Abstract
The vesicular monoamine transporter can protect against toxins that induce an acute parkinsonian syndrome. It has been hypothesized that cytoplasmic dopamine has subacute toxic effects in Parkinson Disease (PD) leading to neuronal death and clinical symptoms. Regulatory polymorphisms in the brain form of the vesicular monoamine transporter (VMAT2) which affect its quantitative expression might therefore serve as genetic risk factors for PD. We have screened the promoter region of the gene for VMAT2 (SLC18A2) and identified several novel polymorphisms that form discrete haplotypes. We have tested the common halpotypes in SLC18A2 for functional effects in reporter gene assays and found that there are several gain-of-function haplotypes that display significantly increased transcriptional activity from the reference element. These gain-of-function haplotypes were tested for association with PD and found to confer a protective effect that was selective for females. This finding is consistent with the prediction that increased sequestration of dopamine in secretory vesicles by VMAT2 is protective for PD.
INTRODUCTION
The brain form of the vesicular monoamine transporter (VMAT2) is an essential molecule for synaptic transmission of all of the biogenic amines: serotonin, norepinephrine, histamine and dopamine (1,2). VMAT2 is the single transporter in the central nervous system available for packaging biogenic amines into synaptic vesicles (3). Quantitative alteration of the expression of VMAT2 has marked effects on synaptic neurotransmission in heterozygous knockout mice. These effects include reducing the content of secretory vesicles, decreasing the magnitude of quantal release and modifying behaviors related to biogenic amines (4,5). Furthermore, pharmacological inhibition of VMAT2 by reserpine induces severe depressive symptoms in a substantial portion of humans given this medication (6).
SLC18A2, the gene for VMAT2, is located on chromosome 10q25 and is a candidate gene for Parkinson Disease (PD). The peripheral vesicular monoamine transporter (VMAT1) was initially identified and cloned by exploiting its protective effects against the neurotoxin N-methyl-4-phenyltetrahydro-pyridine (MPTP) in cell cultures (7,8). VMAT2 has been shown to possess similar properties (9). In vivo, MPTP causes an acute and severe form of parkinsonism due to selective killing of dopaminergic neurons in the substantia nigra (10). The mechanism of the VMAT’s protective effects against MPTP toxicity has been determined. Both VMAT1 and VMAT2 possess transport activity for N-methyl-4-phenyl-pyridinium (MPP+), the active toxic metabolite of MPTP. Through this activity, VMAT2 sequesters MPP+ in synaptic vesicles away from mitochondria where it exerts its toxic effects on nigral neurons. Owing to quantitative reduction of activity and therefore increased cytoplasmic concentrations of MPP+, heterozygous VMAT2 knockout mice display increased sensitivity to MPTP toxicity in vivo (2,4,11). The occurrence of MPTP as an environmental toxin is quite rare and it is unlikely that MPTP toxicity plays a role in PD (12). It has been proposed that VMAT2 may play an analogous role in protecting against chronic toxic effects of cytoplasmic dopamine (13). In this model, cytoplasmic dopamine forms oxidative metabolites that increase the level of oxidative stress leading to apoptosis and/or form covalent adducts of proteins within nigral neurons (14,15). In particular, oxidized dopamine quinones have been shown to form adducts with α-synuclein, stabilizing it in the toxic protofibrillar state (16).
We have previously screened the coding sequence of SLC18A2 in a non-PD population sample and a large sample of PD patients (17). In both of these studies, we found that polymorphisms in coding sequence which predict alterations in the amino acid structure of VMAT2 are very rare and therefore cannot contribute in a substantial way to the population prevalence of sporadic PD. We now report the identification of common functional regulatory polymorphisms in the promoter region of SLC18A2 and demonstrate a protective role for gain-of-function haplotypes in VMAT2 in sporadic PD.
RESULTS
Promoter variant characterization
Our screening of the core promoter of SLC18A2 identified a high degree of genetic variation in terms of both the number of variant positions and the number of polymorphic chromosomes (Table 1). We identified six variants within 219 base pairs 5′ of the transcription initiation site, three of which displayed an average population frequency of 0.10 or more in the total sample (African American and Caucasian combined). This high degree of variability is reflected in high values for nucleotide diversity (θ = 1.86 × 10−3 and average heterozygosity (π) = 1.92 × 10−3. Both of these values are several times higher than those seen in our screen of the coding sequence of SLC18A2 (θ = 3.47 × 10−4, π = 1.72 × 10−4) that was notable for the very low level of variation (15).
Table 1.
Variants identified in the promoter region of SLC18A2 (ref/variant)
| Name | Variation | Position | Sequence context | Overall frequency | AA frequency | CAU frequency |
|---|---|---|---|---|---|---|
| VMAT.1 | G/T | −219 | CTCTTCCCCAGGCCTGGGTCC | 0.01 | 0.01 | 0.00 |
| VMAT.2 | G/A | −112 | CAGCGACGGCGCGGGCGGGCG | 0.01 | 0.01 | 0.00 |
| VMAT.3 | G/A | −106 | CGGCGCGGGCGGGCGGAGGCC | 0.10 | 0.16 | 0.04 |
| VMAT.4 | C/A | −103 | CGCGGGCGGGCGGAGGCCGGG | 0.47 | 0.61 | 0.32 |
| VMAT.5 | C/T | −74 | GCCCCCCGCCCCCGCTCCCTC | 0.05 | 0.06 | 0.04 |
| VMAT.6 | G/A | −62 | CGCTCCCTCCGGCCGTGACGT | 0.15 | 0.07 | 0.23 |
Variant positions are relative to transcription initiation: position chr10:118 990 624 human genome release May 2004. Reference nucleotides are defined as the residue present in the human genome browser (http://www.genome.ucsc.edu/cgi-bin/hggateway?org=human). Frequencies reported are for the variant allele.
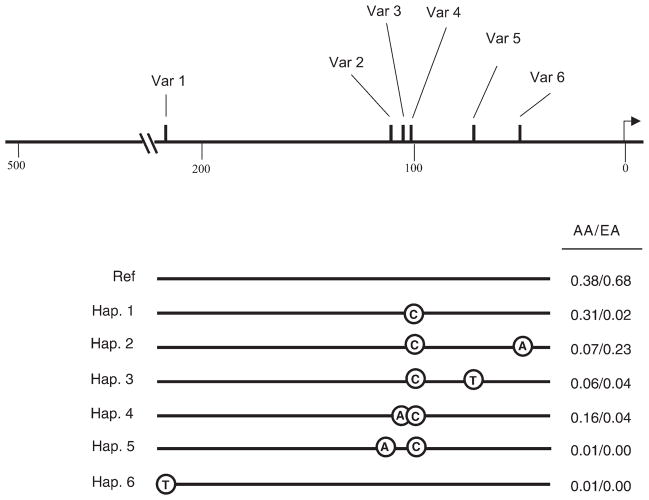
There was a marked difference in the frequencies of promoter polymorphisms between the African American and Caucasian samples we screened (Table 1). The two most rare polymorphisms (VMAT.1 and VMAT.2) were singletons seen only in African American samples. All variants except VMAT6 were more common in the African American population than in the Caucasian sample. Although the distribution of variants is quite different between the African American and Caucasian samples, nucleotide diversity and average heterozygosity were similar for both populations.
We used Phase to predict the haplotypes into which these polymorphisms assemble (Fig. 1) (18). All promoter variants are in complete linkage disequilibrium, which is not surprising given the close proximity of all variants to each other. As with the individual variants, haplotype frequencies varied between the African American and Caucasian populations (Fig. 1).
Figure 1.
Haplotypes formed by variants in the promoter region of SLC18A2. Ref indicates the human genome reference sequence. Variant haplotypes are numbered arbitrarily. Variants comprising each haplotype are placed according to their physical location. Haplotype frequencies are presented for the African American population (AA) and European American population (EA). Arrow marks transcription initiation site.
Reporter gene assays of polymorphic SLC18A2 promoters
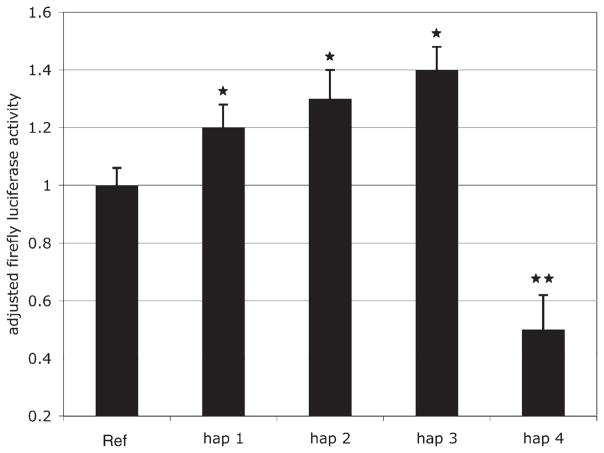
We performed dual luciferase reporter gene assays in JAR cells, a human choriocarcinoma cell line, to determine the transcriptional activity of the polymorphic SLC18A2 promoter haplotypes (Fig. 2). We tested only the most common haplotypes (reference, haps 1–4) for transcriptional activity, as these are most likely to have a substantial impact on the population prevalence of phenotypes related to variability in SLC18A2. We found that three of the four common variant haplotypes (haps 1–3) demonstrated significantly increased transcriptional activity in reporter gene assays when compared with reference. In contrast, hap 4 demonstrated a significantly reduced level of transcriptional activity. Differences in transcriptional activity of variant haplotypes remain significant after Bonferroni correction for multiple pair-wise comparisons (19). There was no obvious relationship between the position or sequence content of the polymorphic haplotypes and predicted transcription factor binding sites that could explain the functional effects we identified.
Figure 2.
Transcriptional activity of variant promoter haplotypes in SLC18A2. Dual luciferase assays were performed on lysates from JAR cells transfected with plasmids containing reference or variant SLC18A2 promoter elements. Relative luciferase activities (firefly/renilla luciferase activity) for variant haplotypes were adjusted to the relative activity of the reference haplotype. *p < 0.01, **p < 0.005.
Association analysis
Table 2 presents a summary of the demographic characteristics for PD cases and controls. The population was primarily Caucasian (83.4%), but also included Asians (2.7%), Hispanics (9.0%) and Native Americans (4.8%). As a result of the small African American population in the tri-county area, we accrued no African American cases. We enrolled slightly more men (54%) than women and the median age of cases at diagnosis was 70 years. All polymorphisms were in Hardy–Weinberg equilibrium in the control sample (χ2 values 0.019–0.561, p < 1.0 for all).
Table 2.
Demographic characteristics of the parkinson disease association sample
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| Total (n) | Males | Females | Total (n) | Males | Females | |
| Total (n = 476) | 248 | 134 | 114 | 228 | 123 | 105 |
| Age (at exam) | ||||||
| ≤ 55 years | 30 | 18 (13.4%) | 12 (10.5%) | 49 | 15 (12.2%) | 34 (32.4%) |
| > 55 years | 218 | 116 (86.6%) | 102 (89.5%) | 179 | 108 (87.8%) | 71 (67.6%) |
| Race/ethnicity | ||||||
| White | 202 | 109 (81.3%) | 93 (81.6%) | 195 | 108 (87.8%) | 87 (82.8%) |
| Hispanic/Latino | 28 | 17 (12.7%) | 11 (9.7%) | 15 | 8 (6.5%) | 7 (6.7%) |
| Native American | 14 | 8 (6.0%) | 6 (5.2%) | 9 | 3 (2.4%) | 6 (5.7%) |
| Asian | 4 | 0 (0.00%) | 4 (3.5%) | 9 | 4 (3.3%) | 5 (4.8%) |
Our association analysis was guided by our hypothesis that increased cytoplasmic dopamine is a risk factor for PD. We therefore grouped subjects on the basis of the presence or absence of a gain-of-function haplotype defined as one of the three haplotypes that demonstrated increased transcriptional activity in the reporter gene assays (haps 1–3). This grouping provides improved statistical power. Haplotype 4, the loss-of-function haplotype, was rare in our sample (20 observations, 4.2%) and therefore was not included in formal association analyses.
Crude associations for homozygous overexpressors compared with wild-type individuals suggested no associations with PD for these promoter haplotypes (Table 3). We performed a subanalysis by gender based on the known differences in the prevalence and incidence of PD in men versus women (20). When stratifying by gender, we observed a strong protective association in females but not in males for overexpressors [female 95% OR = 0.38; CI (0.15, 0.96)].
Table 3.
Association analysis of gain-of-function haplotypes in the promoter region of SLC18A2 and Parkinson disease
| Total population (n = 456) | Males (n = 243) | Females (n = 213) | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| Homozygote overexpressors | 25 | 26 | 17 | 10 | 8 | 16 |
| Heterozygote overexpressors | 108 | 98 | 60 | 55 | 48 | 43 |
| WT | 105 | 94 | 49 | 52 | 56 | 42 |
| OR of homozygote versus WT | 0.86 (0.47, 1.59) | 1.80 (0.75, 4.32) | 0.38 (0.15, 0.96) | |||
| OR of heterozygote versus WT | 0.99 (0.67, 1.46) | 1.16 (0.68, 1.98) | 0.84 (0.47, 1.49) | |||
Gain-of-function refers to one of the three haplotypes (haps 1–3) that were more transcriptionally active than reference in reporter gene assays. Odds ratios are adjusted for race and are presented as OR(95% confidence interval). B-D test of homogeneity: homozygote versus Wt X2 = 5.93, P = 0.01; heterozygote versus Wt X2 = 0.66, P = 0.42.
The association was still protective when we restricted our analyses to Caucasian females [50% reduction in risk; OR = 0.50; 95% CI (0.18, 1.42)], but the analyses were less informative because of the reduced sample size and the confidence interval included the null value of 1. However, in multivariate adjusted logistic regression models, the associations for female homozygote overexpressors when stratifying on gender were not only protective but also the CIs excluded the null even when adjusting for race (OR = 0.36; CI: 0.14, 0.94). Similar results were obtained when adjusting for epidemiological factors thought to influence the heritability of PD risk: age of onset and affected first-degree relatives. We did not see any independent associations to age of onset or affected first-degree relatives. None of our results were sensitive to the inclusion or exclusion of subjects reporting first-degree relatives with PD.
A gene dosage effect was suggested because the associations were more strongly protective in homozygote than in heterozygote overexpressors in females. This gene-dosing effect was also observed when we restricted our sample to Caucasians only.
DISCUSSION
We have identified a high degree of genetic variability in the promoter region of SLC18A2. This finding is in stark contrast to our previous studies of the coding sequence of SLC18A2 that found a very low degree of variability and suggested that the coding sequence of SLC18A2 is under significant negative selective pressure (17). Our reporter gene assays of expression constructs under the transcriptional control of the polymorphic SLC18A2 promoter elements demonstrate that the variant haplotypes display a complex pattern of functional effects. Three of four variant haplotypes displayed increased transcriptional activity, whereas a fourth displayed decreased activity. Lin et al. (21) have recently reported a similar analysis of the promoter region of SLC18A2. In their study, they screened a 17.4 kb segment of genomic sequence upstream of SLC18A2 in a smaller sample comprising European Americans only. That survey identified several of the common variants we present in this report and a number of additional polymorphisms in sequence beyond the region we have screened. Lin et al. also reported increased transcriptional activity of a 6.3 kb fragment that includes our variant haplotype 2 over reference. Although their data are similar to our reporter gene data, it is difficult to make direct comparisons, as there are several methodological differences between the two studies. The quantitative effect of the variant haplotypes in SLC18A2 on transcriptional activity is relatively small, and it is unclear how these findings might relate to in vivo RNA and protein levels. Nonetheless, quantitative reduction of VMAT2 activity in genetically modified mice results in marked effects on neurotransmission and behavior (4,5,22). Genetic variability in VMAT2 expression may be of particular relevance in light of findings suggesting regulatory compensation of VMAT2 expression in PD (23,24).
We have used the functional data we collected on the variant haplotypes in the promoter region of SLC18A2 to guide our association analysis of PD. Our reporter gene analyses of the polymorphic SLC18A2 promoter regions have identified subsets of the population (carriers of haps 1–3) that are functionally similar despite molecular genetic heterogeneity. These analyses allow us to rationally group multiple haplotypes for association analysis on the basis of the hypothesis that increased VMAT2 activity is protective for PD. Grouping haplotypes provides additional statistical power over analyses that compare the prevalence of individual haplotypes in cases and controls.
Our approach of grouping functionally similar haplotypes has allowed us to identify a gender-specific effect of gain-of-function promoter elements in PD. Although the association we have identified is of only nominal statistical significance, the degree of the protective effect of homozygous gain-of-function haplotypes for women is large (OR = 0.37). There appears to be an additive effect of gain-of-function haplotypes as we identify a non-significant trend towards an intermediate protective effect for female carriers of a single gain-of-function haplotype.
The gender-specific nature of our association finding is supported by data demonstrating gender differences in PD prevalence, in vitro and in vivo model systems of PD and studies of VMAT2 function. A number of epidemiological studies have found increased rates of PD in men (25–28). A recent meta-analysis found that PD is roughly 1.5 times more prevalent in men than in women (20). Further, the relative risk for male first-degree relatives of PD probands is greater than that for females, suggesting that shared genetic factors may exert different degrees of risk or protection on men versus women (29). The basis of the gender difference in PD prevalence is unclear but has been attributed to differential exposure to environmental toxins, head trauma and the effects of estrogen (20). In epidemiological studies, estrogen has been found to modify the protective effect of caffeine on PD mortality, and one prospective study of hormone replacement therapy demonstrated that estrogen reduced the risk for dementia in PD (30,31). These findings are consistent with a study that found gender differences in the symptom profile of PD (32).
Estrogen has been shown to have selective protective actions on nigrostriatal dopamine neurons in vivo and in vivo. Male mice are more sensitive to MPTP toxicity than females (33). Estrogen treatment of gonadectomized female and male rodents reduces the degree of nigrostriatal neuron loss due to MPTP, methamphetamine or 6-hydroxydopamine treatment as measured by striatal dopamine concentrations (34–38). The protective effects of estrogen appear to be related, at least in part, to modulation of the dopamine transporter (DAT). Estrogen reduces dopamine uptake in superfusion experiments, and direct kinetic analysis of [3H]dopamine transport by DAT is inhibited by 17B-estradiol through an apparent allosteric interaction (39,40). The fact that estrogen exerts neuroprotective effects on nigrostriatal neurons by reducing DAT activity supports our hypothesis that quantitative reduction of cytoplasmic dopamine levels are protective for nigrostriatal neurons. There are no reports of estrogen effects directly on VMAT2; however, it is interesting to note that heterozygous VMAT2 knockout mice display sex-dependent changes in ethanol consumption, which are presumably related to effects on the dopamine system (39).
Although data from a variety of experiments support the plausibility of our findings, the conclusions from association studies of neurobehavioral phenomena remain tentative until replication in independent samples is obtained. The findings of Lin et al. that regulatory haplotypes in SLC18A2 are associated with a different dopamine-related phenotype, alcohol dependence, supports our hypothesis that these haplotypes have in vivo physiological relevance to dopamine homeostasis (21). Nonetheless, we believe that informing association analyses with functional data on genetic variants contributes to the feasibility and reliability of neurogenetic studies.
MATERIALS AND METHODS
SLC18A2 core promoter variant screening
We screened the core promoter region, defined as the 500 base pairs 5′, to the empirically determined transcription initiation site (41). Promoter regions were amplified from genomic DNA obtained from Coriell ethnic variation panel samples. Forty-eight African American and 48 Caucasian samples were screened. PCR amplification used primers VMATpromF2 (5′-TGCAAAGGGTGGCTTCTTCA-3′) and VMATpromR1 (5′-GCAGTGGGCTCCGTCAGT-3′). PCR reactions were performed in a final volume of 20 μl using 2× Amplitaq Gold master mix (ABI), 10 pmol of each primer, 50 ng genomic DNA and 6% DMSO. The thermal cycling protocol was a touchdown protocol: 95°C for 5 min, 10 cycles of 95°C for 20 s, 61°C for 15 s decremented by 0.5°C per cycle, 72°C for 30 s, 30 cycles of 95°C for 20 s, 56°C for 15 s and 72°C for 30 s. Aliquots of all PCR reactions were run on 2% agarose gel electrophoresis to ensure amplification.
Variant screening was performed by direct sequencing. PCR products were prepared for sequencing by treatment with exonuclease I (0.2 U/rxn) and shrimp alkaline phosphatase (2 U/rxn) in the PCR rxn mix and incubation for 1 h at 37°C, followed by inactivation at 95°C for 5 min. All samples were sequenced in both directions using the same primers as the initial PCR (VMATpromF2 and VMAT-promR1). Sequencing was performed with BigDye Chemistry (ABI) on an ABI 3700 capillary electrophoresis sequencer. Variants were identified by manual inspection of sequencing traces. Variants were called only if seen in both directions.
Analysis of variant distribution
Nucleotide diversity (θ) for the SLC18A2 promoter and coding sequence was estimated as the number of segregating sites, S, divided by a1, where and n is the number of chromosomes analyzed divided by the total number of nucleotides screened. Average heterozygosity (π) was estimated by divided by 1 − (1/n), divided by the number of nucleotides screened, where n is the number of chromosomes screened and pj the observed frequency of the jth variant. Multilocus haplotype frequencies were estimated from the variant data using the Phase program (18).
Cloning reporter gene constructs
The human genome reference SLC18A2 promoter element was amplified from a genomic DNA sample, which was homozygous for the reference haplotype, using VMATpromF2 and VMATpromR1 primers modified to include BglII and HindIII restriction sites, respectively, for downstream cloning. The reference promoter PCR product was cloned into pCR2.1-TOPO (Invitrogen). Variant haplotypes were created by site-directed mutagenesis (QuickChange Multi, Stratagene). Clones were verified by sequencing of plasmid DNA from positive transformants.
Reference and variant promoter elements were cloned upstream of the coding sequence of firefly luciferase in pGL3 basic (Promega). Inserts were released from the pCR2.1-TOPO plasmid by double digestion with BglII and HindIII and ligated into double-digested pGL3 basic. All reporter gene clones were verified by sequencing of purified plasmid DNA.
Reporter gene assays
Reporter gene assays used the dual luciferase system (Promega). We assayed luciferase expression in a human choriocarinoma cell line (JAR, Coriell Cell Repository). JAR cells are derived from a human choriocarcinoma line and are often used to study the serotonin system of which VMAT2 is a component. JAR cells were maintained at 37°C in a 5% CO2 incubator and growth medium consisting of 1× RPMI 1640 without L-glutamine (MT), 1% fetal bovine serum, 1× penicillin–streptomycin and 2 mM L-glutamine. For transfections, cells were plated in 24-well tissue culture plates at a density of 5× 104 cells per well in 500 μl growth medium without serum or antibiotics and allowed to become adherent for 3–4 h. Transfections were performed using lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Promoter constructs in pGL3 basic (1.85 μg total) were mixed with 25 ng of a control plasmid consisting of renilla luciferase under the transcriptional control of the thymidine kinase promoter—pRLTK (Promega), 2 μl lipofectamine 2000 and 25 μl RPMI 1640 and allowed to incubate for 5 min at room temperature and added to the JAR cells. Transfections proceeded at 37°C for 3–4 h after which the medium was changed back to complete growth medium without plasmid. Cells were allowed to express for 36–48 h prior to luciferase assays.
For luciferase assays, cells were lysed with passive lysis buffer (Promega) 200 μl per well for 5 min at room temperature with shaking. Lysed homogenates were aliquotted into flat-bottom 96-well plates in triplicate. Dual luciferase assays were performed according to manufacturer’s protocol in a 96-well automated luminometer (Lmax, Molecular Devices). All assays were performed with duplicate transfections and six replicates of luciferase assays. Transcriptional activity of the reference SLC18A2 promoter element was compared to variant haplotypes by pair-wise Student’s t-tests.
Association study population
We used a population-based approach, drawing from the populations of Fresno, Tulare and Kern Counties, California to recruit newly diagnosed (within 3 years of diagnosis) Parkinson’s patients with the help of local health care providers. Altogether, 28 (90%) of the 31 practicing neurologists in these counties, who provide care for PD patients participated. In addition, we solicited collaboration from large medical groups (including Kaiser Permanente, Kern and Visalia Medical Centers, the Veteran’s Administration and rural community clinic), Parkinson’s disease support groups, local newspapers and radio stations. Participating neurologists notified their patients about the study through mailings to all PD patients and/or passing out study brochures to patients at office visits. PD support groups in Bakersfield, Visalia and Fresno distributed information about our study to their members, and our study’s neurologists attended support group meetings. Between January 1998 and March 2005, we recruited and confirmed the diagnosis for 253 idiopathic Parkinson’s disease cases from the tri-county area. A UCLA movement disorder specialist confirmed a diagnosis of clinically probable or possible PD if patients met these criteria: (i) presence of at least two of the following signs: bradykinesia and cogwheel rigidity resting tremor; at least one of which must have been resting tremor or bradykinesia; (ii) no suggestion of a parkinsonian syndrome due to trauma, brain tumor, infection, cerebrovascular disease or other known neurological disease or treatment in the past with dopamine-blocking or dopamine-depleting agents; (iii) no atypical features such as: prominent oculomotor palsy, cerebellar signs, vocal cord paresis, severe orthostatic hypotension, pyramidal signs, amyo-trophy or limb apraxia; (iv) asymmetric onset and (v) if treatment with levodopa had been initiated, symptomatic improvement after treatment. Probable cases met criteria 1–4 ± 5. Possible cases had at least one sign from category 1 and fulfilled the criteria described in 2–3. The criteria of Hughes et al. (42) previously employed (43) include postural reflex impairment under category 1. We excluded this sign as a criterion because it usually occurs late in PD, but may typically occur early in other parkinsonian disorders (i.e. multiple system atrophy, vascular parkinsonism). The 248 subjects who met the criteria for probable or possible PD were included in our association analysis. A random selection of subjects from Medicare enrollees living in the three counties in 2001 provided us with an elderly control population, whereas controls aged 65 and younger were selected from residents whose homes we identified at random from all housing units listed on county parcel maps for the same tri-county area. Altogether we enrolled 228 controls that were marginally matched to the cases by age, race/ethnicity and gender. All subjects, cases and controls, provided blood samples and extensive demographic data. The UCLA Ethics Committee approved this study.
Genotyping the PEG sample
Genotyping was performed by direct sequencing of PEG samples. We developed a more robust and reliable PCR protocol for genotyping the PEG sample using Advantage-GC 2 reagents (Clontech). Reactions were performed in a final volume of 10 μl consisting of 2 μl 5× Advantage-GC 2 buffer, 2 μl GC-melt reagent, 10 ng genomic DNA, 0.1 μl 50× Advantage 2 polymerase mix, 0.4 μl dNTP mix (10 mM/dNTP) and 15 pmol each of VMATpromF10 (5′-TCTCTGACCTCTTTCCCTCT-3′) and VMATpromR1. Thermal cycling was the same as described for variant identification. After amplification, an aliquot of all reactions was run on 2% agarose gel electrophoresis to confirm amplification. Reactions were prepared for sequencing as described for variant identification and sequenced using VMATpromF10 as the sequencing primer (Macrogen). Genotype assignments were made by visual examination of sequencing traces.
Association analyses
We compared haplotype frequencies in cases and controls and examined gene-dosing effects for homozygous versus heterozygous haplotypes compared to wild-type individuals. We also conducted analyses after stratifying by age at PD onset (≤ 55 years, >55 years) and gender. We performed the same analyses after restricting our sample to White-Caucasian subjects only to account for potential population stratification. We calculated odds ratios and 95% confidence intervals on the basis of logistic regression models in which we also adjusted for race, gender and age of onset. The Breslow Day (B-D) test was used to evaluate the null hypothesis that the gender effect is uniform. All statistical analyses were performed using SAS software.
Acknowledgments
This work was supported by National Institutes of Health (NIH)—National Institute of Environmental Health Sciences Grants U54ES12078 and ES98-05-030-03A.
Footnotes
Conflict of Interest statement. None of the authors in this study have any interest, financial or otherwise, in the outcomes reported in this article.
References
- 1.Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- 2.Uhl GR, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, Sora I. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14(15):2459–2465. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- 3.Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995;15(9):6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19(6):1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 6.Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27(Suppl 3):S49–S54. doi: 10.1097/00005344-199627003-00007. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1992;89(19):9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70(4):539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Peter D, Merickel A, Krantz D, Finn JP, Edwards RH. A molecular analysis of vesicular amine transport. Behav Brain Res. 1996;73(1–2):51–58. doi: 10.1016/0166-4328(96)00069-1. [DOI] [PubMed] [Google Scholar]
- 10.Langston JW, Irwin I. MPTP: current concepts and controversies. Clin Neuropharmacol. 1986;9(6):485–507. [PubMed] [Google Scholar]
- 11.Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21(16):5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern Y. MPTP-induced parkinsonism. Prog Neurobiol. 1990;34(2):107–114. doi: 10.1016/0301-0082(90)90003-y. [DOI] [PubMed] [Google Scholar]
- 13.Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson’s disease. Ann Neurol. 1998;43(5):555–560. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- 14.Hastings TG, Lewis DA, Zigmond MJ. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv Exp Med Biol. 1996;387:97–106. doi: 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- 15.Hastings TG, Zigmond MJ. Loss of dopaminergic neurons in parkinsonism: possible role of reactive dopamine metabolites. J Neural Transm Suppl. 1997;49:103–110. doi: 10.1007/978-3-7091-6844-8_11. [DOI] [PubMed] [Google Scholar]
- 16.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294(5545):1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 17.Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat Genet. 2001;27(4):435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- 18.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25(12):1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 20.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiat. 2004;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Walther D, Yu XY, Li S, Drgon T, Uhl GR. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet. 2005;14(10):1393–1404. doi: 10.1093/hmg/ddi148. [DOI] [PubMed] [Google Scholar]
- 22.Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70(5):1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, Wudel J, Pal PK, de la Fuente-Fernandez R, Calne DB, Stoessl AJ. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47(4):493–503. [PubMed] [Google Scholar]
- 24.Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol. 1999;156(1):138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- 25.Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson’s disease. Neurology. 1993;43(9):1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- 26.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52(6):1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 27.Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, Stern Y. A population-based investigation of Parkinson’s disease with and without dementia. Relationship to age and gender. Arch Neurol. 1992;49(5):492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- 28.Wang YS, Shi YM, Wu ZY, He YX, Zhang BZ. Parkinson’s disease in China. Coordinational Group of Neuroepidemiology, PLA. Chin Med J (Engl) 1991;104(11):960–964. [PubMed] [Google Scholar]
- 29.Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, Groves J, Mayeux R. Risk of Parkinson’s disease among first-degree relatives: A community-based study. Neurology. 1996;47(1):155–160. doi: 10.1212/wnl.47.1.155. [DOI] [PubMed] [Google Scholar]
- 30.Ascherio A, Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Rodriguez C. Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 31.Marder K, Tang MX, Alfaro B, Mejia H, Cote L, Jacobs D, Stern Y, Sano M, Mayeux R. Postmenopausal estrogen use and Parkinson’s disease with and without dementia. Neurology. 1998;50(4):1141–1143. doi: 10.1212/wnl.50.4.1141. [DOI] [PubMed] [Google Scholar]
- 32.Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson’s disease symptom profile. Acta Neurol Scand. 2000;102(1):37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- 33.Yu YL, Wagner GC. Influence of gonadal hormones on sexual differences in sensitivity to methamphetamine-induced neurotoxicity. J Neural Transm Parkinson Dis Dement Sect. 1994;8(3):215–221. doi: 10.1007/BF02260942. [DOI] [PubMed] [Google Scholar]
- 34.Miller DB, Ali SF, O’Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- 35.Dluzen DE, McDermott JL, Liu B. Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/B1 mice. Neurotoxicol Teratol. 1996;18(5):603–606. doi: 10.1016/0892-0362(96)00086-4. [DOI] [PubMed] [Google Scholar]
- 36.Dluzen DE, McDermott JL, Liu B. Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem. 1996;66(2):658–666. doi: 10.1046/j.1471-4159.1996.66020658.x. [DOI] [PubMed] [Google Scholar]
- 37.Dluzen DE, McDermott JL. Neuroprotective role of estrogen upon methamphetamine and related neurotoxins within the nigrostriatal dopaminergic system. Ann NY Acad Sci. 2000;914:112–126. doi: 10.1111/j.1749-6632.2000.tb05189.x. [DOI] [PubMed] [Google Scholar]
- 38.Dluzen D. Estrogen decreases corpus striatal neurotoxicity in response to 6-hydroxydopamine. Brain Res. 1997;767(2):340–344. doi: 10.1016/s0006-8993(97)00630-6. [DOI] [PubMed] [Google Scholar]
- 39.Disshon KA, Dluzen DE. Use of in vitro superfusion to assess the dynamics of striatal dopamine clearance: influence of estrogen. Brain Res. 1999;842(2):399–407. doi: 10.1016/s0006-8993(99)01863-6. [DOI] [PubMed] [Google Scholar]
- 40.Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17 beta-estradiol. Eur J Pharmacol. 1998;345(2):207–211. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- 41.Xu W, Liu L, Mooslehner K, Emson PC. Structural organization of the human vesicular monoamine transporter type-2 gene and promoter analysis using the jelly fish green fluorescent protein as a reporter. Brain Res Mol Brain Res. 1997;45(1):41–49. doi: 10.1016/s0169-328x(96)00218-5. [DOI] [PubMed] [Google Scholar]
- 42.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 43.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]