Abstract
Background & Aims
Oral 5-aminosalicylic acid drugs (5-ASA) are widely used for mild to moderate ulcerative colitis (UC). However, these drugs are costly, and long-term adherence is poor. The purpose of this study was to quantify the cost-utility of inflammation-targeted, intermittent therapy versus universal, continuous maintenance therapy with 5-ASA agents in patients with mild to moderate UC.
Methods
We developed a Markov cohort model that simulated a population of adult patients with newly diagnosed, quiescent UC after induction of remission with 5-ASA agents. Model inputs were obtained from published literature. The perspective taken was that of a short-term payer (health insurance provider) over a 5 year time period. Three treatment strategies were modeled: (1) symptom-targeted treatment (i.e., treatment for symptomatic disease flares only) (SYMPT); (2) continuous 5-ASA maintenance for all patients (CONT); and, (3) inflammation-targeted treatment (i.e., 5-ASA therapy only for patients with a positive stool inflammatory marker) (INFLAM). Disease flares, quality-adjusted life years (QALYs), costs (2009 US dollars), and incremental cost-effectiveness ratios (ICERs) were measured.
Results
INFLAM was the least costly strategy, with a cumulative per patient cost of $22,798, compared to $24,378 and $25,621 for the SYMPT and CONT strategies, respectively. Despite the lower cost, INFLAM was comparable to SYMPT and CONT in terms of effectiveness (4.4986 versus 4.5014 QALYs, respectively), making it the optimal strategy. Several variables proved to be important in sensitivity analysis, but the CONT strategy (current standard of care) was optimal only if the cost of 5-ASA medications was markedly reduced.
Conclusions
Inflammation-targeted treatment of UC has the potential to produce favorable clinical outcomes while limiting costs. Prospective trials of inflammation-targeted treatment are warranted.
Keywords: Ulcerative colitis, cost-effectiveness analysis, health services research
Background & Aims
Though ulcerative colitis (UC) does not have a high population prevalence, its early age of onset and associated long-term morbidity results in substantial negative effects on quality of life and high health care utilization and costs 1-3. Patients with newly diagnosed UC are commonly treated with a 5-aminosalicylic acid (5-ASA) agent to reduce the likelihood of subsequent disease flares 4. Such agents are generally safe and well tolerated. However, patients often discontinue these medications during prolonged periods of disease remission 5, 6. Furthermore, they are expensive, with prior studies suggesting that continuous 5-ASA maintenance therapy may be cost-ineffective 7. Sulfasalazine, a less expensive option, is cost-effective, but it is poorly tolerated in up to 30% of patients 8. Sulfasalzine must also be taken two to three times per day, compared to once daily for newer 5-ASA compounds, again contributing to non-adherence 9. Reports suggest that 5-ASA compounds are the agents of choice in both the United States and Europe, accounting for up to 88% of the market for such medications 10.
One approach that can improve the cost-effectiveness of 5-ASA therapy is to target therapy to patients who are at increased risk for disease flare. Identifying patients at increased risk for a flare could improve clinical outcomes while reducing costs and resource utilization. Recent studies have suggested that stool tests for bowel inflammation can detect subclinical UC and identify patients who are at risk for impending flare before symptoms develop 11, 12. Though the accuracy of these stool tests varies, some (e.g., fecal calprotectin) 13 are reasonably sensitive and specific12, 14, 15and could be used to predict imminent disease flare in individual patients before symptoms develop, allowing treatment to be targeted to at-risk patients to prevent a symptomatic disease flare. The paradigm of targeted therapy has been shown to reduce resource utilization and costs while improving clinical outcomes in a variety of other disease states 16, 17. However, it remains unknown whether targeted therapy has a role in the management of UC.
The purpose of this study was to model the clinical and economic effects of inflammation-targeted, intermittent 5-ASA therapy for mild to moderate UC compared to universal, continuous maintenance therapy or symptom-targeted therapy. We also sought to identify the characteristics of predictive tests (such as fecal calprotectin) that would be required to yield cost-effective inflammation-targeted treatment strategies in UC.
Methods
Decision Analytic Model
We constructed a Markov cohort model using TreeAge Pro decision modeling software (TreeAge Software, Inc., Williamstown, MA). We modeled disease states and outcomes (quality-adjusted life-years, or QALYs) in a simulated cohort of adult patients with newly diagnosed UC. The perspective taken was that of an insurance provider with a time horizon of 5 years. The basic structure of the model is outlined in Figure 1.
Figure 1. Schematic of Markov Model Structure.
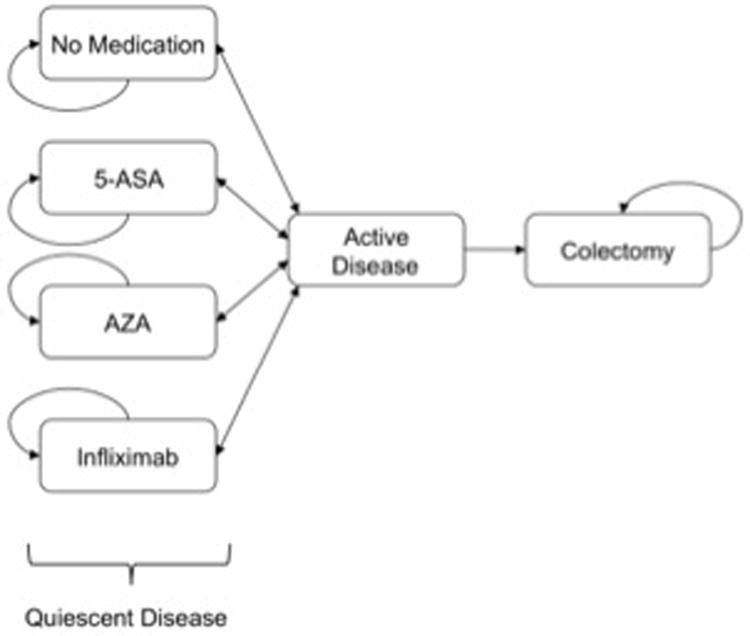
Terminal (death) states (not shown) can be reached from any state; patients with active disease can undergo modification in medical therapy before returning to a quiescent disease state.
At its inception, the cohort consisted of an otherwise healthy population of 22 year-old patients with recently diagnosed 5-ASA-responsive UC, after induction of clinical remission. Patients cycled between the principal health states every three months, with transition probabilities derived from published literature. During a three-month cycle, disease could remain quiescent or become active (clinical flare) (Figure 2).
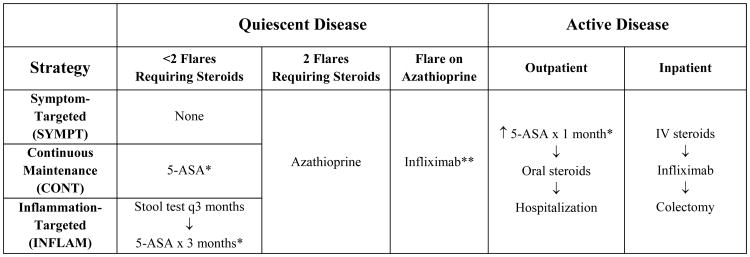
Figure 2. Treatment Strategies.
* In quiescent disease, the 5-ASA dose was mesalamine 2.4 g po qD (“maintenance 5-ASA”). In active disease, the oral 5-ASA dose was doubled, and mesalamine enemas (4 g per rectum each day) were added (“intensive 5-ASA”). ** Flare on infliximab therapy led to colectomy.
Modeled patients who developed disease flare were initially treated as outpatients with a combination of “intensive” oral and rectal 5-ASA compounds, and treatment was escalated in non-responders as outlined in Figure 2. Patients who did not respond to intensive 5-ASA therapy after 1 month were placed on oral corticosteroids. Non-response to oral corticosteroids resulted in admission for intravenous corticosteroids. Those with disease that was refractory to intravenous steroids were treated with infliximab. Finally, lack of response to infliximab resulted in colectomy. To minimize complexity, we did not include cyclosporine rescue therapy in the model, particularly in light of recent data suggesting that cyclosporine and infliximab have equivalent efficacy for treatment of acute steroid-refractory UC 18. We assumed that outpatients successfully treated with 5-ASA compounds incurred 2 physician office visits for management of the disease flare, and those who required corticosteroids incurred 2 additional physician office visits.
Successful medical treatment of a flare resulted in return to a quiescent disease state. If a patient experienced two flares that required oral or intravenous corticosteroids, they were started on azathioprine (AZA). If a patient experienced a flare on AZA, the patient was placed on infliximab therapy. If a patient experienced a flare on infliximab, they proceeded to colectomy. If a colectomy was performed, medical therapy was discontinued, and the patient entered a colectomy state. We did not model specific colectomy related complications; instead, we assumed that patients in a colectomy state incurred ongoing long-term costs due to these complications based on observed data from a recent study of post-colectomy patients 19. Any state in the model could transition to a death state (due to natural causes, with rates obtained from National Center For Health Statistics life tables) 20. Treatment adherence was assumed to be 100% in the base-case analysis (and was varied widely in an exploratory analysis).
Strategies Modeled
We explored three strategies for management of UC: (1) a symptom-targeted treatment strategy, where 5-ASA medications were only used for symptomatic disease flares (SYMPT); (2) a continuous maintenance strategy, where continuous 5-ASA maintenance therapy was used in all patients with quiescent disease (CONT) (current standard of care); and, (3) an inflammation-targeted treatment strategy, where all patients underwent predictive stool testing every 3 months and those with a positive test were treated with a 3 month course of 5-ASA medication to potentially avert a symptomatic disease flare (INFLAM). Under the INFLAM strategy, we assumed that a subset of patients were destined to clinically flare within a 3 month period (22% probability in those on no therapy and 12% probability in those on 5-ASA, based on published data). Stool test sensitivity and specificity were then applied to determine what proportion of patients would have positive and negative stool tests, and inflammation-targeted treatment with 5-ASAs was applied accordingly. Due to a paucity of data on the topic, we did not assume a within-patient correlation of false negative or false positive results. An overview of treatment strategies is displayed in Figure 2.
Transition Probabilities
Probabilities were derived from published literature, with systematic reviews utilized if available. All probabilities were converted to three-month probabilities to match the cycle length of the model (Table 1).
Table 1. Clinical and Economic Variables.
| Variable | Estimate | Range* | Reference | |||
|---|---|---|---|---|---|---|
| Clinical Inputs | ||||||
| Probability of Disease Flare On Maintenance Therapy (q3 months) | ||||||
| No medical therapy | 0.22 | 0.19-0.25 (B) | 4 | |||
| 5-ASA | 0.12 | Calculated | 4 | |||
| Azathiopurine | 0.13 | 0.10-0.18 (B) | 21 | |||
| Infliximab | 0.21 | 0.17-0.26 (B) | 22 | |||
| Odds Ratios | ||||||
| Flare on 5-ASA maintenance therapy | 0.47 | 0.36-0.62 (N) | 4 | |||
| Probability of Response To Treatment During Active Disease | ||||||
| Intensive 5-ASA | 0.64 | 0.50-0.76 (B) | 23 | |||
| Corticosteroids (oral) | 0.84 | 0.50-0.92 (B) | 24 | |||
| Corticosteroids (intravenous) | 0.67 | 0.48-0.85 (B) | 25 | |||
| Infliximab | 0.70 | 0.50-0.90 (B) | 26, 27 | |||
| Utilities | ||||||
| Quiescent disease | 0.98 | 0.91-1.00 (B) | 36 | |||
| Active disease without hospitalization | 0.90 | 0.79-0.99 (B) | 36 | |||
| Active disease with hospitalization | 0.59 | 0.47-0.71 (B) | 1, 37 | |||
| Post-colectomy | 0.92 | 0.84-0.99 (B) | 36 | |||
| Maintenance medication (disutility) | 0.005 | 0-0.01 (U) | 17 | |||
| Stool testing (disutility) | 0.0025 | 0-0.01 (U) | 38 | |||
| Stool Test Diagnostic Test Characteristics | ||||||
| Sensitivity for impending flare | 0.77 | 0.5-1.0 (B) | 15 | |||
| Specificity for impending flare | 0.71 | 0.5-1.0 (B) | 15 | |||
| Death | ||||||
| Colectomy (peri-operative period) | 0.023 | 0.01-0.10 (B) | 30 | |||
| Non-colectomy death | Age dependent | Not varied | 20 | |||
| Economic Inputs | ||||||
| Outpatient Costs (2009 US Dollars) (per year) | ||||||
| Mesalamine (2.4 g po qD) | $3,408 | $1,704 - $6,816 (G) | 31 | |||
| Mesalamine (4 g pr qD) | $9,415 | $4,707 - $18,830 (G) | 31 | |||
| Azathioprine (200 mg po qD) | $961 | $480 - $1,922 (G) | 31 | |||
| Infliximab (5 mg/kg q8 weeks) | $19,800 | $9,900 - $39,600 (G) | 32 | |||
| Post-colectomy care (initial 6 months) | $26,660 | $13,330 - $53,320 (G) | 19 | |||
| Post-colectomy care (subsequent years) | $3,329 | $1,664 - $6,658 (G) | 19 | |||
| Stool test (q3 months) (annual cost) | $740 | $370 - $1,480 (G) | See text | |||
| Inpatient Costs (2009 US Dollars) | ||||||
| Hospitalization for disease flare (DRG 387) | $5,861 | $2,930 - $11,722 (G) | 33 | |||
| Infliximab 5 mg/kg induction (3 doses) | $9,900 | $4,950 - $19,800 (G) | 32 | |||
| Colectomy (ICD-9 code 45.8) | $25,623 | $12,811 - $51,246 (G) | 33 | |||
| Other | ||||||
| Prednisone (3 month taper) | $6 | $3 - $12 (G) | 31 | |||
| Outpatient physician visit (HCPCS 99214) | $69 | $34 - $138 (G) | 34 | |||
| Discount rate | 0.03 | 0.00-0.05 (B) | 39 | |||
B, G, N, and U signify type of distribution used for probabilistic sensitivity analysis (beta, gamma, normal, or uniform).
Quiescent Disease
The natural history of disease activity without 5-ASA therapy and the effectiveness of 5-ASA maintenance therapy were obtained from a 2006 Cochrane meta-analysis 4. Specifically, we extracted the 3-month probability of disease flare in the absence of 5-ASA therapy (0.22, range: 0.19 – 0.25) and the odds of disease flare during 5-ASA maintenance therapy (0.47, range: 0.36-0.62) (yielding a base-case probability of disease flare of 0.12 for patients using 5-ASA therapy). The probability of disease flare during AZA maintenance therapy was obtained from a 2007 Cochrane review on this topic (0.13, range: 0.10-0.18) 21. The probability of flare during infliximab maintenance therapy was obtained from a randomized controlled trial (ACT I) that enrolled patients who had failed corticosteroids +/- azathioprine (0.21, range: 0.17-0.26) 22.
Active Disease
The probability of response (induction of remission during flare) to intensive 5-ASA therapy was obtained from a 2005 study on this topic (0.64, range: 0.50-0.76) 23. The probability of response to oral corticosteroids was obtained from a 2001 retrospective cohort study of patients with ulcerative colitis (0.84, range: 0.50-0.92) 24. Finally, the probabilities of response to intravenous corticosteroids and infliximab were obtained from recent systematic reviews of studies enrolling patients who had failed usual outpatient disease management (0.67, range: 0.48-0.85 and 0.70, range: 0.50-0.90, respectively) 25-27.
Stool Tests
A variety of stool tests could be used to assess inflammation in patients with ulcerative colitis. Some of these tests, such as fecal leukocytes, are widely available and inexpensive but have limited accuracy 28, 29. Others, such as fecal calprotectin, are more expensive but have better predictive test characteristics 11, 15. Because fecal calprotectin has the most robust data supporting its use as a predictor of impending disease flare in inflammatory bowel disease patients, we used fecal calprotectin as the “prototype” stool test in our base-case analysis 11. Specifically, we assumed a stool test sensitivity of 77% and specificity of 71% based on results of a recent meta-analysis 15. In sensitivity analysis, these values were varied widely to examine the potential role of both less accurate tests (such as fecal leukocytes) and more accurate tests (such as future generation fecal calprotectin).
Probability of Death
Probability of death from natural causes was obtained from National Center for Health Statistics life tables and was age-dependent 20. Probability of death in the peri-operative period after colectomy was assumed to be 2.3% based on an analysis of data from the Nationwide Inpatient Sample 30.
Cost Inputs
The costs of outpatient medications were obtained from the 2009 Red Book (Table 1) 31. The cost of outpatient infliximab infusion was obtained from 2006 study that utilized insurance claims data 32. Costs of inpatient medications were generally assumed to be included in the total costs of hospitalization, described below. Finally, because fecal calprotectin is not specifically reimbursed by Medicare (see HCPCS code 83993), the cost of this test ($185) was obtained from our local hospital laboratory and was varied widely in an exploratory analysis.
The costs of medical care were obtained from the 2009 Medicare Physician Fee Schedule, the Healthcare Cost and Utilization Project (HCUP), and published literature on long-term costs after colectomy in patients with UC 19, 33-35. Specifically, the cost of an outpatient physician visit (beyond the cost of medications) was assumed to be $69 (HCPCS code 99214, facility price for outpatient visit) 34. The cost of hospitalization for a course of intravenous corticosteroids was assumed to be $5,861 (2008 mean hospital cost for diagnosis related group (DRG) 387 for inflammatory bowel disease) 33. Patients with steroid refractory disease (requiring infliximab) incurred additional costs for ongoing hospitalization (HCPCS 99232 for 7 days) and infliximab induction 32, 34. For the in-hospital cost of colectomy, we assumed the mean cost for ICD-9 code 45.8 (total colectomy) in patients between ages 18-44, yielding a value of $25,623 33. This value was corroborated by a recent study that reported hospital charges of $49,739 for colectomy with ileal pouch anal anastamosis (IPAA) 35. Finally, long-term post-colectomy costs were obtained from a recent study by Holubar and colleagues in which authors assessed immediate and long-term costs associated with colectomy with IPAA 19. Specifically, the combined direct costs of surgery and the 6 month period following surgery were reported to total $52,283 per patient. We subtracted the cost of colectomy obtained from HCUP from this value, yielding an initial 6 month “recovery period” cost of $26,660 after colectomy with IPAA. The mean total cost in the 2 years after recovery from surgery in this study was $6,659, yielding an annual long-term cost of $3,329 after colectomy with IPAA 19.
We did not specify additional costs related to routine laboratory monitoring, though these relatively small expenditures were addressed within the ranges of our sensitivity analyses. All costs were varied between half and twice the base-case value in sensitivity analysis (Table 1). Costs were also discounted at a rate of 3% per year and adjusted for inflation to 2009 U.S. dollars based on the Consumer Price Index (CPI).
Utility Valuations
Health state utility valuations were obtained from published studies in patients with inflammatory bowel disease that used the time trade-off technique. Specifically, we assumed utilities of 0.98, 0.90, and 0.92 for quiescent disease, active disease not requiring hospitalization, and the post-colectomy state. These values were obtained from a recent study examining health state utilities in patients with UC 36. The utility of active disease requiring hospitalization was obtained from two studies of patients with severe UC awaiting colectomy (0.59, range: 0.47-0.71) 1, 37.
To model the decrease in quality of life associated with chronic medication use, we assumed a small disutility of 0.005 in patients taking 5-ASA maintenance therapy, similar to that used by Hayward in patients taking high-dose statins 17. Notably, patients with UC have repeatedly been shown to discontinue their maintenance medications in remission, suggesting an inherent disutility of these medications 5, 9. Similarly, because stool testing requires collection of stool by the patient (which is time consuming and potentially unpleasant), we also incorporated a small disutility for stool testing. Specifically, we conservatively assumed that one “stool testing day” (when a stool sample was collected for the stool test, for a total of 4 days per year) was similar to one day of life with severe back pain (utility = 0.79), yielding an annual stool testing disutility of 0.0025 38. Because the true values of these disutilities are not well defined, base-case assumptions were widely varied in sensitivity analysis (from zero to 0.01). All utilities and disutilities were discounted at a rate of 3% per year 39.
Clinical and Economic Outcomes
For each strategy, clinical and economic outcomes were measured and reported. Clinical outcomes included: (1) average number of flares per patient; (2) proportion of patients who had undergone colectomy; (3) proportion of simulation cycles where asymptomatic patients were assigned 5-ASA therapy; and, (4) quality-adjusted life-years (QALYs). Economic outcomes included costs and incremental cost-effectiveness ratios (ICERs).
Model Validation
To validate that our model output was predictive of observed data, we compared our results with those of long-term observational studies of the natural history of treated mild to moderate UC 40-42. Though natural history studies have not traditionally reported the volume and frequency of disease flares, these studies do commonly report on the proportion of patients undergoing colectomy, with 5-year cumulative rates ranging from 7.5% in adult patients 41 to 24% in older pediatric patients 40. We compared these observed colectomy rates to the colectomy rates predicted by our model.
Sensitivity Analysis
Sensitivity analysis was used to identify variables that had important effects on the choice of optimal management strategy. One-way sensitivity analyses were performed on each variable in the model. Two-way sensitivity analyses were performed using variables identified in one-way analyses. Probabilistic sensitivity analysis was also performed, where 35 variables were simultaneously varied over their sensitivity analysis ranges according to specified probability distributions (10,000 Monte Carlo trials). Beta, gamma, and normal distributions were assumed for proportions, costs, and log odds ratios, respectively 43. For disutilities (for which the distribution is undefined), we assumed a uniform distribution. For each distribution, we assumed that the mean was equal to the point estimate and that the standard deviation was equal to the sensitivity analysis range / [ 2 ×1.96]. Acceptability curves were generated for each strategy across a wide range of willingness-to-pay (WTP) thresholds ($0 to $100,000) using net health benefit calculations.
Results
Model Validation
The model predicted 5-year cumulative colectomy rates ranging from 10.6% (for continuous maintenance therapy (CONT) with 100% adherence) to 24.4% (symptom-targeted treatment (SYMPT)). Assuming a realistic adherence rate of 60% 9, 41, the model predicted a 5-year colectomy rate of 16.2% (95% CI: 7.8%-27.2%) under the CONT strategy, a value between the observed colectomy rates for adult and older pediatric patients (7.5% and 24%, respectively) 40, 41.
Base-Case Analysis
Results of the base-case analysis are shown in Table 2. The CONT strategy resulted in the fewest flares (2.14 per patient) and the lowest cumulative colectomy rate of the three strategies (10.6%), albeit at the expense of maximum exposure to 5-ASA agents (mean exposure time 55 months). This strategy resulted in 4.5014 QALYs per patient per 5 years. The SYMPT strategy yielded the most flares (3.49 per patient) and the highest colectomy rate (24.4%) of the various strategies. However, by avoiding the disutility of chronic medication use incurred by those under the CONT strategy, this strategy resulted in the same number of QALYs per patient as the CONT strategy.
Table 2. Base-Case Analysis: Clinical Outcomes Under Each Treatment Strategy (Cumulative Over 5 Years).
| Strategy | Moderate Flares per Patient | Severe Flares per Patient | Colectomy Rate | Average 5-ASA Exposure (months) | QALYs | Cost | ICER |
|---|---|---|---|---|---|---|---|
| Inflammation-Targeted Therapy (INFLAM)** | 2.35 | 0.14 | 13.6% | 24 (69%*) | 4.4986 | $22,798 | - |
| Symptom-Targeted Therapy (SYMPT) | 3.29 | 0.20 | 24.4% | 10 (0%) | 4.5014 | $24,378 | $575,894 |
| Continuous Maintenance Therapy (CONT) | 2.02 | 0.12 | 10.6% | 55 (88%) | 4.5014 | $25,621 | Dominated |
Proportion of 5-ASA exposure that occurred during disease quiescence.
Under the INFLAM strategy, 35% of stool tests were positive and led to 5-ASA therapy.
The inflammation-targeted treatment (INFLAM) strategy resulted in 2.49 flares per patient and a colectomy rate of 13.6%. Though these values were slightly greater than those observed under the CONT strategy, patients were exposed to 5-ASA agents for only 24 months on average under the INFLAM strategy (a 56% reduction compared to 55 months under the CONT strategy). As a result of this substantial improvement in 5-ASA efficiency, INFLAM resulted in a similar number of QALYs per patient to the other two strategies (4.4986 versus 4.5014 QALYs per patient per 5 years) at lower cost, making INFLAM the optimal strategy (i.e., SYMPT and CONT were cost-ineffective compared to INFLAM) (Table 2).
Sensitivity Analysis
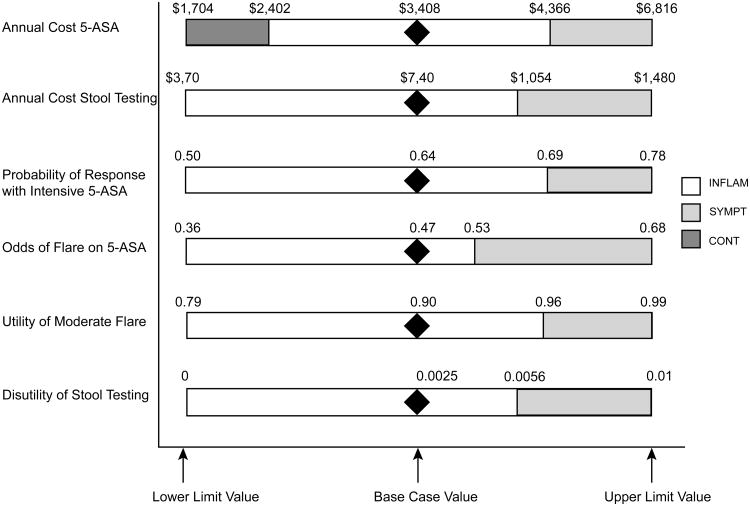
In one-way sensitivity analysis, several variables emerged as having potentially important effects on the choice of optimal strategy (WTP = $100,000 per QALY) (Figure 3): (1) the annual cost of oral mesalamine (2.4 g po qday); (2) the annual cost of stool testing; (3) the effectiveness of 5-ASA maintenance therapy (expressed as an odds ratio); (4) probability of response to treatment with intensive 5-ASA (high-dose oral and rectal mesalamine) during active disease; (5) the utility of active disease without hospitalization; and, (6) the disutility of stool testing.
Figure 3. Variables Found to be Important in One-Way Sensitivity Analysis.
Diamond indicates base-case value for given variable. White region indicates values for which INFLAM is the optimal strategy. Light gray region indicates values for which SYMPT is the optimal strategy. Dark gray region indicates values for which CONT is the optimal strategy.
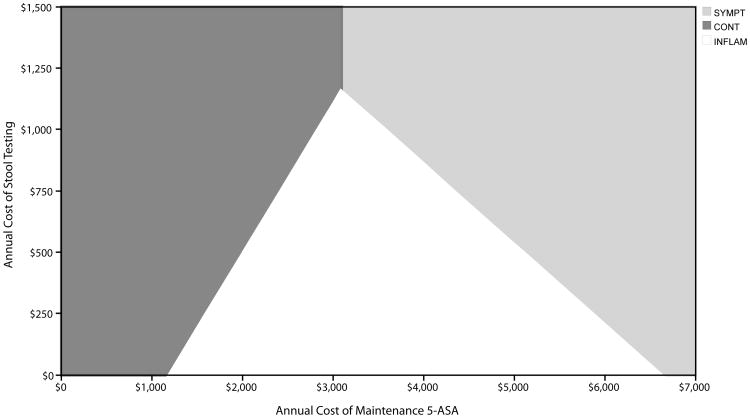
First, the optimal strategy varied across assumptions about the annual cost of mesalamine (base = $3,408), with CONT favored at costs less than $2,402 per year, INFLAM favored at costs between $2,402 and $4,366 per year, and SYMPT favored at costs greater than $4,366 per year. Similarly, the annual cost of stool testing also had important effects, with INFLAM being the optimal strategy unless the cost exceeded $1,054 per year. A two-way sensitivity analysis on these variables is shown in Figure 4.
Figure 4. Two-Way Sensitivity Analysis on Cost of 5-ASA Maintenance Medication and Cost of Stool Testing.
White region indicates values for which INFLAM is the optimal strategy. Light gray region indicates values for which SYMPT is the optimal strategy. Dark gray region indicates values for which CONT is the optimal strategy.
Two variables related to effectiveness of 5-ASA therapy had important effects on choice of optimal strategy. Namely, as 5-ASA therapy became more effective (odds of flare < 0.53), INFLAM was the favored strategy, compared to SYMPT when odds of flare was > 0.53 (Figure 3). Similarly, the effectiveness of intensive 5-ASA therapy for aborting an active disease flare also had important effects, with INFLAM favored when the probability of remission with intensive therapy was < 0.69 (and SYMPT favored when the probability was > 0.69) (Figure 3).
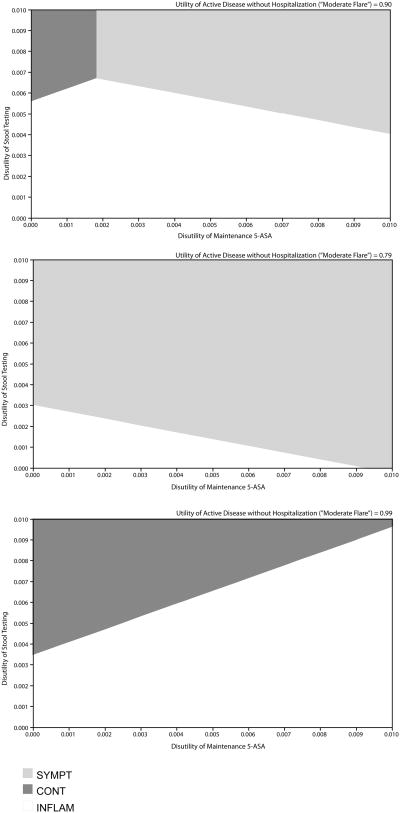
Finally, variables related to the utility and disutility of UC disease states and treatments also had important effects. Specifically, as the disutility of stool testing increased to > 0.006 (base = 0.0025), the SYMPT strategy was favored (and INFLAM was favored when this disutility was < 0.006). The utility of active disease without hospitalization (“moderate flare” state) also impacted the choice of optimal strategy, with SYMPT favored when the utility was > 0.96 (base = 0.90) and INFLAM favored when this variable was < 0.96. In addition, the choice of strategy was further affected by the disutility of maintenance medication. A three-way sensitivity on these variables is shown in Figure 5.
Figure 5. Three-Way Sensitivity Analysis on Disutility of 5-ASA Maintenance Medication, Disutility of Stool Testing, and Utility of Moderate Disease Flare.
White region indicates values for which INFLAM is the optimal strategy. Light gray region indicates values for which SYMPT is the optimal strategy. Dark gray region indicates values for which CONT is the optimal strategy.
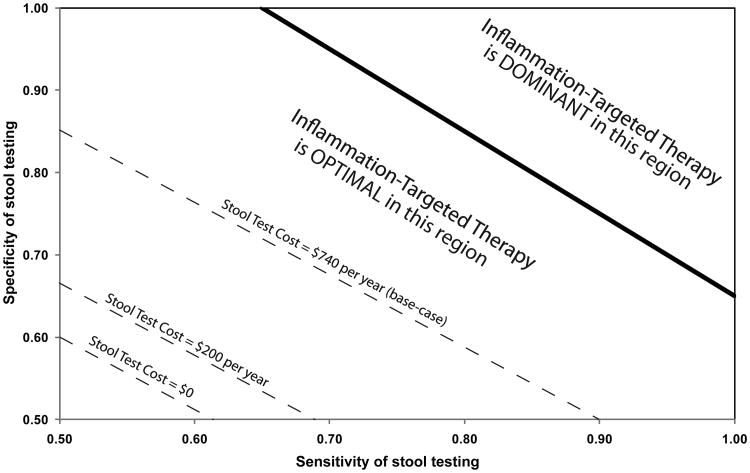
In an exploratory analysis, we assessed the impact of the cost, sensitivity, and specificity of stool testing. Under base-case assumptions for test sensitivity and specificity (77% and 71%, respectively), we found that INFLAM remained the optimal strategy unless predictive stool testing cost more than $1,054 per year ($264 per test) (base: $740 per year, or $185 per test). At lower stool testing costs, INFLAM was optimal even if the sensitivity and specificity of stool testing were reduced below base case values (Figure 6). Specifically, at the base-case stool testing cost of $740 per year, INFLAM was the optimal strategy when test sensitivity and specificity were each at least 70%. At a stool testing cost of $200 per year (the approximate cost of fecal leukocytes at our local hospital laboratory), INFLAM was optimal when test sensitivity and specificity were each at least 60% (the approximate sensitivity and specificity for fecal leukocytes reported in a prior meta-analysis) 44. If stool testing cost $80 per year (or $20 per test, comparable to a home pregnancy test, which uses similar technology), INFLAM was optimal if the sensitivity and specificity of stool testing were each at least 57%. Finally, if stool testing were free (cost = $0), INFLAM was optimal provided that the sensitivity and specificity of stool testing were each at least 55%. Below these values, INFLAM was no longer an optimal strategy compared to CONT and SYMPT. Finally, if sensitivity and specificity were high (∼ 85% each), INFLAM became a dominant strategy (meaning that it cost less and was more effective than other strategies).
Figure 6. Two-Way Sensitivity Analysis on Stool Test Sensitivity and Specificity.
The dark gray area at the upper right indicates the sensitivity/specificity ranges over which inflammation-targeted therapy (INFLAM) was dominant over other strategies. The light gray area (center) indicates the sensitivity/specificity ranges for which INFLAM was the optimal strategy (i.e., the cost of other strategies exceeded $100,000 per QALY). The dark gray area at the bottom left indicates the sensitivity/specificity ranges where INFLAM was no longer the optimal strategy, with the threshold differing depending on the cost of stool testing (dashed lines).
Probabilistic sensitivity analysis (multivariable Monte Carlo selection of model parameters) corroborated these findings, finding that INFLAM or SYMPT was the favored strategy in 78% of iterations, while CONT (the current standard of care) was favored in only 22% of iterations. Additionally, incorporating realistic rates of 5-ASA non-adherence and/or stool testing non-adherence had no effect on which strategy was optimal.
Discussion
Patients with newly diagnosed mild to moderate UC are commonly treated with 5-ASA compounds to maintain disease remission. These medications, though effective, are costly and carry a small risk of adverse events, such as acute interstitial nephritis. Less costly drugs, such as sulfasalazine, are poorly tolerated in up to 30% of patients and may require frequent dosing 8, making 5-ASA agents the maintenance treatment of choice in both the United States and Europe 10. In this study, we found that inflammation-targeted therapy (INFLAM) was the optimal strategy under base-case assumptions, resulting in similar outcomes to the other modeled strategies but at lower cost, primarily due to less use of 5-ASA medications. However, if a patient had low aversion to disease flares and stool testing, and had high aversion to taking daily medication for flare prevention, SYMPT was the favored strategy. Furthermore, SYMPT was also favored if the cost of maintenance 5-ASA and/or stool testing were high, or if effectiveness of maintenance 5-ASA was low and/or effectiveness of intensive 5-ASA “rescue” therapy was high. Probabilistic sensitivity analysis found that INFLAM and SYMPT were optimal in 40% and 38% of trials, respectively. Therefore, based on our current knowledge of treatment costs and effectiveness, the choice of which of these strategies is optimal for a given patient appears to largely be driven by patient preference. Notably, under most circumstances, CONT (current standard of care) was not the optimal strategy. Exceptions include situations when the cost of 5-ASA is low (such as with sulfasalazine) or when a patient has a strong aversion to disease flares and stool testing, and has little aversion to taking daily medication for flare prevention.
Studies on the use of targeted therapy in inflammatory bowel disease and other high expense, low prevalence (HELP) diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus) are limited. However, the paradigm of targeted therapy has been widely advocated in the management of chronic diseases and preventive care. Multiple studies have shown that targeted, benefit-based treatment has the potential to enhance the efficiency of care, improving clinical outcomes while appropriately limiting costs and resource utilization. In a recent modeling study, Hayward and colleagues demonstrated that targeting statin therapy for primary prevention to patients based on overall cardiovascular risk rather than lipid profile produced better outcomes with less statin use 17. Similarly, Saini and colleagues demonstrated that targeting surveillance colonoscopy to patients at high risk for colorectal cancer was a safe and highly cost-effective preventive strategy compared to untargeted surveillance in all patients with colorectal adenomas 16. Finally, Zulman and colleagues demonstrated that targeted prevention for cardiovascular disease was preferred to universal, population-based prevention when the preventive strategy carried even a minimal disutility 45.
Our study, building on this work and the work of other investigators, demonstrates that inflammation-targeted therapy in UC has the potential to be cost-effective while achieving similar quality of life and comparable rates of disease flare and colectomy when compared to those on either universal, untargeted continuous maintenance therapy or on symptomatic treatment. Though not a primary outcome in our study, the decrease in overall medication usage under an inflammation-targeted strategy (56% reduction) could also reduce both costs to the patient (through reduced copayments) and medication-related adverse events.
Several important limitations of our study should be highlighted. First, our model is limited by the logic and assumptions of the model. However, these assumptions were tested and were robust in multiple sensitivity analyses. We also validated our model by comparing predicted colectomy rates to observed colectomy rates, finding that our model was consistent with real-world observations. Though we focused on short-term (5 year) outcomes and did not model potential long-term benefits of 5-ASA medications, such as the controversial possibility of colorectal cancer prevention 46, studies suggest that any chemopreventive effects of 5-ASAs are mediated through treatment of inflammation and the prevention of flares (the aim of our targeted approach) 47. Furthermore, carefully done population-based studies have found no reduction in colorectalcancer in patients using 5-ASA medications 48. Second, data on the disutility of stool testing and the effectiveness of serial stool testing to predict and prevent disease flares are limited. However, we were conservative in our base-case disutility estimate.
In summary, our study shows that inflammation-targeted treatment with 5-ASA in patients with mild to moderate ulcerative colitis may be an optimal strategy compared to continuous maintenance or symptom-targeted therapy. Such an approach appropriately limits medication exposure and costs while achieving similar or better outcomes than the more costly continuous maintenance approach that is the current standard of care. Our results were robust to a wide range of assumptions in sensitivity analysis. Future studies should prospectively examine the clinical and economic effects of inflammation-targeted therapy in patients with ulcerative colitis.
Acknowledgments
Dr. Saini's research is funded by a VA HSR&D CDA-2 Career Development Award. Dr. Waljee's research is supported by grant number UL1RR024986 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, the NCRR, or the National Institutes of Health.
Abbreviations
- UC
Ulcerative colitis
- 5-ASA
5-aminosalicylic acid
- QALY
quality-adjusted life year
- ICER
incremental cost-effectiveness ratio
- WTP
willingness to pay
Footnotes
The authors have no relevant financial disclosures (no conflicts of interest exist). All authors participated in study concept and design, data analysis, and drafting and revision of the manuscript.
References
- 1.Muir AJ, Edwards LJ, Sanders LL, Bollinger RR, Koruda MJ, Bachwich DR, Provenzale D. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:1480–5. doi: 10.1111/j.1572-0241.2001.03801.x. [DOI] [PubMed] [Google Scholar]
- 2.Sewell JL, Yee HF, Jr, Inadomi JM. Hospitalizations are increasing among minority patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2010;16:204–7. doi: 10.1002/ibd.21008. [DOI] [PubMed] [Google Scholar]
- 3.Gibson TB, Ng E, Ozminkowski RJ, Wang S, Burton WN, Goetzel RZ, Maclean R. The direct and indirect cost burden of Crohn's disease and ulcerative colitis. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2008;50:1261–72. doi: 10.1097/JOM.0b013e318181b8ca. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006:CD000544. doi: 10.1002/14651858.CD000544.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Higgins PD, Rubin DT, Kaulback K, Schoenfield PS, Kane SV. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Alimentary pharmacology & therapeutics. 2009;29:247–57. doi: 10.1111/j.1365-2036.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DT, Siegel CA, Kane SV, Binion DG, Panaccione R, Dubinsky MC, Loftus EV, Hopper J. Impact of ulcerative colitis from patients' and physicians' perspectives: Results from the UC: NORMAL survey. Inflamm Bowel Dis. 2009;15:581–8. doi: 10.1002/ibd.20793. [DOI] [PubMed] [Google Scholar]
- 7.Yen EF, Kane SV, Ladabaum U. Cost-effectiveness of 5-aminosalicylic acid therapy for maintenance of remission in ulcerative colitis. Am J Gastroenterol. 2008;103:3094–105. doi: 10.1111/j.1572-0241.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen OH. Sulfasalazine intolerance. A retrospective survey of the reasons for discontinuing treatment with sulfasalazine in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1982;17:389–93. doi: 10.3109/00365528209182073. [DOI] [PubMed] [Google Scholar]
- 9.Kane SV, Accortt NA, Magowan S, Brixner D. Predictors of persistence with 5-aminosalicylic acid therapy for ulcerative colitis. Aliment Pharmacol Ther. 2009;29:855–62. doi: 10.1111/j.1365-2036.2009.03941.x. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed March 16, 2011]; www.shire.com/shireplc/uploads/report/10K.
- 11.Gisbert JP, Bermejo F, Perez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, Lopez P, Lopez-Palacios N, Calvo M, Gonzalez-Lama Y, Carneros JA, Velasco M, Mate J. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–8. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 12.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1746–54. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 13.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364–8. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22861. [DOI] [PubMed] [Google Scholar]
- 16.Saini SD, Schoenfeld P, Vijan S. Surveillance colonoscopy is cost-effective for patients with adenomas who are at high risk of colorectal cancer. Gastroenterology. 2010;138:2292–9. 2299 e1. doi: 10.1053/j.gastro.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Annals of internal medicine. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 18.Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, Zerbib F, Nachury M, Savoye G, Moreau J, Delchier JC, Ricart E, Cosnes J, Román ALS, Dewit O, Carbonnel F, Coffin B, Assche GAV, Esteve M, Färkkilä MA, Gisbert JP, Bommelaer G, Marteau P, Nahon S, Vos MD, Franchimont D, Mary JY, Colombel JF, Lémann M. Cyclosporin versus infliximab in severe acute ulcerative colitis refractory to intravenous steroids: a randomized trial. Gastroenterology. 2011;140:S–112. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 19.Holubar SD, Long KH, Loftus EV, Jr, Wolff BG, Pemberton JH, Cima RR. Long-term direct costs before and after proctocolectomy for ulcerative colitis: a population-based study in Olmsted County, Minnesota. Diseases of the colon and rectum. 2009;52:1815–23. doi: 10.1007/DCR.0b013e3181b327a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias E. National vital statistics reports. Vol. 56. National Center for Health Statistics; 2007. United States life tables, 2004. [PubMed] [Google Scholar]
- 21.Timmer A, McDonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane database of systematic reviews. 2007:CD000478. doi: 10.1002/14651858.CD000478.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 23.Marteau P, Probert CS, Lindgren S, Gassul M, Tan TG, Dignass A, Befrits R, Midhagen G, Rademaker J, Foldager M. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54:960–5. doi: 10.1136/gut.2004.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 25.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2007;5:103–10. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Shibolet O, Regushevskaya E, Brezis M, Soares-Weiser K. Cyclosporine A for induction of remission in severe ulcerative colitis. Cochrane database of systematic reviews. 2005:CD004277. doi: 10.1002/14651858.CD004277.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane database of systematic reviews. 2006;3:CD005112. doi: 10.1002/14651858.CD005112.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Seva-Pereira A, Franco AO, de Magalhaes AF. Diagnostic value of fecal leukocytes in chronic bowel diseases. Sao Paulo Med J. 1994;112:504–6. doi: 10.1590/s1516-31801994000100006. [DOI] [PubMed] [Google Scholar]
- 29.Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Diseases of the colon and rectum. 2007;50:1697–706. doi: 10.1007/s10350-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134:680–7. doi: 10.1053/j.gastro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Red Book. Thomson Healthcare Inc.; 2009. [Google Scholar]
- 32.Ollendorf DA, Lidsky L. Infliximab drug and infusion costs among patients with Crohn's disease in a commercially-insured setting. Am J Ther. 2006;13:502–6. doi: 10.1097/01.mjt.0000245223.43783.45. [DOI] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) [Accessed March 1, 2011]; http://hcupnet.ahrq.gov/ [PubMed]
- 34.Centers for Medicare and Medicaid Services. Physician Fee Schedule Lookup. [Accessed March 1, 2011]; http://www.cms.hhs.gov/PFSlookup/
- 35.Loftus EV, Jr, Friedman HS, Delgado DJ, Sandborn WJ. Colectomy subtypes, follow-up surgical procedures, postsurgical complications, and medical charges among ulcerative colitis patients with private health insurance in the United States. Inflamm Bowel Dis. 2009;15:566–75. doi: 10.1002/ibd.20810. [DOI] [PubMed] [Google Scholar]
- 36.Waljee AK, Higgins PD, Waljee JF, Tujios SR, Saxena A, Brown LK, Chaudhary MN, Morris AM. Perceived and actual quality of life with ulcerative colitis: a comparison of medically and surgically treated patients. Am J Gastroenterol. 2011;106:794–9. doi: 10.1038/ajg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLeod RS, Churchill DN, Lock AM, Vanderburgh S, Cohen Z. Quality of life of patients with ulcerative colitis preoperatively and postoperatively. Gastroenterology. 1991;101:1307–13. doi: 10.1016/0016-5085(91)90081-u. [DOI] [PubMed] [Google Scholar]
- 38.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, Martin PA. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 39.Gold MR. Cost-effectiveness in health and medicine. Oxford University Press; 1996. [Google Scholar]
- 40.Gower-Rousseau C, Dauchet L, Vernier-Massouille G, Tilloy E, Brazier F, Merle V, Dupas JL, Savoye G, Balde M, Marti R, Lerebours E, Cortot A, Salomez JL, Turck D, Colombel JF. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104:2080–8. doi: 10.1038/ajg.2009.177. [DOI] [PubMed] [Google Scholar]
- 41.Henriksen M, Jahnsen J, Lygren I, Sauar J, Kjellevold O, Schulz T, Vatn MH, Moum B. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study) Inflamm Bowel Dis. 2006;12:543–50. doi: 10.1097/01.MIB.0000225339.91484.fc. [DOI] [PubMed] [Google Scholar]
- 42.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 43.Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford University Press; 2006. [Google Scholar]
- 44.Huicho L, Campos M, Rivera J, Guerrant RL. Fecal screening tests in the approach to acute infectious diarrhea: a scientific overview. Pediatr Infect Dis J. 1996;15:486–94. doi: 10.1097/00006454-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Zulman DM, Vijan S, Omenn GS, Hayward RA. The relative merits of population-based and targeted prevention strategies. Milbank Q. 2008;86:557–80. doi: 10.1111/j.1468-0009.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubenstein JH, Waljee AK, Jeter JM, Velayos FS, Ladabaum U, Higgins PD. Cost effectiveness of ulcerative colitis surveillance in the setting of 5-aminosalicylates. Am J Gastroenterol. 2009;104:2222–32. doi: 10.1038/ajg.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein CN, Nugent Z, Blanchard JF. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. Am J Gastroenterol. 2011;106:731–6. doi: 10.1038/ajg.2011.50. [DOI] [PubMed] [Google Scholar]