Abstract
Processing motor errors is essential for on-line control of goal-directed movements and motor learning. Evidence from psychophysical and imaging studies supports the long standing view that error processing is central to cerebellar function. The dominant view is that error related signals are encoded in the complex spike discharge of Purkinje cells. However, the findings are inconsistent on whether complex spike activity correlates with motor errors. Recently, we examined if simple spike firing carries error signals in monkeys trained to manually track a randomly moving target. The task requires continuous processing of motor errors characterized by the relative movements between the hand-driven cursor and the target center. Linear regression models show that error parameters are robustly represented in the simple spike activity of most Purkinje cells. At the single cell level, the error signals are encoded independently and integrated with kinematic signals. In a large majority of Purkinje cells, correlation strengths between the simple spike discharge and an error parameter have bimodal profiles with respect to time, exhibiting a local maxima corresponding to firing leading the behavior and another one corresponding to firing lagging behavior. The bimodal temporal profiles suggest that individual error parameters are dually encoded as both an internal prediction used for feedback-independent, compensatory movements and the actual sensory feedback used to monitor performance. Approximately 75% of the dual representations have opposing modulations of the simple spike activity, one increasing firing and the other depressing firing, as reflected by the reversed signs of the regression coefficients corresponding to the local maxima of the R2 profile. These dual representations of individual parameters with opposing modulation of the simple spike firing are consistent with the signals needed to generate sensory prediction errors used to update an internal model.
Introduction
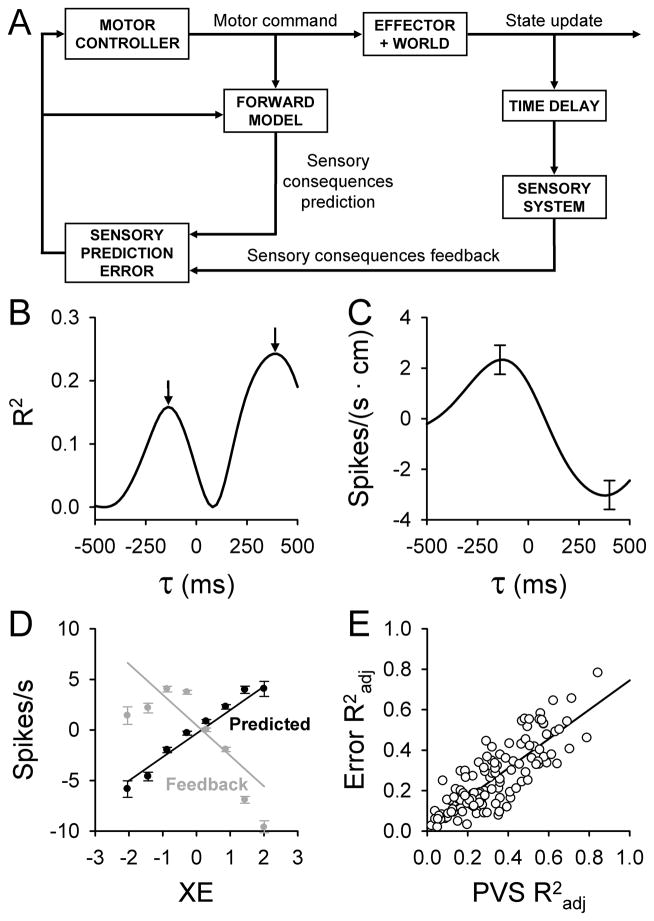
Both motor adaptation and on-line control of goal-directed movements require detecting and correcting performance errors [1, 2]. Notably, compensatory corrections for errors occur before or in the absence of sensory feedback (see review [1]). This suggests the central nervous system computes internal predictions of upcoming errors by implementing internal models. The key aspect of a forward internal model is that it predicts the sensory consequences of motor commands (Fig. 1A). These internal predictions are compared with actual sensory feedback to compute sensory prediction errors (Fig. 1A) used for motor control and learning [1–4].
Figure 1.
A) Schematic of motor control based on a forward internal model and sensory prediction errors. Adapted from [12]. B – C) Example temporal R2 and regression coefficient profiles as a function of lead/lag (τ) for an individual error parameter (XE) from a single Purkinje cell. Error bars in C represent the confidence intervals at the times of the local maxima in B. D) Plots of the simple spike modulation with XE at the times of the local maxima in B (black = prediction, gray = feedback). E) Correlation between maximal R2adj values computed by fitting firing residuals (kinematic variability removed) to the error model (ER) versus maximal R2adj values computed by fitting firing residuals (error variability removed) to the kinematic model (PVS). B, C, and D are adapted with permission from [11].
The cerebellum has been implicated as the substrate for a forward internal model [1, 3, 4], but whether cerebellar neurons provide the necessary predictive and feedback error signals remains unknown. Psychophysical, patient and imaging results suggest cerebellar involvement in motor error processing [5, 6]. The dominant view is that Purkinje cell complex spike discharge signals motor errors [7, 8]. However, this concept is not universally accepted (see review [9]), and there is no evidence showing that complex spikes encode predictive signals. Alternatively, a less examined hypothesis is that errors are encoded in the simple spike activity.
Simple spike activity both predicts and conveys motor errors
To test if Purkinje cell simple spike discharge encodes performance errors, monkeys were trained to manually track a randomly moving target using a planar manipulandum [10, 11]. Successful tracking requires that animals compensate for errors induced by unexpected changes in target kinematics. Four performance error measures describe cursor movements relative to the target center and include position (XE, YE), distance (i.e., radial error, RE) and direction (i.e., position direction error, PDE) errors. PDE indicates the relative direction the hand should move to return to the target center. Behavior analyses demonstrate that the monkeys continuously use these (or analogous) error parameters to position the cursor in the target center [10, 11].
In evaluating single error parameter encoding, it is essential that each error signal is independent from other error and kinematic signals found in the simple spike firing [10]. We determined the firing residuals for each error parameter (e.g. XE) by removing variability associated with the kinematics and remaining error parameters (e.g. YE, RE and PDE) from the simple spike discharge [11]. These residuals were regressed against the associated error parameter (e.g. XE). Correlation strength between the firing residuals and each error parameter was evaluated as a function of lead/lag (τ) from −500 to 500 ms. Negative τ values indicate neural signals leading or predicting behavior, while positive values are consistent with encoding sensory feedback. Similar regression analyses using the actual firing produced almost identical results [11], demonstrating independence of the individual error signals.
Regression results reveal two remarkable features of simple spike error encoding [11]. First, the correlation of simple spike firing with individual error parameters (e.g. XE in Fig. 1B) exhibits a bimodal R2 profile, with two local maxima located at both predictive and feedback timing. Similar bimodal profiles exist for 72% of the Purkinje cells, suggesting that individual cells impart both predictive and feedback information about an error parameter. Decoding analyses confirm the simple spike discharge conveys highly accurate predictions of the upcoming errors [11], consistent with the expected output of a forward internal model. Secondly, regression coefficients for the predictive and feedback maxima reverse sign (Fig. 1C). The sign change reflects opposing modulation so that the predictive and feedback representations of the same error parameter counter each other, one signal increasing and the other decreasing the simple spike firing. Analysis of the firing discharge at the times of the two maxima reveals highly anti-correlated firing patterns (Fig. 1D) [11]. These anti-correlated signals are precisely what is needed to compute sensory prediction errors (Fig. 1A) [2, 12].
Purkinje cells encode both error signals and kinematic signals
In addition to encoding robust error signals, firing from the same cells also modulates with movement kinematics [10]. This raises the question of how different signals are simultaneously encoded in a single cell. First, we fit simple spike firing residuals in which error variability was removed to a multi-linear regression model with terms for position, velocity, and speed (PVS model). A second analysis fit the firing residuals with PVS kinematic variability removed to a multi-linear model based on the four error parameters (ER model). Summing the R2 temporal profiles for individual kinematic or error parameters yields the same result as the PVS or ER regression models, respectively, demonstrating mutual independence of the parameters [10, 11]. The linear relationship between the ER model and PVS model R2adj values (Fig. 1E) shows that Purkinje cells are not functionally segregated by signal type. Across the range of R2 values, simple spike firing is similarly modulated by both error and kinematic signals, confirming that the two classes of information are represented together within a single cell. Presumably, a Purkinje cell’s capacity to carry numerous signals arises from its large number of parallel fiber inputs and unique morphology and physiology.
Kinematic signals are stable across a range of movements and conditions and are viewed as the output of a forward internal model of the limb [10, 13]. Error signals likely represent outputs of an additional feed-forward internal model that drives task-specific on-line corrections and learning. The presence of both kinematic and error signals may appear redundant in an internal model framework focused mainly on error processing (Fig. 1A). However, optimal feedback control theory [14] suggests that signal redundancy could improve the accuracy of goal-directed movements using the variability present in the motor system.
Implications of predictive and feedback error signals
Finding rich performance error representations in the simple spike discharge brings into question the information carried by complex spikes. Evidence for complex spike error signaling is inconsistent. Several studies document error-related complex spike modulation [7, 8], while others fail to show reliable error responses (see [9]). Because complex spikes have limited bandwidth due to low frequency discharge and occur only in response to errors, they are unlikely to convey the high resolution, predictive error signals described for the simple spike firing. One possibility is that complex spikes provide a critical signal needed for engaging synaptic plasticity that updates internal models [15].
The presence of kinematic and error signals in the simple spike firing of Purkinje cells conveys predictions about the state of the effector and motor performance (Fig. 1A) to the many output targets of the cerebellum including the cerebral cortex. These signals are hypothesized to optimize motor commands [1–4, 12]. Furthermore, dual signals could facilitate direct comparison between internal predictions and sensory feedback representations of the same motor parameter within a single Purkinje cell. An intriguing possibility is whether the cerebellum provides similar signals in the cognitive domain, although this remains to be investigated. However, the observations do not fit perfectly into the proposed computational framework for forward internal model implementation (Fig. 1A). For example, the predictive and feedback error signals are separated in time, raising the question of if and where the two signals are compared. Individual Purkinje cells continuously encode a large number of motor and error signals, suggesting that downstream decoding of specific signals is exceedingly complicated. Understanding how these signals are modified during learning, then are transformed and compared within and downstream of the Purkinje cells will be critical to further testing the role of the cerebellum in motor control.
Acknowledgments
We wish to thank Lijuan Zhou and Michael McPhee for technical and graphics support and Kris Bettin for manuscript preparation. Supported in part by NIH grants NS18338, NS062158, GM 008244 and NS071686.
Footnotes
Conflict of Interest
There are no current or potential conflicts of interest for the three authors, Laurentiu S. Popa, Angela L. Hewitt and Timothy J. Ebner
Reference List
- 1.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 2.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3 (Suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- 3.Kawato M, Wolpert D. Internal models for motor control. Novartis Found Symp. 1998;218:291–304. doi: 10.1002/9780470515563.ch16. [DOI] [PubMed] [Google Scholar]
- 4.Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 5.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 8.Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–497. doi: 10.1038/33141. [DOI] [PubMed] [Google Scholar]
- 9.Ebner TJ, Hewitt A, Popa L. What features of movements are encoded in the discharge of cerebellar neurons during limb movements? Cerebellum. 2011;10:683–693. doi: 10.1007/s12311-010-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt A, Popa L, Pasalar S, Hendrix CM, Ebner TJ. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol. 2011;106:2232–2247. doi: 10.1152/jn.00886.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popa LS, Hewitt AL, Ebner TJ. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci. 2012;32:15345–15358. doi: 10.1523/JNEUROSCI.2151-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- 14.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nature Neuroscience. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 15.Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. J Neurophysiol. 2012;108:1752–1763. doi: 10.1152/jn.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]