Abstract
The p75 neurotrophin receptor is highly expressed in the developing nervous system and is required for neuronal survival, growth and synaptic transmission. In young mice, p75 is present in both granular cells and Purkinje cells of the cerebellum. Although p75 has been implicated in modulation of neuronal excitability in several neuronal types, whether and how it affects the excitability of cerebellar Purkinje neurons remained unclear. Using extracellular recordings of spontaneous firing of Purkinje neurons in cerebellar slices prepared from wild type and p75 knockout mice, we measured intrinsic firing properties in the presence of fast synaptic blockers of more than 200 Purkinje cells, each for a period of 5 min, for each genotype. We detected a significant increase in the mean firing frequency in p75−/− neurons comparing to the wild type littermates. Upon separating tonically firing from phasically firing cells, i.e. cells with firing pauses of longer than 300 msec, we observed that the change mainly arose from phasic firing cells and can be explained by an increase in the firing/silence ratio and a decrease in the number of long pauses during the 5-min recording period. We conclude that p75 plays an important role in regulating the firing-to-silence transition during the phasic firing period of the spontaneous firing of Purkinje cells. Thus, p75 exerts a modulatory function on Purkinje cell firing patterns, through which it may act as a key player in motor coordination and other cerebellum-regulated activities since Purkinje cells represent the sole neuronal output of the cerebellar cortex.
Introduction
The p75 neurotrophin receptor is expressed at high levels in the developing nervous system during periods of axon outgrowth and dendritic arborization, but its expression decreases in the postnatal period and adulthood, except in the basal septum cholinergic neurons and Purkinje cells in the cerebellum [1, 2]. Various injuries in the adult, however, often induce p75 expression in ectopic regions [3], which led to the concept that the function of p75 is mostly associated with regulating cell survival, programmed cell death and axonal growth during development or after injury [3–5].
On the other hand, p75 has also been implicated to affect neuronal excitability and neural transmission. For instance, in dorsal root ganglion neurons, p75 mediates the increase in neuronal excitability induced by brain-derived neurotrophic factor (BDNF) through the sphingomyelin signaling cascade [6]. In sympathetic neurons, signaling through the p75 pathway causes phasic firing while that through the TrkA receptor induces tonic firing [7]. In the hippocampus, proBDNF facilitates long-term depression through activation of p75 [8]. In the cerebellum, p75 has been reported to mediate the effect of nerve growth factor (NGF) on glutamate release in developing cerebellar neurons [9] and on survival of cultured Purkinje cells in conjunction with activation of metabotropic glutamate receptors [10]. Supporting the role of p75 in cerebellar development, p75−/−:BDNF+/− mice display defects in foliation and Purkinje cell morphology [11]. However, whether, and to what extent, p75 modulates Purkinje cell excitability was unknown. Therefore, we examined the contribution of p75 to the firing properties of cerebellar Purkinje cells. Our data show a significant increase in the firing/silence ratio during the phasic firing periods in Purkinje neurons from p75−/− mice as compared to that from the wild types.
Increased spontaneous firing frequency in phasic firing Purkinje cells from p75−/− mice
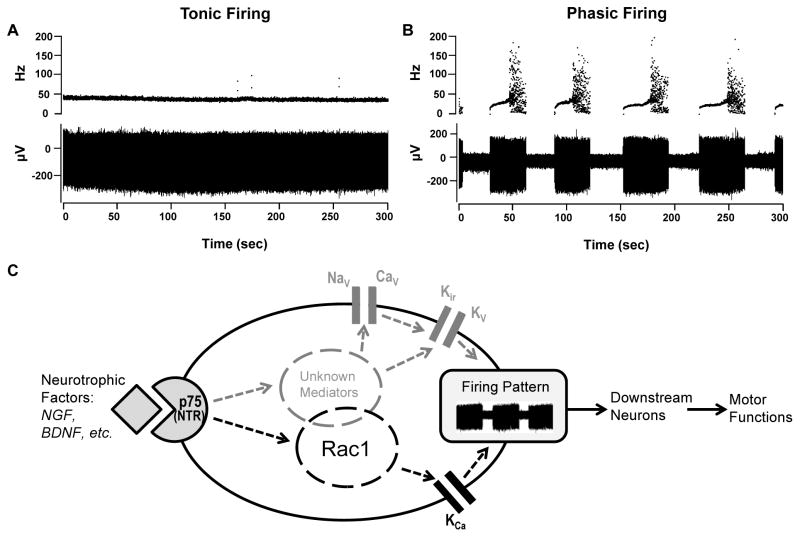
Extracellular recordings were performed on Purkinje cells in cerebellar slices from a total of 8 littermate pairs of p75+/+ and p75−/− mice ranging from postnatal days 18 to 25. For each mouse, 20–40 Purkinje neurons from the apex of lobule VI were randomly selected for recordings. Fast synaptic transmission was blocked by 5 mM kenurenic acid and 100 μM picrotoxin. Only well isolated, single cell unit recordings were counted and included in data analyses. Each cell was recorded continuously for 5 minutes. For simplicity, if no pause of the spontaneous firing lasting for more than 300 msec was seen during the 5-min recording, the cell was categorized as tonic firing (Fig. 1A); otherwise, it was considered as phasic firing (Fig. 1B), which included cells with a trimodal firing pattern, as described before by others [12, 13], and cells that fired irregularly.
Figure 1. Cerebellar Purkinje cell firing patterns and schematic of p75-mediated signaling pathways involved in the modulation of Purkinje cell firing.
(A, B) Representative traces of tonic (A) and phasic (B) firing Purkinje neurons in cerebellar slices from wild type mice. Upper traces illustrate instantaneous firing frequency while lower traces show field potential changes during the 5-min recording periods. The sharp increase in the instantaneous firing frequency and a broader distribution of such frequency at the end of each firing phase in (B) represents a burst firing period, which typically precedes the silent phase. (C) Schematic of p75-mediated signaling pathways in the cerebellar Purkinje cell. Neurotropic factors, such as NGF and BDNF, bind to p75, causing activation of Rac1 and other intracellular pathways, which in turn modulate ion channel activities. Candidate channels include calcium-activated potassium channels (KCa), inwardly rectifying potassium channels (Kir), and voltage-gated sodium, potassium and calcium channels (NaV, KV and CaV). The interplay among these channels regulates the firing-to-pause transition and thus Purkinje cell firing patterns, which critically control motor functions.
Mean firing frequency was determined for each cell over a 5-min recording and the average values for all cells (20–40 for each animal) from individual animals were compared between the littermates. We found that the mean firing frequency was higher in p75−/− than p75+/+ Purkinje cells for each littermate pair. Pooled data from all recorded neurons (220, p75+/+ and 224, p75−/− cells) revealed a 15% increase of spontaneous firing frequency in p75−/− compared to the wild type neurons. Interestingly, separation of tonic firing neurons from phasic firing ones (46 and 38% of total recorded cells for p75+/+ and p75−/−, respectively) revealed that the observed increase in firing frequency was mainly due to the change in phasic firing cells, in which 7 of the 8 littermate pairs showed obvious increases of the mean firing frequency, with ~41% overall increase in p75−/− compared to p75+/+. By contrast, no significant change in firing frequency was detected in tonic firing Purkinje neurons between p75−/− and p75+/+ mice. These results demonstrate a role of p75 in modulating intrinsic firing properties of cerebellar Purkinje neurons, especially during the phasic period of spontaneous firing.
Higher firing/silence ratio accounts for the increased phasic firing frequency in p75−/− Purkinje neurons
While no significant change was found for interspike and interburst intervals of all phasic firing cells between p75−/− and p75+/+, analysis of interphasic intervals (pause intervals >300 ms) revealed less number of pauses, especially for those of longer durations, e.g. >4 sec, in p75−/− neurons during the 5-min recording period. Related to this, the total firing/silence ratio was, on average, 71% higher in p75−/− than in p75+/+. The total silence time included a summation of all interspike intervals that were longer than 300 msec. The total firing time was the difference between the 5-min recording time and the total silence time. Because no significant change was observed between p75−/− and p75+/+ neurons in the distributions of interspike intervals longer than 300 msec, it is unlikely that the loss of p75 caused a defect in maintaining the silence period. By contrast, the reduction in the pause numbers is consistent with extended firing period during the phasic firing cycles. Thus it can be generally concluded that compared to the wild type counterparts, p75−/− Purkinje cells are more reluctant to enter the silence phase, i.e. to stop firing. This view is supported by the fact that more p75−/− cells (62%) fired tonically (no silence) during the 5-min recording than wild type cells (54%). Our data thus suggest a role for p75 in promoting the firing to silence transition, regulating the spontaneous firing patterns of cerebellar Purkinje cells.
Discussion
We show for the first time that the neurotrophin receptor p75 plays a role in regulating spontaneous firing patterns of cerebellar Purkinje neurons. This occurs through an effect at the transition from firing to silence. As such, deletion of p75 resulted in prolonged firing periods, which are reflected as an increased proportion of tonic firing cells, enhanced firing/silence ratio and decreased pause numbers during a given time period. This modulation is independent of fast synaptic transmissions as it happened in the presence of fast synaptic blockers. Therefore, p75 modulates intrinsic firing properties of Purkinje cells.
Substantial changes in ion channel activities are expected during the firing-to-silence transition. In sympathetic neurons, p75 induced firing pattern changes may be mediated by at least four voltage-gated currents: sodium current and M-type, delayed rectifier, and calcium-activated potassium currents [7]. Tetradotoxin-resistant sodium channels and G protein-activated inwardly rectifying potassium channels have also been suggested to be involved in p75-mediated excitability changes of dorsal root ganglion neurons and cerebellar Purkinje neurons [6, 14, 15]. It would be interesting to know which channel(s) and which signaling pathway(s) are involved in p75-mediated regulation of Purkinje cell firing activities (Fig. 1C). Our other results, not reported here, suggest the importance of Rac1 signaling and calcium-activated potassium channels in this regulation.
Since Purkinje cells represent the sole signal output of cerebellar cortex, changes of their firing patterns substantially impact the activity of deep cerebellar nuclei neurons, which directly receive signal inputs from Purkinje cells. Prolonged firing of Purkinje cells in p75−/− mice may strengthen the inhibitory input to the deep nuclei neurons, causing defects in motor-related functions. Indeed, we have observed moderate ataxia in p75−/− mice (data not shown), supporting the critical role of p75 in regulating Purkinje cell function and motor coordination. More detailed studies of the p75 signaling pathway on Purkinje cell activity will shed new lights on how neurotrophins regulate cerebellum and motor functions.
Acknowledgments
This work was supported by NIH grants R01NS039472 (to S.O.Y.), R01GM081658 (to M.X.Z.), and P30NS045758 (The Ohio State Neuroscience Center Core).
Footnotes
Conflicts of interest: There are no current or potential conflicts of interest for the all authors.
References
- 1.Yan Q, Johnson E. An immunohistochemical study of the nerve growth factor receptor in developing rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8:3481–98. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urschel B, Hulsebosch C. Distribution and relative density of p75 nerve growth factor receptors in the rat brain as a function of age and treatment with antibodies to nerve growth factor. Brain Research. 1992;591:223–38. doi: 10.1016/0006-8993(92)91702-g. [DOI] [PubMed] [Google Scholar]
- 3.Chao M. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 4.Lu B, Pang P, Woo N. The yin and yang of neurotrophin action. Nature reviews Neuroscience. 2005;6:603–4. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zeng J, Cen L, Wang X, Yao G, Wang W, Qi W, Kong K. Multiple roles of the p75 neurotrophin receptor in the nervous system. The Journal of international medical research. 2009;37:281–8. doi: 10.1177/147323000903700201. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Chi X, Nicol G. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. The Journal of Physiology. 2008;586:3113–27. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luther J, Birren S. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:5411–24. doi: 10.1523/JNEUROSCI.3503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo N, Teng H, Siao C-J, Chiaruttini C, Pang P, Milner T, Hempstead B, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature Neuroscience. 2005;8:1069–77. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 9.Numakawa T, Nakayama H, Suzuki S, Kubo T, Nara F, Numakawa Y, Yokomaku D, Araki T, Ishimoto T, Ogura A, Taguchi T. Nerve growth factor-induced glutamate release is via p75 receptor, ceramide, and Ca(2+) from ryanodine receptor in developing cerebellar neurons. The journal of biological chemistry. 2003;278:41259–69. doi: 10.1074/jbc.M304409200. [DOI] [PubMed] [Google Scholar]
- 10.Mount H, Elkabes S, Dreyfus C, Black I. Differential involvement of metabotropic and p75 neurotrophin receptors in effects of nerve growth factor and neurotrophin-3 on cultured Purkinje cell survival. Journal of Neurochemistry. 1998;70:1045–53. doi: 10.1046/j.1471-4159.1998.70031045.x. [DOI] [PubMed] [Google Scholar]
- 11.Carter A, Berry E, Segal R. Regional expression of p75NTR contributes to neurotrophin regulation of cerebellar patterning. Molecular and cellular neurosciences. 2003;22:1–13. doi: 10.1016/s1044-7431(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 12.Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:10603–12. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Womack M, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2600–7. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulson E, May L, Osborne S, Reid K, Underwood C, Meunier F, Bartlett P, Sah P. p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:315–24. doi: 10.1523/JNEUROSCI.2699-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damarjian T, Craner M, Black J, Waxman S. Upregulation and colocalization of p75 and Nav1. 8 in Purkinje neurons in experimental autoimmune encephalomyelitis. Neuroscience letters. 2004;369:186–90. doi: 10.1016/j.neulet.2004.07.023. [DOI] [PubMed] [Google Scholar]