Abstract
Purpose
There is compelling evidence to suggest that drugs that function as pure estrogen receptor (ERα) antagonists, or that down regulate the expression of ERα, would have clinical utility in the treatment of advanced tamoxifen and aromatase resistant breast cancer. Whereas such compounds are currently in development, we reasoned, based on our understanding of ERα pharmacology, that there may already exist among the most recently developed Selective Estrogen Receptor Modulators (SERMs) compounds that would have utility as breast cancer therapeutics. Thus, our objective was to identify among available SERMs those with unique pharmacological activities and to evaluate their potential clinical utility using predictive models of advanced breast cancer.
Experimental Design
A validated molecular profiling technology was used to classify clinically relevant SERMs based on their impact on ERα conformation. The functional consequences of these observed mechanistic differences on (a) gene expression, (b) receptor stability, and (c) activity in cellular and animal models of advanced endocrine resistant breast cancer was assessed.
Results
The high affinity SERM bazedoxifene was shown to function as a pure ERα antagonist in cellular models of breast cancer, and effectively inhibited the growth of both tamoxifen sensitive and resistant breast tumor xenografts. Interestingly, bazedoxifene induced a unique conformational change in ERα that resulted in its proteasomal degradation, although the latter activity was dispensible for its antagonist efficacy.
Conclusion
Bazedoxifene was recently approved for use in the European Union for the treatment of osteoporosis and thus may represent a near-term therapeutic option for patients with advanced breast cancer.
Keywords: estrogen receptor, SERM, bazedoxifene, SERD
Introduction
The primary goal of endocrine therapies in breast cancer is to block the transcriptional activity of estrogen receptor (ESR1, ERα) by either (a) inhibiting CYP19A1 (aromatase), the enzyme responsible for the conversion of androgens to estrogens, or (b) directly interfering with the transcriptional activity of the receptor. When used as primary interventions both approaches are similarly efficacious and improve outcome to the same degree. Notable, however, is the observation that a significant number of patients that exhibit de novo or acquired resistance to the antiestrogen tamoxifen subsequently respond to aromatase inhibitors (1–3). This finding highlights the continued dependence on ERα signaling within tumors in advanced disease, raising the possibility that even in tumors that are resistant to tamoxifen and aromatase inhibitors, the ERα signaling axis may remain a viable target.
The primary mechanisms underlying resistance to tamoxifen and aromatase inhibitors are somewhat different. However, it now appears that a common feature in either case involves the reprograming of ER signaling pathways within tumor cells, allowing them to continue to capitalize on progrowth and survival pathways downstream of ERα. The currently available aromatase inhibitors effectively reduce the production of both peripherally and intratumorally generated estrogens, and resistance to these agents is not associated with an inability to effectively suppress estrogen production (4, 5). Rather, there is accumulating evidence that exposure of ERα-positive breast cancer cells to aromatase inhibitors renders them hypersensitive to either residual amounts of steroidal estrogens, dietary/environmental compounds with estrogenic activity, or to endogenously produced molecules that exhibit estrogenic activity but which do not require aromatization (6–8). With respect to the latter, we and others have shown that the oxysterol 27-hydroxycholesterol, a primary metabolite of cholesterol, exhibits robust estrogenic activity and is produced at levels that are likely to promote substantial tumor growth [(9) and unpublished data]. Thus, it is anticipated that high affinity ERα antagonists, or compounds that ablate ERα expression, would have clinical activity in aromatase resistant disease.
The mechanisms underlying resistance to tamoxifen are complex. When originally developed, tamoxifen was classified as an “antiestrogen”, a compound that competitively inhibited the binding of estrogens to ERα, thus freezing it in an apo-conformation. However, this simple model failed to explain how tamoxifen could manifest agonist activity in bone and in the endometrium, and did not explain the withdrawal responses noted in some breast cancer patients who progressed while on tamoxifen (10–13). It is now known that tamoxifen is not an “antiestrogen” per se, but rather a Selective Estrogen Receptor Modulator (SERM), a compound whose relative agonist/antagonist activity can differ between cells. Thus, while it is capable of functioning as an antagonist in the breast, tamoxifen can also manifest agonist activity in other tissues. This observation was significant in light of the early studies that suggested that resistance to tamoxifen represented a “switch” within breast cancer cells that enabled them to recognize tamoxifen as an agonist as opposed to an antagonist (14). Supported by a wealth of more recent preclinical and clinical data, it now appears that it is the ability of breast cancer cells to support the agonist activity of tamoxifen that is the primary driver of resistance. The mechanisms underlying the molecular pharmacology of tamoxifen in breast cancer are now well understood and have been informative with respect to the development of resistance. Specifically, it has been determined that tamoxifen does not freeze ERα in an apo-state but rather it induces a conformational change that enables the presentation of unique protein-protein interaction surfaces on the receptor that dictate its transcriptional coregulator binding preferences (15). Thus, the agonist activity of tamoxifen depends on the relative expression and/or the activity of functionally distinct coregulators in target tissues (16, 17). Whereas the specific coregulators that enable the agonist activity of tamoxifen remain elusive, there is considerable additional data to support this hypothesis. Most notably, by screening for compounds that induced a conformational change in ERα that did not present the protein-protein interaction surfaces required for tamoxifen action, we identified DPC974/GW5638 (18, 19). This compound was shown to be an effective inhibitor of ER action in xenograft models of both tamoxifen sensitive and tamoxifen resistant breast cancers and yielded positive results in a heavily treated population of breast cancer patients with endocrine treatment resistant disease (20). Similarly, it has been shown that fulvestrant (ICI 182,780) also induces a unique conformational change in ERα, likewise functions as an antagonist in tamoxifen resistant xenograft models, and demonstrates efficacy in patients with advanced disease (15, 21, 22). Together these data suggest that by manipulating ERα structure and influencing coregulator engagement it is possible to develop compounds with useful activities in breast cancer.
Clearly, there is a very strong rationale to support the targeting of ERα in the setting of tamoxifen and aromatase resistant disease. Considering the current state of the art in this field, it is likely that drugs with utility in these disease states can be identified by screening for agents that (a) bind ERα with an affinity high enough to outcompete both endogenous and exogenous estrogens, (b) exhibit minimal agonist activity on those genes on which tamoxifen agonist activity is manifest in resistant breast cancer cells and (c) induce a structural change in ERα that disables the protein-protein interaction surfaces required for tamoxifen agonist activity. With these criteria in mind, we performed a comparative functional analysis of clinically relevant ER ligands, a study that revealed that bazedoxifene, a recently developed high affinity orally active SERM, inhibits ERα action in both tamoxifen sensitive and tamoxifen resistant xenograft models. This drug was recently approved for use in the EU for the treatment of osteoporosis. The findings presented herein should inform near term clinical studies of this drug in patients with advanced breast cancer.
Materials and Methods
Reagents
ER ligands included 17β-estradiol (E2 – Sigma), ICI 182,780 (ICI – Tocris), 4-hydroxytamoxifen (4OHT – Sigma), raloxifene (Ral – Sigma) and endoxifen (endox – Sigma). Lasofoxifene (Laso) and bazedoxifene (BZA) were gifts from Wyeth, Inc (now Pfizer). Ligands were dissolved in ethanol or DMSO. Cycloheximide and MG132 were purchased from Sigma.
Cell culture
Cell lines were maintained in DMEM/F12 (MCF7) or RPMI (BT483, rBT474, and SKBR3) media (Invitrogen) supplemented with 8% FBS (Gemini), non-essential amino acids (Invitrogen), and sodium pyruvate (Invitrogen). Parental cell lines were obtained from ATCC, which authenticates cell lines by short tandem repeat profiling. Lapatinib resistant rBT474 cells and LTED MCF7 cells were maintained as described (23, 24). Cells were plated for experiments in media lacking phenol red and supplemented with 8% charcoal stripped FBS (Gemini). After 48 hours cells were treated with ER ligands and/or inhibitors as indicated, and were harvested for immunoblot or real time quantitative PCR analysis 24 hours after treatment.
Immunoblot analysis
Protein expression was analyzed as described (25) using antibodies sc-6259 (cytokeratin 18), sc-20680 (lamin A), sc-5546 (α-tubulin) and sc-8005 (ERα) (Santa Cruz Biotechnology).
RNA isolation and real time quantitative PCR
RNA isolation and analysis was performed as described (26). mRNA abundance was calculated using the ΔΔCT method (27). Primer sequences are available upon request.
Mammalian 2 hybrid analysis
Transfections and analysis were performed as previously described (26).
Proliferation assays
3×103 MCF7 cells were plated per well in 96 well culture plates. One plate was decanted and frozen on day 1. Remaining plates were treated as indicated on days 1, 4, and 6, with identically treated plates harvested on days 1, 6, and 8. DNA content was detected using a FluoReporter assay (Invitrogen) per manufacturer's instructions.
Adenovirus production
Creation, production, and purification of an adenovirus expressing human ERα were previously described (28). 48 hours after plating, cells were infected with ERα adenovirus using multiplicity of infection (MOI) 0 or 100. Cells were treated with ER ligands as indicated immediately following infection and were harvested for immunoblot or real time quantitative PCR analysis 24 hours later. For proliferation assays, cells were infected and treated immediately and 2 or 5 days later.
Xenograft tumor analyses
All procedures were approved by the Duke University Institute for Animal Care and Use Committee. E2 dependent MCF7 or tamoxifen stimulated TamR tumors were initiated in the axial mammary gland of female NU/NU mice (~6 weeks age) by serial transfer. Briefly, ovariectomized recipient mice received hormone treatment via a timed release pellet (0.72 mg E2 or 5 mg tamoxifen/60 days – Innovative Research of America) implanted sc. 2 days later, an MCF7 or TamR tumor ~0.8–1 cm3 volume was excised under sterile conditions from a euthanized donor mouse, diced to ~2mm3 sections and implanted into the axial mammary gland under anesthesia (10g trochar). Tumor growth was measured 3× weekly by caliper (tumor volume = (A2 × B)/2, where A is the longer axis). When tumor volume reached ~0.2cm3 mice were randomized to continued E2 or tamoxifen treatment with placebo or BZA (5 mg/60 days) pellets implanted sc, or weekly injection with 5mg/mouse ICI 182,780 suspended in corn oil. For the BZA treatment only group E2 pellets were surgically removed simultaneous with BZA pellet insertion.
Analysis of tumor tissue
Tumors were excised upon reaching ~1cm3 and cryopreserved, and frozen tissues were pulverized. Protein expression was analyzed essentially as above. RNA was extracted using Trizol (Invitrogen) per manufacturer's instructions, and mRNA expression was detected as above.
Results
BZA induces a unique conformational change in ERα and inhibits its transcriptional activity in cellular models of breast cancer
We and others have demonstrated that tamoxifen partial agonist activity, and by inference tamoxifen resistance, can be attributed to specific ligand-induced conformational changes in ERα that presents a unique protein-protein interaction surface(s) that facilitates coregulator recruitment (15). This hypothesis was confirmed in studies which demonstrated that compounds that do not present the “tamoxifen surface” are effective in inhibiting the growth of tamoxifen resistant xenograft models of breast cancer (20). In recent years, several new high affinity SERMs have been developed for use in the treatment and prevention of osteoporosis. The goal of this study was to evaluate whether these clinically relevant SERMs could have activity as breast cancer treatments and explore the potential of their near term use in this disease.
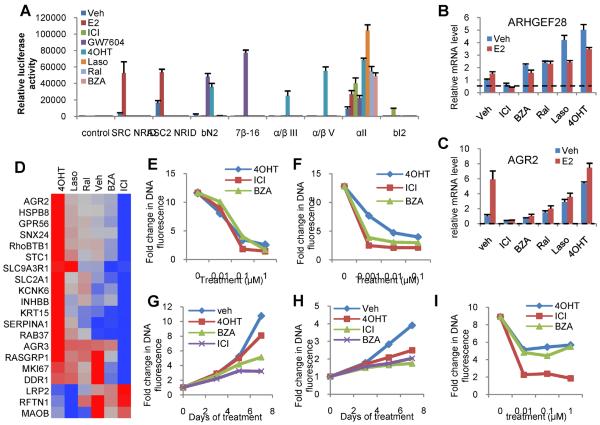
The impact of a series of chemically distinct SERMs on ERα conformation was evaluated using a previously described peptide-profiling technology that reads on the differential availability of protein-protein interactions presented on the receptor following ligand binding (15, 20). Specifically, peptide probes were selected that could identify those surfaces on ERα that were required for the agonist activity of estradiol and or tamoxifen. Using an in-cell two-hybrid assay, we determined that the SERMs bazedoxifene (BZA), lasofoxifene (Laso), and raloxifene (Ral) facilitate conformational changes in ERα that are distinct from those observed in the presence of 4-hydroxytamoxifen (4OHT) or the agonist 17β-estradiol (E2). Furthermore, neither BZA, Laso or Ral occupied ERα interacted with probes that identified surfaces that were uniquely presented on the receptor upon binding ICI 182,780 (ICI) and GW7604 (Figure 1A). These results demonstrate the conformational flexibility of ERα and highlight a potential opportunity to identify compounds whose impact on receptor structure results in a favorable activity in breast cancer. These findings are in agreement with a prior study in which hydrogen/deuterium exchange (HDX) mass spectrometry was used to interrogate the impact of ligands on ERα conformation; studies which highlighted the structural uniqueness of the ERα-BZA complex (29).
Figure 1. BZA induces a unique conformational change in ERα and inhibits ERα activity in cellular models of breast cancer.
A) Interaction between ERα and conformation-specific peptides in a mammalian two-hybrid system. Triplicate wells of SKBR3 cells were transfected with plasmids expressing ERα fused to VP16 together with Gal4DBD alone (control) or Gal4DBD fused to ER interacting peptides noted on the horizontal axis. Cells were then treated with the indicated ER ligands (100 nM). Interaction of ERα with the Gal4DBD peptide constructs was detected through activation of a Gal4 responsive luciferase reporter construct and was normalized to detected β-galactosidase activity expressed in a constitutive manner using a second vector. Normalized response is expressed as fold increase over the detected level of interaction between Gal4DBD alone and ER-VP16 in the absence of ligand (Veh). B–D) MCF7 cells were treated for 24 hours with vehicle or 100nM ligand - ICI 182,780 (ICI), Bazedoxifene (BZA), Raloxifene (Ral), Lasofoxifene (Laso), or 4-hydroxytamoxifen (4OHT) - prior to RNA isolation and RT-qPCR analysis of the expression of mRNAs shown previously to be responsive to SERMs. mRNA expression was normalized to the expression of the 36B4 housekeeping gene. D) Fold changes as compared to vehicle of mRNAs of interest were transformed and normalized as previously described and are presented as a dendogram constructed in JMP 9.0. E–F) MCF7 cells were plated in phenol red free media supplemented with charcoal stripped FBS (E) or in complete media supplemented with FBS (F) and were treated with the indicated ligands (100nM) on days 1, 4, and 6 of an 8 day proliferation assay. Cells in E were simultaneously treated with 1nM E2. DNA content as assessed by fluorescence serves as an indicator of cell proliferation. G) MCF7 cells were plated and treated as in E, but were treated with 20nM insulin instead of E2. H) Lapatinib resistant BT474 cells (rBT474) were plated in complete media supplemented with FBS and with 1μM GW2974 (EGFR inhibitor), and were then treated as in F. I) LTED MCF7 cells were plated in media supplemented with FBS that was stripped of growth factors twice using charcoal. Cells were treated with 0.01–1μM ligands on days 1, 4, and 6 of an 8 day proliferation assay and analyzed as in E. Values (relative increase in DNA fluorescence) in E–I were normalized to values detected in a duplicate plate of cells that was harvested on day 1 prior to the initial treatment. Data are representative of at least 3 independent experiments.
E,F – 756 1nM E2 and complete media
703 – H RBT474 in complete media
G – 875 GF prolif
Data from 647
H – prolif in complete media
F/G – gene expression and degr. coordinated
Previously, we performed a broad survey of the transcriptional responses that occur within breast cancer cells when treated with different SERMs and identified a) genes regulated similarly by all SERMs tested, b) genes regulated only by 4OHT, and c) genes whose response differentiated SERMs (26). Using the most informative representative genes from this study we evaluated how differences in ligand-induced changes in ERα conformation translate into differences in gene expression. Several genes, ARHGEF28 (RGNEF) for example, respond in a graded manner to different SERMs, with 4OHT exhibiting the greatest agonist activity, Ral and BZA being significantly less active, and ICI functioning as an inverse agonist (Figure 1B). Most notable, however, was the observation that the basal expression and estrogen dependent induction of AGR2, a gene associated with breast cancer progression during tamoxifen therapy, is repressed by ICI and BZA, but induced by Ral, Laso, and 4OHT (Figure 1C). Considering these data, a more comprehensive survey of the activity of different SERMs on the expression of a large number of genes whose expression was induced or repressed by tamoxifen was performed. Analysis of the data revealed that among the SERMs studied, the pharmacological profile of BZA was most comparable to the pure antagonist ICI, and thus it was brought forward for a more complete analysis in relevant models of breast cancer (Figure 1D). Importantly, it was shown that BZA inhibited estrogen dependent proliferation of MCF7 cells with efficacy similar to that of ICI and 4OHT (Figure 1E). As a more stringent test of activity, we assessed MCF7 cell proliferation in growth factor replete FBS media. Under these conditions 4OHT manifests significant partial agonist activity whereas ICI and BZA were effective antagonists (Figure 1F). It was further demonstrated that 4OHT was unable to inhibit insulin-stimulated proliferation of MCF7 cells whereas both ICI and BZA effectively inhibited this activity (Figure 1G). It was further demonstrated that ICI and BZA effectively inhibited the proliferation of lapatinib resistant rBT474 cells, tamoxifen sensitive subline of the HER2+ BT474 cells in which reactivation of ERα signaling is associated with the development of lapatinib resistance (24) (Figure 1H). Finally, BZA, 4OHT, and ICI similarly inhibited the proliferation of LTED MCF7 cells (Figure 1I), a cellular model of resistance to aromatase inhibitors. When taken together these data suggest that the conformational changes induced in ER upon binding ICI or BZA enable these agents to exhibit favorable activities in relevant models of breast cancer. These findings encouraged us to perform a comparative analysis of these drugs in in vivo models of breast cancer.
BZA attenuates estrogen dependent growth of MCF7-cell derived tumor xenografts
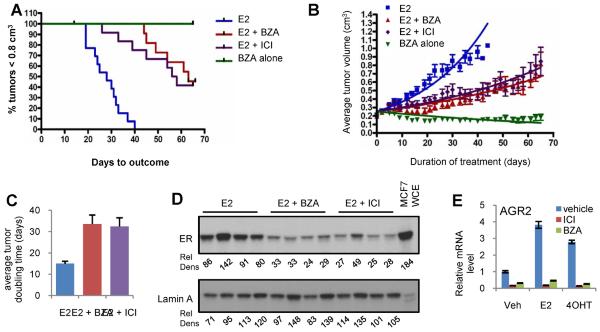
As an initial test of the therapeutic potential of BZA, its ability to inhibit the growth of E2 dependent MCF7 derived tumors was compared to ICI. For these experiments, MCF7 derived tumors were harvested from estrogen treated donor mice and similar sized tumor fragments were implanted into ovariectomized athymic nu/nu mice. All mice received estrogen supplementation until tumors reached 0.2cm3 at which time the tumor bearing mice were randomized to either of four groups. In three groups the estrogen supplementation was continued (1) alone or together with (2) BZA or with (3) ICI. In a fourth group of animals, the estradiol pellet was removed and the animals were administered BZA, a regimen intended to reveal any agonist activity of BZA not evident from the studies performed in vitro. The results of this analysis clearly indicate that both BZA and ICI significantly inhibit tumor growth rate with a delay in both tumor progression (Figure 2A–B) and tumor doubling time noted (Figure 2C). Furthermore, when administered alone, BZA did not exhibit agonist activity in this model (Figure 2B). It is important to note that ICI was administered at a dose of 5 mg/mouse injected subcutaneously once weekly, which translates to a dose ~1000 fold greater than that currently achievable in breast cancer patients. This dose of ICI greatly exceeds the dose of BZA used (5 mg/mouse in a 60 day continuous release pellet = ~83ug/kg/day), yet these drugs inhibited tumor growth and reduced tumor doubling time in a similar manner despite being administered at a dose only 7-fold greater than the daily dose of estradiol. Previously, it has been shown that ICI induces turnover of ERα in breast tumors and accordingly this drug, and others that exhibit similar activities, are now classified as Selective Estrogen Receptor Degraders (SERDs). Given the established SERD activity of ICI, the expression of ERα was measured in a subset of tumors harvested at the end of the study. Notwithstanding minor differences between tumors in each group, it was noted that the dosing regimen used to deliver ICI effected a substantial, although not absolute, turnover of the receptor. Interestingly, we also observed that ERα expression was similarly reduced in BZA treated tumors (Figure 2D). This was not totally unexpected as previously we and others have shown that BZA exhibits some SERD activity in vitro (30).
Figure 2. BZA attenuates ER and estrogen dependent growth of MCF7-cell derived tumor xenografts.
Mice bearing MCF7 xenograft tumors were randomized (11–12 mice per group) at 0.2±0.025cm3 tumor volume to receive continued E2 stimulation (E2 alone), treatment with E2 together with either BZA (sc pellet) or ICI (weekly injection) or E2 withdrawal (pellet removed) together with BZA treatment (BZA alone). A) Days required for tumors to reach 0.8 cm3 by Kaplan Meier analysis. Logrank test indicated significance (p<0.0001) in comparison of all treatments to the estrogen control. Differences observed between the E2+ICI and E2+BZA groups were not significant. B) Tumor growth for each group is presented as the average tumor volume for each treatment group +/− SEM at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Non-linear regression analysis for each growth curve is presented. C) Average doubling time for tumors within each group. Data for each replicate was fitted to an exponential growth regression model [Y=Start*exp(K*X), where we constrained the Start to being shared between all groups]. Doubling time was estimated from these curves and plotted as mean +/−SEM. Non-parametric Kruskal-Wallis analysis followed by the Dunn's test indicated significant differences (P< 0.0001) between both groups and E2. D) ER expression detected by immunoblot of whole cell extracts from tumors harvested from four representative mice from each group. Relative density (Rel Dens) of detected bands were calculated by mean density*pixels and normalized to, and expressed as a percentage of, the average density detected for the vehicle treated samples. E) MCF7 cells were treated for 24 hours with vehicle, 17β-estradiol (1nM) or 4OHT (10nM) in the presence or absence of ICI or BZA (100nM). AGR2 mRNA expression was detected and normalized as in Figure 1.
BZA attenuates ERα dependent growth of a tamoxifen resistant xenograft tumor model
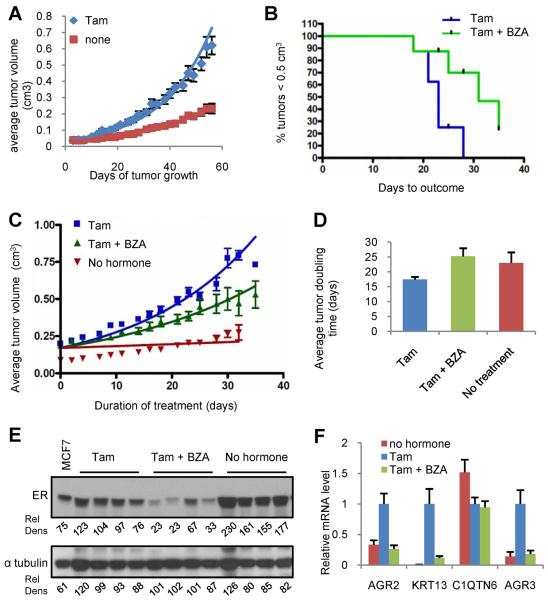
The observation that BZA and ICI functioned similarly as inhibitors of either tamoxifen or estradiol induced gene expression in MCF7 cells in vitro led us to examine whether BZA would exhibit efficacy in an in vivo model of tamoxifen resistance (Figure 2E). Previously, we reported on the development and characterization of a xenograft model (TamR) in which resistance to tamoxifen is manifest (20). In brief, in this very stable tumor model, tamoxifen functions as an agonist whereas both of the SERDs, ICI and DPC974/GW5638, effectively inhibit growth (20). In contrast to the parental MCF7 tumors, which are unable to grow without E2 stimulation (data not shown), TamR tumors grow, albeit slowly, in the absence of estradiol supplementation, and their growth is dramatically stimulated upon administration of tamoxifen (5mg/60 day release pellet = ~83 μg/kg/day) (Figure 3A). Analysis of tumor volume over time (~30 days) indicated that BZA significantly reversed tamoxifen stimulation of these tumors (Figure 3B), resulting in a growth rate that was equivalent to that observed when tumors were grown in the absence of tamoxifen (Figure 3C–D). Immunoblot analysis of whole cell extracts prepared from tumors harvested at the end of the study indicated that ER levels were significantly reduced in those treated with BZA whereas expectedly tamoxifen had a minimal effect on receptor expression (Figure 3E). To further differentiate the actions of BZA and Tam and to confirm adequate drug exposure, we assessed the expression of several ER target genes in tumor mRNA, selected for their ability to read on the agonist activity of tamoxifen. As expected from our previously published work (26), it was determined that the mRNAs encoding AGR2, AGR3 and KRT13 were significantly induced in tumors exposed to tamoxifen and that this activity is completely inhibited by BZA. The inhibitory activity of tamoxifen on the basal expression of the estrogen responsive gene C1QTN6 was unaffected by BZA, indicating, not surprisingly, that these drugs are likely to similarly impact some target genes (26, 31, 32) (Figure 3F). These data indicate that BZA does not facilitate tumor growth in a validated model of tamoxifen resistance but rather inhibits tamoxifen stimulated growth of these tumors. These findings highlight the potential clinical utility of BZA as a breast cancer therapeutic.
Figure 3. BZA attenuates ER dependent growth of tamoxifen resistant breast tumor xenografts.
A) Average tumor volume of tamoxifen resistant xenograft tumors implanted with or without tamoxifen stimulation (5 mg sc pellet). B–D) When Tam stimulated tumors attained 0.2±0.025cm3 tumor volume, animals were randomized (8–10 mice per group) to receive continued tamoxifen stimulation (tam alone) or treatment with Tam together with BZA (5mg sc pellet). B) Days required for tumors in each group to reach 0.5 cm3 volume (Kaplan Meier analysis). Logrank test indicated significant difference (p=0.0175). C) Tumor growth for each group is presented as average tumor volume +/− SEM per study arm at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Tumor volume for the unstimulated (no hormone) group is plotted corresponding to the first day of treatment for the +/− BZA groups. Non-linear regression analysis for each growth curve is presented D) Average doubling time for tumors in each group. Data for each replicate was fitted to an exponential growth regression model [Y=Start*exp(K*X), where we constrained the Start to being shared between all groups]. Doubling time was estimated from these curves and plotted as mean +/−SEM. Non-parametric Mann Whitney test indicated a significant difference (P=0.0207) between Tam and Tam+BZA. E) ER expression detected by immunoblot of whole cell extracts of tumors harvested from four representative mice from each group. Relative density (Rel Dens) was calculated as in Figure 2. F) Average expression level (+/− SEM) of tamoxifen regulated genes in tumors (n=8–10) from each treatment group. mRNA levels were detected and normalized to human 36B4 as in Figure 1. Data are plotted relative to the tamoxifen treated group.
Graph – exp 558
BZA exhibits SERD activity in cellular models of ERα-positive breast cancer
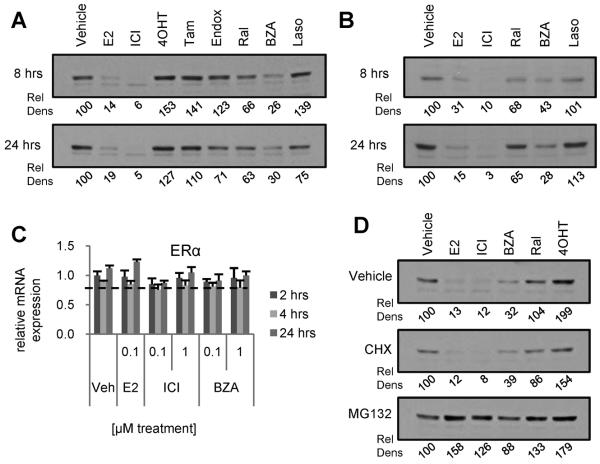
BZA and ICI exhibited similar activities on gene regulation in MCF7 cell derived tumors and in their TamR variants, and both compounds resulted in a similar downregulation of ERα expression in these tumors. Of specific interest was the observation that treatment of MCF7 cell derived tumors with either BZA or ICI resulted in a similar downregulation of ERα expression. The pharmacology of ICI has been probed in detail and it has been concluded from these studies that this compound induces a conformational change in the receptor that targets it for degradation. Whereas the conformational change in ERα induced by BZA is distinct from that observed upon binding ICI [Figure 1A and (29)], we were interested in determining if the down regulation of ERα observed in BZA treated tumors reflects SERD activity, as is the case for ICI, or occurs in an indirect manner (i.e. decreased ERα mRNA expression). Notably, the impact of ligands on ERα stability/expression observed in tumors was recapitulated in cellular models of breast cancer. As shown, treatment of MCF7 (Figure 4A) or BT483 (Figure 4B) cells for 8 or 24 hours with BZA or ICI resulted in a loss of ERα expression, while 4OHT, endoxifen, Tam, Ral and Laso had little effect on the level of the receptor. For comparative purposes, and to validate the cell models, we demonstrated that treatment of cells with 17β-estradiol resulted in a quantitative down regulation of ERα expression.
Figure 4. BZA reduces ERα expression in ERα-positive breast cancer cells.
MCF7 (A) and BT483 (B) breast cancer cells were treated for 8 or 24 hours with E2 (100 nM) or ER antagonists (1 μM). ERα levels in these whole cell extracts were analyzed by immunoblot.. C) MCF7 cells were pretreated 2 hours with MG132 (30μm) or cycloheximide (CHX – 10μg/ml) prior to 4 hours treatment with ER ligands (100nM E2, 1μM others). ER expression was analyzed by Western immunoblot analysis of whole cell extracts. D) MCF7 cells were treated for 2, 4 or 24 hours with 0.1 or 1μM E2, BZA, or ICI prior to RNA isolation and detection of ERα mRNA by RT-qPCR. ER mRNA levels were normalized to the housekeeping gene 36B4. E) MCF7 cells were treated for 24 hours with E2 (100nM) or SERDs (1μM) and ERα levels were analyzed as in (A). Loading controls for panels A–B are illustrated in Supplementary Figure S1. Relative density (Rel Dens) of blot images was calculated as in Figure 2, but normalized to, and expressed as a percentage of, the density detected for the vehicle treated control.
We next explored the mechanisms underlying the apparent SERD activity of BZA. Firstly, it was demonstrated that the effects of BZA, ICI or 17β-estradiol on ERα expression occurred in a post-transcriptional manner as no differences in ERα mRNA expression were observed following drug treatment (Figure 4C). It was further demonstrated that ligand dependent downregulation of ERα expression was not affected by cotreatment with cycloheximide suggesting that receptor stability was an intrinsic property of the receptor ligand complex and did not require the de novo synthesis of ancillary proteins required for receptor turnover. Furthermore, 17β-estradiol, ICI and BZA-mediated turnover of the receptor could be blocked by cotreatment of cells with the proteasome inhibitor MG132 (Figure 4D). Together these results suggest that, similar to ICI, BZA exhibits proteasome dependent SERD activity.
Inhibition of ERα turnover does not compromise the antagonist activity of BZA
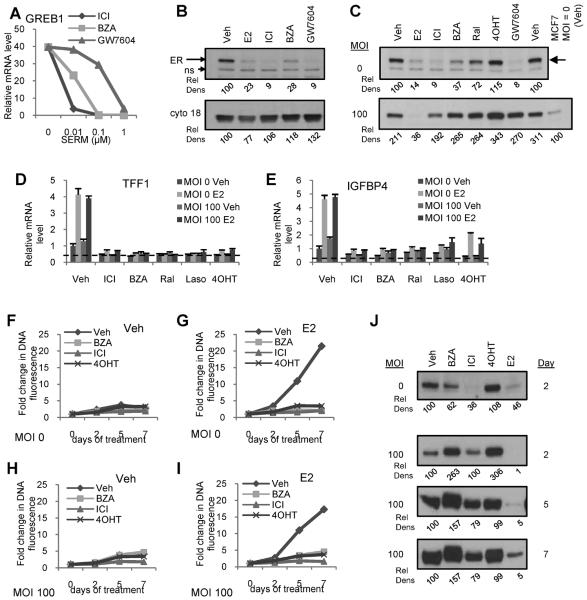
Having demonstrated that BZA binding induces ERα turnover, we next performed a comparative analysis of this compound with ICI and GW7604, another SERD that has been evaluated previously in patients with breast cancer. When evaluated for their ability to inhibit 17β-estradiol mediated induction of the transcription of GREB1 mRNA in MCF7 cells, it was observed that all three compounds were equally effective as antagonists with potencies that reflected their affinity for the receptor (Figure 5A); similar results were observed on other ER-responsive genes (26). However at saturating, maximally efficacious doses of each compound, it was noticed that, unlike what occurs in ICI or GW7604 treated cells, ERα expression was not completely down regulated in cells treated with BZA (Figure 5B). This raised the interesting question as to the requirement of receptor turnover for maximal antagonist activity, an important issue to address as it speaks to the utility of ERα measurements in tumors as a surrogate for the antiestrogenic actions of BZA.
Figure 5. BZA dependent inhibition of ER-mediated transcriptional activity is not impacted by loss of ER turnover.
A) MCF7 cells were treated for 24 hours with or without E2 (1nM) in the presence of the indicated SERDs (0.01–1 μM). mRNA expression of target gene GREB1 was detected as in Figure 1. B) MCF7 cells were treated for 24 hours with E2 (100nM) or SERDs (1μM) and ERα levels were analyzed by immunoblot. C–E) MCF7 cells were infected with ERα adenovirus (MOI 100) or mock infected prior to 24 hours treatment with E2 (100 nM) or other ligands (1μM). C) ERα levels were analyzed by immunoblotting of whole cell extracts. D–E) Expression of ER target genes in cells infected in parallel and treated 24 hours with E2 (10 nM) in the presence or absence of SERMs or ICI (1 μM) was analyzed by RT-qPCR as in Figure 1. F–G) MCF7 cells were infected as in C and treated with ligands (1μM) in the presence or absence of E2 (1nM) immediately, and 2 and 5 days following infection, with one replicate plate harvested prior to infection and 2, 5, and 7 days after infection. Cell proliferation in the absence (left) or presence (right) of estrogen was analyzed as in Figure 1. H) MCF7 cells were infected, treated with ligands, and harvested in parallel with F and G. ERα expression was analyzed as in C. Data are representative of at least 3 independent experiments. Relative density (Rel Dens) of blot images was calculated as in Figure 2, but normalized to, and expressed as a percentage of, the density detected for the vehicle treated control. For 5C, values are normalized to, and expressed as a percentage of, the vehicle treated uninfected sample. In 5H, values are normalized to, and expressed as a percentage of, the vehicle control for each MOI and time.
Since proteasomal degradation of ERα, when occupied by different ligands, requires it to engage a specific E3 ligase(s), it stands to reason that if the expression of these latter enzymes are limiting that it should be possible to saturate the turnover process by overexpression of the receptor. Previously, we demonstrated that the ubiquitination pattern of 17β-estradiol and ICI occupied ERα in cells were different (33, 34), suggesting that they may be targeted by different E3 ligases. In follow-up experiments, it was demonstrated that ICI mediated turnover of ERα was reduced upon receptor overexpression (35). Under the same conditions, however, a quantitative down regulation of 17β-estradiol occupied receptor was observed. Thus, the ability to saturate the degradation process by overexpressing ERα afforded us the opportunity to assess whether turnover of the receptor was required for the antagonist activity of BZA. As shown in Figure 5C, the anticipated effects of the various SERMs/SERDs on ERα stability were observed in uninfected cells (MOI=0). However, in cells infected with a virus expressing ERα, up to 10-fold overexpression of the receptor was achieved, and under these conditions the SERD activity of ICI, BZA and GW7604 was saturated. We have previously demonstrated that receptor overexpressed in this manner is active and able to bind ligand (35). As observed previously, 17β-estradiol mediated turnover of ERα was not impacted by any of these manipulations, ruling out non-specific effects of this viral overexpression protocol on the activity of the proteasome. The most important finding, however, is that overexpression of ERα had no significant effects on the antagonist efficacy of the SERMs/SERDs evaluated in this study. As expected overexpression of ERα did lead to an increase in ligand independent activation of transcription by the receptor; however, this activity was attenuated by all of the SERMs and SERDs tested (Figure 5D–E). Furthermore, the inhibition of ERα function by SERMs and SERDs, as measured by comparing the proliferation of infected and uninfected cells, was unaffected as well despite sustained overexpression of ERα (Figure 5F–J). When taken together these results suggest that although the removal of ERα by an ER ligand is a desirable attribute of antagonists, it is not required for the antagonist activity of the existing SERDs.
Discussion
In recent years there has been a resurgence of interest in ERα as a therapeutic in cancer. In part, this has been fueled by recent advances that have been made in the pharmaceutical exploitation of the androgen receptor (AR) in prostate cancer. Until relatively recently it was considered that the AR was not a viable target in castrate resistant prostate cancer. However, the spectacular clinical responses to Cyp17 inhibitors, like abiraterone, and to the new high affinity AR antagonist MDV3100 in late stage disease, have encouraged a reappraisal of ER as a therapeutic target in those breast cancers that have failed both tamoxifen and/or aromatase inhibitors (36, 37). As with AR, our understanding of the molecular pharmacology of ER has advanced tremendously in recent years, enabling the development of highly predictive mechanism-based screens for ER-modulators. Indeed, Laso and BZA emerged from in vitro screens that selected for compounds which (a) bound ERα and competitively displaced 17β-estradiol (b) inhibited estrogen action in cellular models of breast cancer and (c) did not manifest ERα agonist activity in contexts where tamoxifen functioned as an agonist (38–40). In clinical trials, it was determined that both of these compounds exhibited robust estrogenic activity in bone and reduced the incidence of vertebral fractures by ~40% in osteoporotic post-menopausal patients (41, 42). In addition, as a secondary endpoint, Laso was shown to significantly decrease the risk of ER-positive breast cancer (43). However, Laso was subsequently found to have a less favorable clinical profile than BZA in the reproductive tract, in that its use was associated with significantly increased endometrial thickness and increased incidence of endometrial polyps, uterine leiomyoma, and vaginal bleeding (44). Laso has been approved in the EU for the treatment and prevention of osteoporosis but has not yet been registered. Recently, BZA has been approved and marketed in the EU for the same use. Our studies of the molecular pharmacology of BZA and Laso indicate that the former most closely profiled with Fulvestrant (ICI) an approved SERD. Importantly, as demonstrated in this study BZA effectively inhibited the growth of tamoxifen sensitive and tamoxifen resistant breast cancer xenografts. Given these data, and the fact that this drug is currently available for use in humans, we believe that a near-term evaluation of its efficacy in patients with advanced disease is justified.
One of the unexpected findings made in this study is that BZA induces ERα turnover both in vitro and in vivo absent any impact on the expression of ERα mRNA. The results observed in vivo are of particular note as they indicate that a sustained knockdown of ERα protein expression can be accomplished by BZA over time and suggest the absence of feedback mechanism(s) to restore receptor levels. When considering the mechanisms by which resistance to endocrine therapies can arise, it is clear that it would be advantageous to chronically suppress ERα expression. In this manner, ERα, the target for coactivators and signaling pathways that impinge upon the ER-coregulator complex(s), is removed and signaling is attenuated. Importantly, we have shown that although BZA manifests SERD activity, receptor degradation is not required for its antagonist activity. This is an important finding as receptor overexpression has been considered as a possible explanation for resistance to tamoxifen in breast cancer.
Our studies have revealed that ICI and BZA exhibit very similar pharmacology and function similarly in relevant animal models of breast cancer, findings which provide rationale for its clinical evaluation. However, there has been significant concern of late as to the utility of ICI in breast cancer. Although this drug is approved for the treatment of tamoxifen/AI refractory tumors, its response rate as originally determined in the EFECT (Evaluation of Faslodex versus Exemestane) trial was ~10%, similar to intervention with exemestane a second line, steroidal AI (21). This low response rate was unexpected and was initially considered to indicate that SERD intervention would have limited use in the treatment of advanced breast cancer. However, a considerable amount of additional data has emerged of late indicating that the pharmacokinetic properties of fulvestrant, administered by IM injection, and not its mechanism of action, is likely that which limits its efficacy (45, 46). Indeed, despite the use of a loading dose three times higher than that evaluated in the EFECT trial, drug concentrations measured in the vicinity of the tumor were found to be insufficient to saturate the receptor (47). A compelling series of PET imaging studies confirmed poor delivery of fulvestrant to the tumor (43). Regardless, the results of the CONFIRM (Comparison of Fulvestrant in Recurrent or Metastatic Breast Cancer) trial suggested that this SERD may have improved biologic activity and clinical efficacy at higher doses than that originally approved (48). Specifically, it was noted that in the high-dose arm of this study, fulvestrant significantly delays time to cancer progression compared with standard-dose regimens. However, despite these advances in the delivery of fulvestrant, it is likely that the full therapeutic potential of SERD intervention has not been realized with this drug. Being a high affinity, orally bioavailable drug, BZA would not be limited by poor tumor access. Thus, its bio-availability presents the opportunity to evaluate the true potential of SERD activity in late stage disease.
Our studies introduce an apparent paradox in that BZA, originally developed as a SERM for use in the treatment and prevention of osteoporosis, actually profiles as a pure antagonist/SERD in the breast. The latter implies that in the context of bone, BZA exhibits ERα agonist activity in bone, a finding that on first glance is incompatible with it being a SERD. Indeed, DPC974/GW5638, an earlier molecule we developed that exhibited substantial SERD activity, is also bone protective (19). This raises the possibility that within the appropriate cells in bone that ERα stability is not impacted in the same manner as in breast cancer cells, a hypothesis that we are currently testing.
Supplementary Material
Translational Relevance.
Breast cancer remains the most commonly diagnosed cancer among women and the leading cause of cancer mortality in women worldwide. While targeted therapies such as tamoxifen and aromatase inhibitors are effective in treating estrogen receptor (ER) positive tumors, de novo and acquired resistance remain an impediment to durable clinical responses. However, ERα remains a therapeutic target in breast cancers that are resistant to both first and second line endocrine interventions. Evaluation of the molecular pharmacology of clinically relevant ER modulators revealed that the third generation selective estrogen receptor modulator (SERM) bazedoxifene had a unique mechanism of action and inhibited the growth of both tamoxifen sensitive and resistant ERα positive breast cancer xenografts. These findings provide strong support for the clinical evaluation of bazedoxifene in breast cancer patients who relapse while undergoing treatment with tamoxifen and/or an aromatase inhibitor.
Acknowledgements
Lapatinib resistant rBT474 cells and long term estrogen deprived (LTED) cells were kindly provided by Drs. Neil Spector (Duke University) and Richard Santen (University of Virginia), respectively.
Grant support Supporting funding for all authors was provided by Pfizer Pharmaceuticals, Inc. and DK048807.
Footnotes
Disclosure summary: D.P.M and S.E.W. have in the past served as paid consultants for Pfizer, Inc.
References
- 1.Kvinnsland S, Anker G, Dirix LY, Bonneterre J, Prove AM, Wilking N, et al. High activity and tolerability demonstrated for exemestane in postmenopausal women with metastatic breast cancer who had previously failed on tamoxifen treatment. Eur J Cancer. 2000;36:976–82. doi: 10.1016/s0959-8049(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 2.Dodwell D, Wardley A, Johnston S. Postmenopausal advanced breast cancer: options for therapy after tamoxifen and aromatase inhibitors. Breast. 2006;15:584–94. doi: 10.1016/j.breast.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 4.Miller WR, Larionov AA. Understanding the mechanisms of aromatase inhibitor resistance. Breast cancer research : BCR. 2012;14:201. doi: 10.1186/bcr2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodie A, Jelovac D, Sabnis G, Long B, Macedo L, Goloubeva O. Model systems: mechanisms involved in the loss of sensitivity to letrozole. J Steroid Biochem Mol Biol. 2005;95:41–8. doi: 10.1016/j.jsbmb.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, et al. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68:4910–8. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 7.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AM. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–44. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 8.Santen R, Jeng M-H, Wang J-P, Song R, Masamura S, McPherson R, et al. Adaptive hypersensitivity to estradiol: Potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Molec Biol. 2001;79:115–25. doi: 10.1016/s0960-0760(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 9.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallenbach-Hellweg G, Schmidt D, Hellberg P, Bourne T, Kreuzwieser E, Doren M, et al. The endometrium in breast cancer patients on tamoxifen. Arch Gynecol Obstet. 2000;263:170–7. doi: 10.1007/s004040050276. [DOI] [PubMed] [Google Scholar]
- 11.Farnell YZ, Ing NH. Endometrial effects of selective estrogen receptor modulators (SERMs) on estradiol-responsive gene expression are gene and cell-specific. J Steroid Biochem Mol Biol. 2003;84:513–26. doi: 10.1016/s0960-0760(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 12.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–6. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 13.Canney PA, Griffiths T, Latief TN, Priestman TJ. Clinical significance of tamoxifen withdrawal response. Lancet. 1987;1:36. doi: 10.1016/s0140-6736(87)90717-3. [DOI] [PubMed] [Google Scholar]
- 14.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–7. [PubMed] [Google Scholar]
- 15.Norris J, Paige L, Christensen D, Chang C, Huacani M, Fan D, et al. Peptide antagonists of the human estrogen receptor. Science. 1999;285:744–6. doi: 10.1126/science.285.5428.744. [DOI] [PubMed] [Google Scholar]
- 16.Kressler D, Hock MB, Kralli A. Coactivators PGC-1 beta and SRC-1 interact functionally to promote the agonist activity of the selective estrogen receptor modulator tamoxifen. J Biol Chem. 2007;282:26897–907. doi: 10.1074/jbc.M705596200. [DOI] [PubMed] [Google Scholar]
- 17.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–66. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 18.Bentrem DJ, Dardes RC, Liu H, Maccgregor-Schafer J, Zapf JW, Jordan VC. Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen. Endocrinology. 2001;142:838–46. doi: 10.1210/endo.142.2.7932. [DOI] [PubMed] [Google Scholar]
- 19.Willson TM, Norris JD, Wagner BL, Asplin I, Baer P, Brown HR, et al. Dissection of the molecular mechanism of action of GW5638, a novel estrogen receptor ligand, provides insights into the role of ER in bone. Endocrinology. 1997;138:3901–11. doi: 10.1210/endo.138.9.5358. [DOI] [PubMed] [Google Scholar]
- 20.Connor CE, Norris JD, Broadwater G, Willson TM, Gottardis MM, Dewhirst MW, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917–22. [PubMed] [Google Scholar]
- 21.Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 22.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–3. [PubMed] [Google Scholar]
- 23.Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995;80:2918–25. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- 24.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittmann B, Sherk A, McDonnell D. Definition of functionally important mechanistic differences among selective estrogen receptor down-regulators. Cancer Res. 2007;67:9549–60. doi: 10.1158/0008-5472.CAN-07-1590. [DOI] [PubMed] [Google Scholar]
- 26.Wardell SE, Kazmin D, McDonnell DP. Research resource: Transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol. 2012;26:1235–48. doi: 10.1210/me.2012-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Gaillard S, Grasfeder LL, Haeffele CL, Lobenhofer EK, Chu TM, Wolfinger R, et al. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol Cell. 2006;24:797–803. doi: 10.1016/j.molcel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Dai SY, Chalmers MJ, Bruning J, Bramlett KS, Osborne HE, Montrose-Rafizadeh C, et al. Prediction of the tissue-specificity of selective estrogen receptor modulators by using a single biochemical method. Proc Natl Acad Sci U S A. 2008;105:7171–6. doi: 10.1073/pnas.0710802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor alpha and cyclin D1. Mol Pharmacol. 2011;80:610–20. doi: 10.1124/mol.111.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68:7493–501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–33. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 33.Wijayaratne A, McDonnell D. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem. 2001;276:35684–92. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 34.Wijayaratne A, Nagel S, Paige L, Christensen D, Norris J, Fowlkes D, et al. Comparative analysis of mechanistic difference among antiestrogens. Endocrinology. 1999;140:5828–40. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- 35.Wardell SE, Marks J, McDonnell DM. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82:122–30. doi: 10.1016/j.bcp.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 37.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ke HZ, Qi H, Crawford DT, Chidsey-Frink KL, Simmons HA, Thompson DD. Lasofoxifene (CP-336,156), a selective estrogen receptor modulator, prevents bone loss induced by aging and orchidectomy in the adult rat. Endocrinology. 2000;141:1338–44. doi: 10.1210/endo.141.4.7408. [DOI] [PubMed] [Google Scholar]
- 39.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287:40–6. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923–34. doi: 10.1359/jbmr.080710. [DOI] [PubMed] [Google Scholar]
- 42.Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 43.LaCroix AZ, Powles T, Osborne CK, Wolter K, Thompson JR, Thompson DD, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010;102:1706–15. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein SR, Neven P, Cummings S, Colgan T, Runowicz CD, Krpan D, et al. Postmenopausal Evaluation and Risk Reduction With Lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18:17–22. doi: 10.1097/gme.0b013e3181e84bb4. [DOI] [PubMed] [Google Scholar]
- 45.Linden H, Kurland B, Livingston R, Sandararajan L, Peterson L, Schubert E, et al. PET FES Measures Uterine and Tumor In Vivo Pharmacodynamics Of Endocrine Therapy. Meeting abstracts: San Antonio Breast Cancer Meeting. 2009 [Google Scholar]
- 46.Wakeling A, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–73. [PubMed] [Google Scholar]
- 47.Robertson J, Nicholson R, Bundred N, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61:6739–46. [PubMed] [Google Scholar]
- 48.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko I, Khasanov R, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor positive advanced breast cancer. J Clin Oncol. 2010;28 doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.