phosphoprotein encoded by the human ets-2 proto-oncogene is stabilized by activation of protein kinase C. Mol Cell Biol. 1988 Nov;8(11):4700–4706. doi: 10.1128/mcb.8.11.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Koizumi S., Fisher R. J., Bhat N. K., Papas T. S. Phosphorylation of the ETS-2 protein: regulation by the T-cell antigen receptor-CD3 complex. Mol Cell Biol. 1990 Mar;10(3):1249–1253. doi: 10.1128/mcb.10.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Yamaguchi Y., Ogawa E., Shigesada K., Satake M., Ito Y. A ubiquitous repressor interacting with an F9 cell-specific silencer and its functional suppression by differentiated cell-specific positive factors. Cell Growth Differ. 1990 Mar;1(3):135–147. [PubMed] [Google Scholar]
- Gegonne A., Leprince D., Pognonec P., Dernis D., Raes M. B., Stehelin D., Ghysdael J. The 5' extremity of the v-ets oncogene of avian leukemia virus E26 encodes amino acid sequences not derived from the major c-ets-encoded cellular proteins. Virology. 1987 Jan;156(1):177–180. doi: 10.1016/0042-6822(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay J., Introna M., Graf T. A single point mutation in the v-ets oncogene affects both erythroid and myelomonocytic cell differentiation. Cell. 1988 Dec 23;55(6):1147–1158. doi: 10.1016/0092-8674(88)90259-0. [DOI] [PubMed] [Google Scholar]
- Griep A. E., DeLuca H. F. Decreased c-myc expression is an early event in retinoic acid-induced differentiation of F9 teratocarcinoma cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5539–5543. doi: 10.1073/pnas.83.15.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Angel P., Le Beau M. M., Karin M. Structure and chromosomal localization of the functional intronless human JUN protooncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Imler J. L., Ugarte E., Wasylyk C., Wasylyk B. v-jun is a transcriptional activator, but not in all cell-lines. Nucleic Acids Res. 1988 Apr 11;16(7):3005–3012. doi: 10.1093/nar/16.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
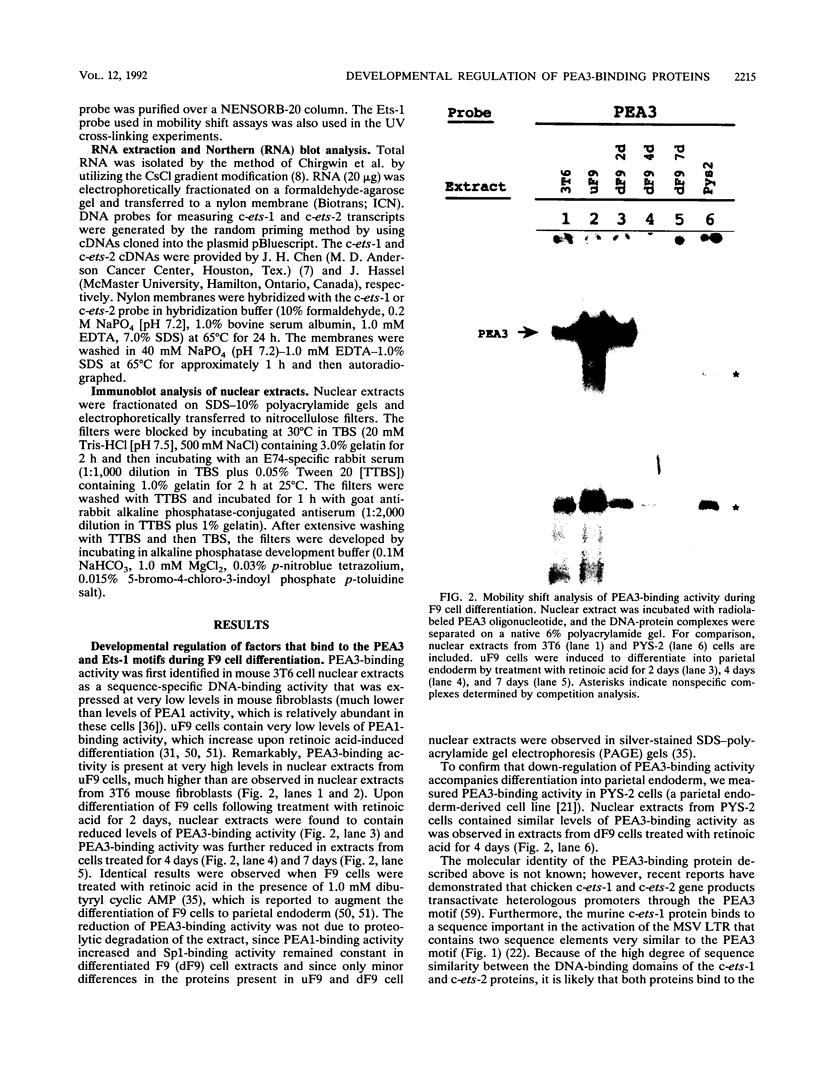
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leprince D., Duterque-Coquillaud M., Li R. P., Henry C., Flourens A., Debuire B., Stehelin D. Alternative splicing within the chicken c-ets-1 locus: implications for transduction within the E26 retrovirus of the c-ets proto-oncogene. J Virol. 1988 Sep;62(9):3233–3241. doi: 10.1128/jvi.62.9.3233-3241.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
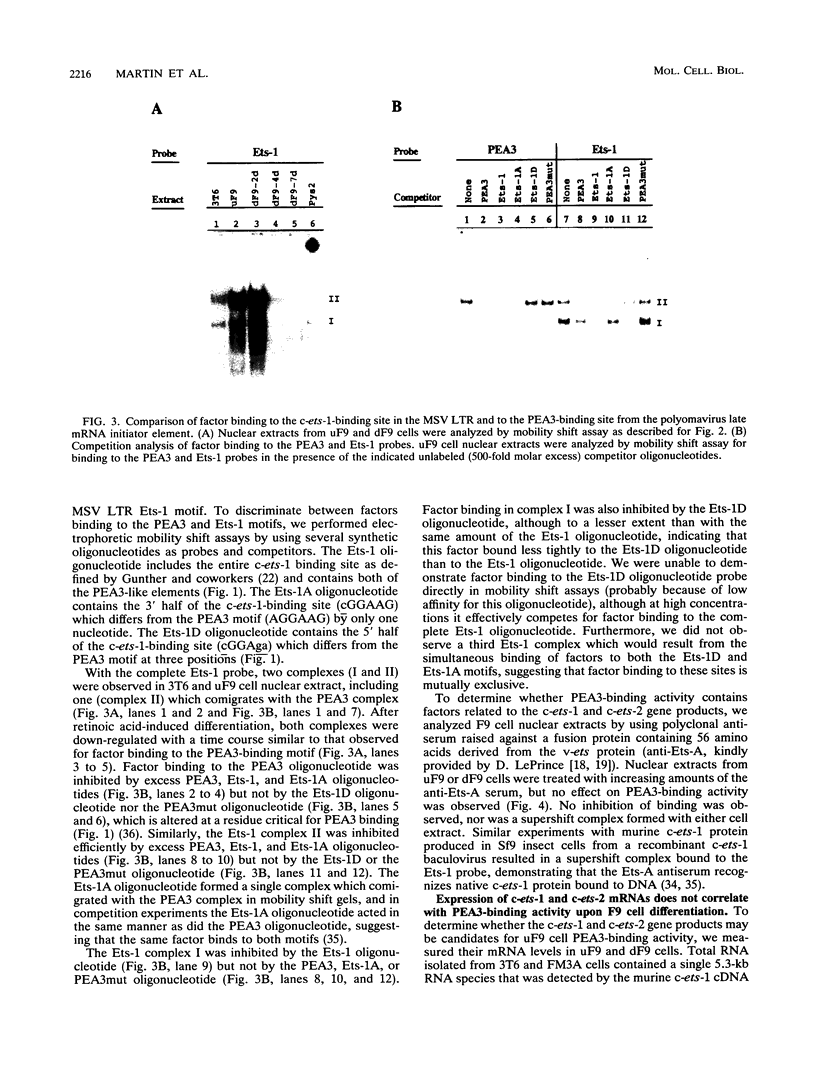
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Burbelo P. D., Sasaki M., Yamada Y. The laminin B2 chain promoter contains unique repeat sequences and is active in transient transfection. J Biol Chem. 1988 Jun 15;263(17):8384–8389. [PubMed] [Google Scholar]
- Paul R., Schuetze S., Kozak S. L., Kozak C. A., Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991 Jan;65(1):464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
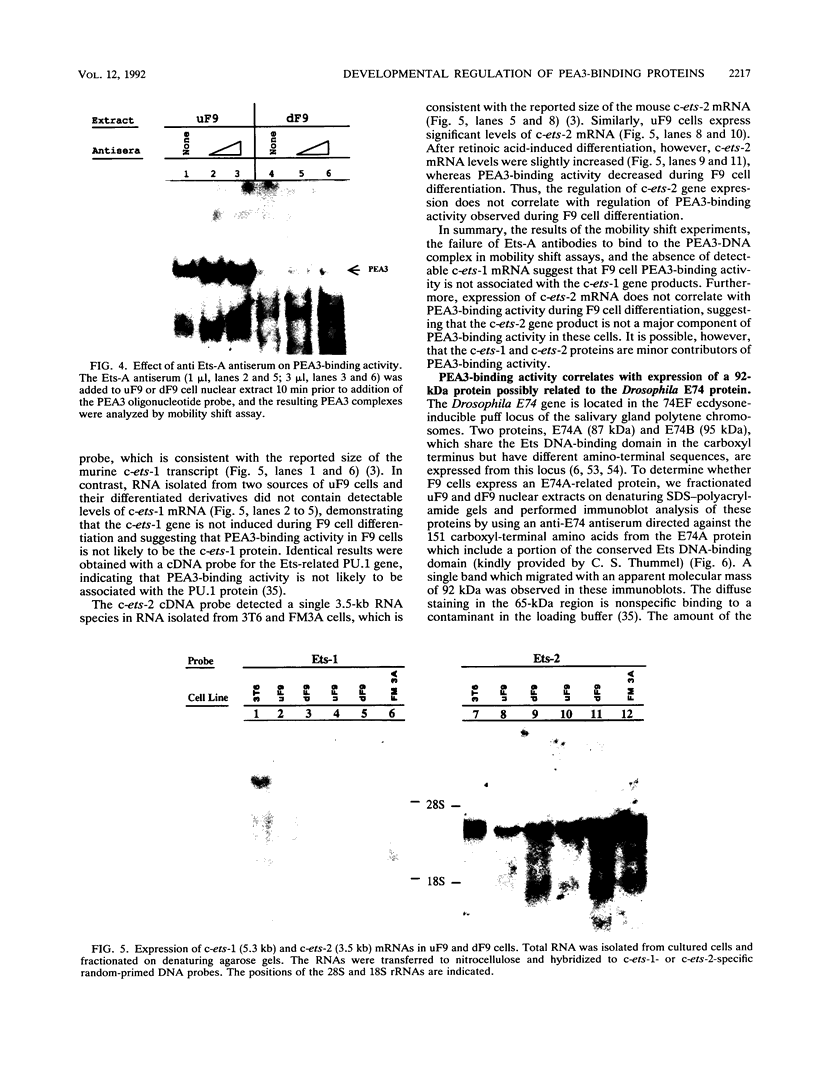
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N., Papas T. S. The erg gene: a human gene related to the ets oncogene. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6131–6135. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Differentiation-responsive elements in the 5' region of the mouse tissue plasminogen activator gene confer two-stage regulation by retinoic acid and cyclic AMP in teratocarcinoma cells. Mol Cell Biol. 1989 Apr;9(4):1691–1704. doi: 10.1128/mcb.9.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
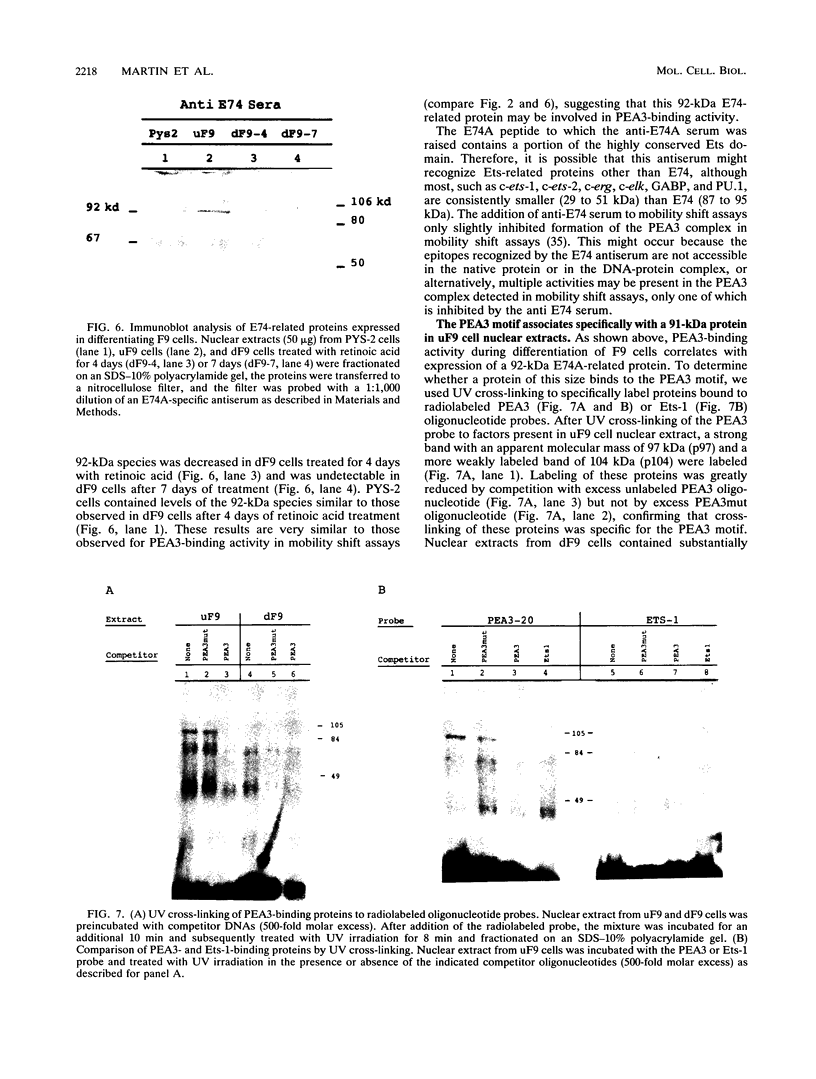
- Rørth P., Nerlov C., Blasi F., Johnsen M. Transcription factor PEA3 participates in the induction of urokinase plasminogen activator transcription in murine keratinocytes stimulated with epidermal growth factor or phorbol-ester. Nucleic Acids Res. 1990 Sep 11;18(17):5009–5017. doi: 10.1093/nar/18.17.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Cohen R. B., Garfinkel S., Thompson J. A. DNA affinity labeling of adenovirus type 2 upstream promoter sequence-binding factors identifies two distinct proteins. Mol Cell Biol. 1988 Jan;8(1):105–113. doi: 10.1128/mcb.8.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Fromental C., Chambon P. A trans-acting factor represses the activity of the polyoma virus enhancer in undifferentiated embryonal carcinoma cells. Oncogene Res. 1987 Jul;1(2):113–119. [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Seth A., Papas T. S. The c-ets-1 proto-oncogene has oncogenic activity and is positively autoregulated. Oncogene. 1990 Dec;5(12):1761–1767. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Brown T. A., McKnight S. L. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Burtis K. C., Hogness D. S. Spatial and temporal patterns of E74 transcription during Drosophila development. Cell. 1990 Apr 6;61(1):101–111. doi: 10.1016/0092-8674(90)90218-4. [DOI] [PubMed] [Google Scholar]
- Thummel C. S. The Drosophila E74 promoter contains essential sequences downstream from the start site of transcription. Genes Dev. 1989 Jun;3(6):782–792. doi: 10.1101/gad.3.6.782. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Urness L. D., Thummel C. S. Molecular interactions within the ecdysone regulatory hierarchy: DNA binding properties of the Drosophila ecdysone-inducible E74A protein. Cell. 1990 Oct 5;63(1):47–61. doi: 10.1016/0092-8674(90)90287-o. [DOI] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. H., Martin G. R., Lee P., Mark D., Wang A., Williams L. T. Developmentally regulated use of alternative promoters creates a novel platelet-derived growth factor receptor transcript in mouse teratocarcinoma and embryonic stem cells. Mol Cell Biol. 1989 Oct;9(10):4563–4567. doi: 10.1128/mcb.9.10.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Flores P., Gutman A., Wasylyk B. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 1989 Nov;8(11):3371–3378. doi: 10.1002/j.1460-2075.1989.tb08500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- Yoo W., Martin M. E., Folk W. R. PEA1 and PEA3 enhancer elements are primary components of the polyomavirus late transcription initiator element. J Virol. 1991 Oct;65(10):5391–5400. doi: 10.1128/jvi.65.10.5391-5400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]