Abstract
5-hydroxytryptamine 3 (5HT3) receptors are important modulators of mesostriatal dopaminergic transmission and have been implicated in the pathophysiology of cocaine reward, withdrawal, and self-administration. In addition, the 5HT3 antagonist ondansetron is effective in treating early-onset, but not late-onset, alcohol-dependent subjects. To explore the role of 5HT3 receptor systems in cocaine addiction using functioning imaging, we administered ondansetron to 23 abstinent, treatment-seeking cocaine-addicted and 22 sex-, age-, and race-matched healthy control participants. Differences between early- (first use before 20 years, n=10) and late-onset (first use after 20 years, n=10) cocaine-addicted subjects were also assessed. On two separate days, subjects were administered ondansetron (0.15 mg/kg intravenously over 15 min) or saline. Regional cerebral blood flow (rCBF) was measured following each infusion with single photon emission computed tomography (SPECT). No significant rCBF differences between the cocaine-addicted and control participants were observed following ondansetron relative to saline. Early-onset subjects, however, showed increased (p < 0.001) right posterior parahippocampal rCBF following ondansetron. In contrast, late-onset subjects showed decreased rCBF following ondansetron in an overlapping region of the right parahippocampal/hippocampal gyrus. Early-onset subjects also displayed increased rCBF in the left anterior insula and subthalamic nucleus following ondansetron; late-onset subjects showed decreased rCBF in the right anterior insula. These findings suggest that age of drug use onset is associated with serotonergic biosignatures in cocaine-addicted subjects. Further clarification of these alterations may guide targeted treatment with serotonergic medications similar to those successfully used in alcohol-dependent patients.
INTRODUCTION
5-hydroxytryptamine 3 (5HT3) receptors modulate dopaminergic transmission in mesolimbic, mesocortical, and nigrostriatal pathways (Alex and Pehek, 2007), suggesting that this serontonergic receptor system may have relevance to the addictive disorders. These receptors mediate the excitatory effect of compounds acting upstream from dopamine neurons, inducing heightened extracellular levels of dopamine in the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Engleman et al., 2008). In addition to dopamine, 5HT3 receptors also modulate the release of cholecystokinin, acetylcholine, GABA, substance P, glutamate and serotonin itself (Farber et al., 2004). High concentrations of 5HT3 receptors have been identified in the amygdalar sub-nuclei, hippocampus (HP) and entorhinal cortex as well as the caudate, thalamus, and other cortical regions (Farber et al., 2004).
A number of preclinical studies support a role of 5HT3 receptors in the addictive disorders. The 5HT3 antagonist ondansetron suppresses withdrawal symptoms of cocaine, nicotine, and ethanol when administered into the amygdala or dorsal raphe nucleus (Costall et al., 1990). The stimulatory effects of cocaine are mediated by the 5HT3 receptors in the core of the NAc (Herges and Taylor, 2000) and these NAc 5HT3 receptors are functionally downregulated following 14 days of continuous cocaine administration (King et al., 2000). Ondansetron also blocks both cocaine-induced sensitization (Davidson et al., 2002; King et al., 2000), the downregulation of 5HT3 receptors induced by continuous cocaine administration (King et al., 1999), and cocaine (Davidson et al., 2002) and cocaine plus alcohol (Ding et al., 2011) self-administration in rodent models. Although it is generally presumed that cocaine’s effects upon 5HT3 receptors are mediated through cocaine-mediated changes in monoamine reuptake, cocaine also directly interferes with the 5HT3 voltage-dependent sodium channel (Fan et al., 1995).
Over the previous decade several clinical studies have suggested that 5HT3 receptor antagonists may be effective in the treatment of substance use disorders. A marked decrease in drinking has been observed in alcohol-dependent subjects with early-onset, but not late-onset, drinking (Johnson et al., 2000b; Kranzler et al., 2003) (early-onset was defined as the onset of alcohol problems prior to the age of 25 years). Hypothesizing that early-onset alcoholism was a result of a high functioning polymorphic variant (LL vs. LS/SS; L= long; S=short) of the serotonin transporter (SERT), Johnson et al. (Johnson et al.) subsequently demonstrated that alcohol-dependent subjects with the LL genotype who are treated with ondansetron drink significantly fewer drinks per drinking day and have more days abstinent than LS/SS individuals. In a human imaging study, ondansetron suppressed the ventral striatal response to cue-induced craving in alcohol-dependent participants (Myrick et al., 2008). While some promise for ondansetron has been reported in the treatment of cocaine dependence (Johnson et al., 2006), follow-up studies are ongoing (Ait-Daoud, 2008).
These preclinical and clinical findings suggest that differences in the modulatory effects of the 5HT3 receptor upon upstream neural processes, particularly upon dopaminergic release, may alter the rewarding properties of cocaine or other addicted substances. However, to our knowledge, there have been no published controlled human studies exploring the interaction between 5HT3 receptor antagonism and cocaine use in human participants. We, therefore, used a well-studied 5HT3 receptor antagonist, ondansetron, to selectively assess 5HT3 receptors. Given the high concentration of 5HT3 receptors in the limbic system, particularly in the amygdala and HP, and the importance of this system to rewarding and addictive behaviors, we hypothesized that limbic regions would be significantly altered in cocaine-dependent individuals compared to healthy controls. We further predicted that areas dominant in 5HT3 receptors would demonstrate a differential response between early- and late-onset (defined by age of first use) cocaine-dependent participants. Regional cerebral blood flow (rCBF) was measured in response to ondansetron against a saline comparison. Whereas neuroreceptor radiotracers are used to study pre-and post-synaptic sites, rCBF is used to assess the integrated response to receptor specific probes, including downstream effects. We also chose this probe because of its extensive history of safety in humans, its specificity for the 5HT3 receptor, and its relevance to treatment in alcohol-dependence.
METHODS
Participants
Twenty-three (12 male) cocaine-addicted and 22 (12 male) healthy control subjects were studied. Cocaine-addicted subjects had a primary DSM-IV diagnosis of cocaine dependence and cocaine was their lifetime drug of choice. They were one to six weeks abstinent [range 8–39 days; 27.5 (SD 7.5)] which offered a relatively circumscribed period of abstinence, acclimation to the residential unit, and adequate time for the most marked effects of cocaine withdrawal to subside. Cocaine-addicted subjects were recruited from patients requesting treatment for cocaine dependence at the Veteran’s Administration Medical Center, Homeward Bound, Inc., and Nexus Recovery Center in Dallas, Texas. Addicted subjects were hospitalized as soon as possible after their last reported use of cocaine, and remained in a structured, residential unit until the study was completed. Exclusion criteria included a substance-use disorder (other than cocaine or nicotine) within the previous six months, present use of any central nervous system active medications (including all psychotropics), or a lifetime history for Affective, Anxiety, or Schizophrenic Disorder, or Organic Brain Syndrome experienced prior to the onset of a substance abuse diagnosis or following a period of at least three months abstinence. Exclusion criteria for healthy controls included the criteria as noted for the cocaine-addicted subjects, as well as a lifetime history of any other Axis I disorder (except nicotine dependence) or a first-degree relative with an addictive disorder (other than nicotine).
After providing a complete description of the study to the subjects, written informed consent was obtained. Subjects were financially compensated for their participation. Approval for the study was obtained from the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas (UTSW) and the VA North Texas Health Care System. Subjects underwent a medical history and physical examination, DSM-IV Structured Clinical Interview, clinical laboratory tests, urine drug screen, electrocardiogram, and clinical MRI. Lifetime cocaine and other substance use history, including age of first use, was obtained from cocaine-dependent subjects using the Time Line Follow Back (TLFB).
Personality measures of impulsivity were obtained from the Revised NEO Personality Inventory (NEO PI-R). The NEO PI-R measures individual differences in normal personality based on 5 major domains and 30 facet scales (Costa and McCrae, 1992). The Temperament and Character Inventory (TCI) (Cloninger et al., 1994) assesses seven personality domains and 25 facets. Facets from the NEO-PI-R and the TCI were selected based on their relevance to impulsive behaviors associated with early-onset typology: NEO-PI-R Impulsiveness (N5), Self-discipline (C5), and Deliberation (C6) and TCI Impulsiveness (NS2), Fear of Uncertainty (HA2), Purposefulness (SD2), and Congruent Second Nature (SD5).
SPECT Study Sessions
Study sessions took place at the Clinical Trials Office at the UTSW. Subjects underwent four study sessions over a ten-day period. For one session only a saline placebo was administered; for the other sessions subjects received either ondansetron, physostigmine, or scopolamine [physostigmine and scopolamine data have previously been reported; (Adinoff et al., 2010)]. Sessions were separated by at least 48 hours to allow time for decay of 99mTcHMPAO, which has a half-life of approximately six hours. The four scans were administered in a pseudo-random order (i.e. randomization was assigned at study initiation to assure that infusion order was balanced within each group and to avoid having the two cholinergic probes in sequence).
Nicotine-dependent subjects were allowed a cigarette 30 min approximately two hours prior to radioligand administration). This timing was long enough to avoid acute effects of nicotine while short enough to avoid the onset of nicotine withdrawal. Subjects were placed supine in a recliner, at rest, with eyes open, and ears unplugged. An intravenous catheter was inserted at approximately 2:00 PM (T=−30 min) and a continuous infusion of saline was given throughout the study (except when medication was being administered). The Brief Symptom Inventory (Derogatis and Melisaratos, 1983) was administered after 30 min of rest (T=0) and again just prior to radioligand administration. The BSI uses 53 questions to assess cognitive, sensory, and affective changes. Blood pressure and heart rate were obtained every 10 minutes throughout the study. Medications were administered through the intravenous line that was hidden behind a curtain, and all participants and the study coordinator were blinded to study drug. At T=60, 0.15 mg/kg ondansetron was administered over 15 minutes at a constant rate of infusion. [This dosing paradigm replicates that used in chemotherapy]. Fifteen minutes later (T=75), 20 mCi of the SPECT rCBF tracer 99mTc HMPAO (GE Healthcare, Princeton, New Jersey) was administered over 30 sec and followed by a 10 ml saline flush over 30 sec. Twenty min after the radioligand infusion the intravenous catheter was removed and a Subjective Symptom Inventory was obtained. The Subjective Symptom Inventory asked subjects to rate (from 0 to 5) twenty signs and symptoms (i.e. shakiness, blurred vision, headache, sadness, anxiety, drowsiness, etc.) potentially experienced during the study. SPECT scans were obtained 90 minutes following 99mTc HMPAO administration to allow time for tracer activity to clear from blood and non-brain tissues.
SPECT Image Analysis
SPECT images were acquired on a PRISM 3000S 3-headed SPECT camera (Picker International, Cleveland, OH) using ultra-high-resolution fan-beam collimators (reconstructed resolution of 6.5mm) in a 128×128 matrix in three-degree increments. For our system, voxels in reconstructed images were 1.9mm3. Reconstructed images were smoothed with a 6th-order Butterworth three-dimensional filter, and attenuation corrected using a Chang first-order method with ellipse size adjusted for each slice.
Statistical Analysis
Demographic and Clinical Data
Demographic, clinical characteristics and personality measures were compared using ANOVA F or chi-square tests, as appropriate. Tukey’s tests were used for post-hoc comparisons. Basal and medication-induced (post- vs. pre-infusion) changes in Brief Symptom Inventory were assessed using ANOVA F tests. False Discovery Rate (FDR) techniques (Benjamini and Hochberg, 1995) were used to control the type I error rate for the large number of comparisons. Standard scores for the NEO-PI-R and raw scores for the TCI were utilized.
Early vs. Late Onset
The cocaine-addicted group’s median age of first cocaine use was 20 years old. Since 20 years of age has used by others to define early- and late-onset alcohol dependence (Babor et al., 1992; Cloninger et al., 1981; Johnson et al., 2000a; Lee and DiClimente, 1985), we used this age to define the early- and late-onset cocaine-addicted subjects in our sample. This resulted in equal groups of ten participants reporting an earlier age (< 20 years old) and ten reporting a later age (> 20 years old) of first cocaine use. There were three cocaine-addicted subjects who reported first use at age 20 years. As there was no a priori reason to include them in either the early- or late-onset groups, these three subjects were excluded from subsequent analyses (Fig. 1).
Fig. 1.
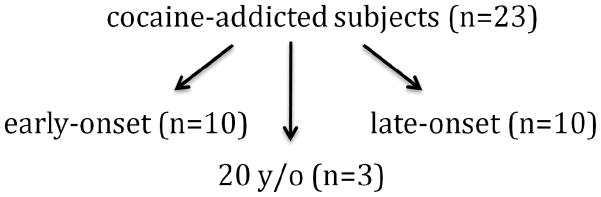
Twenty-three cocaine-addicted subjects were compared as early-onset (younger than 20 years old, n=10) and late-onset (older than 20 years old, n=10) groups. The three 20 years old subjects were not included in the early- vs. late-onset comparisons.
Image Analysis
SPECT images were resliced to 2mm3 voxels, co-registered to Montreal Neurologic Institute (MNI) space using the MNI T1 MRI template, smoothed to a final resolution of 10mm and normalized to whole brain counts (to correct for individual variability in global cerebral blood flow). This approach provides only relative, not absolute, rCBF values. The accuracy of normalization in our data is about 2–4mm, and the anatomic designations assigned to observed rCBF effects are constrained by these limitations, as well as by our spatial resolution and partial volume effects. Voxel-wise analyses (voxel z scores=p<0.001, cluster size>50 voxels) comparing saline- and ondansetron-induced effects were conducted using Statistical Parametric Mapping (SPM2; University College, London, England). Significant changes induced by ondansetron were computed relative to saline. Between-group differences following ondansetron (compared to saline) were also assessed between early- and late-onset cocaine-dependent subjects and between each cocaine-dependent group and controls. In addition, between-group differences were also assessed following saline only. To clarify localization of identified clusters, MNI Space utility (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html) was used to identify anatomical region labels of SPM-derived clusters. MNI coordinates were converted to Talairach coordinates using a nonlinear transformation approach. Because comparisons are for relative measures, significant clusters cannot be associated with an absolute direction of differences.
RESULTS
Demographics and Clinical Characteristics
Control and cocaine-addicted populations were gender matched and did not significantly differ in race or age. The cocaine-addicted group had significantly more smokers relative to the control group and a lower mean level of education. The early- and late-onset groups did not differ from one another in age, gender, race, cocaine use (recent or lifetime), nicotine use, or their severity of cocaine dependence [as measured by the Inventory of Drug Use Consequences (InDUC) (Tonigan and Miller, 2002)] (Table 1). Both the early- and late-onset groups had significantly less education and more nicotine use than the control group.
Table 1.
Demographic Characteristics of Control and Cocaine-Addicted Subjects
| Characteristic | Control (n = 22) | Cocaine-Addicted early-onset (n=10) | Cocaine-Addicted late-onset (n=10) | F value | p-value | |||
|---|---|---|---|---|---|---|---|---|
| mean/n | sd/% | mean/n | sd/% | mean/n | sd/% | |||
| Age in yrs, mean (SD)a | 34.4 | 6.8 | 34.9 | 7.4 | 38.9 | 5.5 | 1.62 | 0.21 |
| Education in yrs, mean (SD) | 15 | 2.7 | 11.9d | 1.9 | 12.1d | 2.4 | 7.1 | 0.002 |
| Male, No. (%)b | 12 | 54.6 | 5 | 50 | 6 | 60 | 0.2 | 0.90 |
| Race, n (%)b | 3.77 | 0.44 | ||||||
| Black | 11 | 50 | 6 | 60% | 8 | 80% | ||
| White (non-Hispanic) | 9 | 40.9 | 4 | 40 | 2 | 20 | ||
| Hispanic | 2 | 9.1 | 0 | 0 | 0 | 0 | ||
| Cigarette Use, mean (SD)a | ||||||||
| Cigarettes/day | 0.3 | 0.8 | 13.5d | 7.7 | 9.5d | 8.0 | 22.0 | <0.001 |
| Pack years | 3.1 | 4.5 | 14.7d | 8.9 | 14.1d | 11 | 3.53 | 0.05 |
| Cocaine Use, mean (SD)a | ||||||||
| Age of onset of cocaine use | --- | --- | 16.4 | 2.4 | 27.4 | 6.0 | 21.9 | <0.001 |
| Days used in previous 90 days | --- | --- | 69.1 | 28.7 | 74.4 | 24.3 | 0.66 | 0.43 |
| Yrs used in lifetime | --- | --- | 11.4 | 6.7 | 8 | 6.7 | 1.28 | 0.27 |
| Lifetime dollars spent on cocaine | --- | --- | 206330 | 141578 | 219836 | 337509 | 0.01 | 0.91 |
| InDUCa | 2.3 | 3.1 | 36.5 | 5.5 | 36 | 6 | 122.6 | <0.001 |
| Days abstinent at testinga | --- | --- | 29.3 | 8.6 | 25.6 | 7.2 | 1.09 | 0.31 |
| Personality Measuresa | ||||||||
| Impulsiveness N5 (NEO-PI-R) | 48.1 | 10.6 | 58.4c | 6.63 | 51.1 | 5.7 | 4.28 | 0.022 |
| Self-discipline C5 (NEO-PI-R) | 51.4 | 13.0 | 40.9 | 16.2 | 41.7 | 8.5 | 2.75 | 0.078 |
| Deliberation C6 (NEO-PI-R) | 54.5 | 12.3 | 41.0c | 8.8 | 45.5 | 9.8 | 5.13 | 0.011 |
| Impulsiveness NS2 (TCI) | 3.2 | 2.1 | 5.6c | 3.3 | 4.7 | 0.49 | 3.39 | 0.045 |
| Fear of Uncertainty HA2 (TCI) | 2.9 | 1.7 | 3.2 | 1.8 | 4.3 | 1.6 | 2.25 | 0.120 |
| Purposefulness S2 (TCI) | 6.8 | 1.4 | 5.5 | 2.0 | 6.3 | 1.3 | 2.39 | 0.106 |
| Congruent Second Nature S5 (TCI) | 10.4 | 1.8 | 7.6d | 2.5 | 7.0d | 1.6 | 12.49 | <0.001 |
values obtained by ANOVA
values obtained by Chi-square test
p<0.05 (control vs. early- or late-onset)
p<0.01 (control vs. early- or late-onset)
rCBF following ondansetron in control and cocaine-addicted subjects
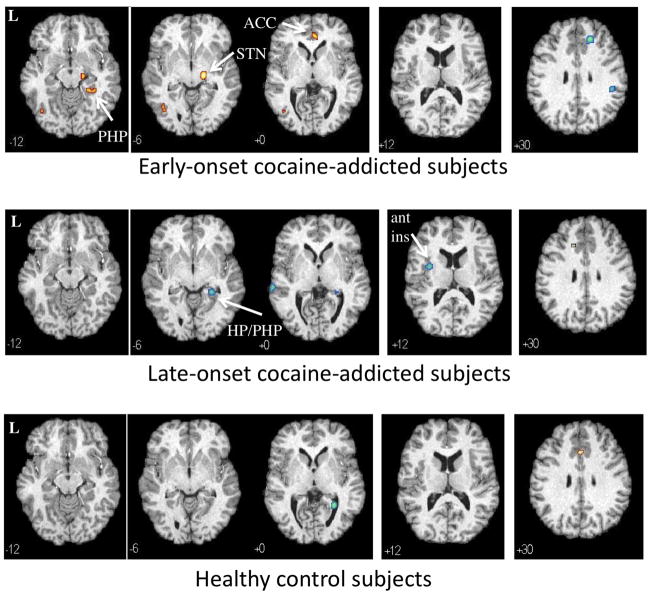
Following administration of ondansetron, compared to saline, control subjects demonstrated increased rCBF in the anterior cingulate (−2, 24, 32; 61) and in the left cerebellum (−10, −72, −34; 79) (Fig. 2, bottom panel; Supplement 1). There were no significant rCBF changes observed in the cocaine-dependent subjects. Comparison of rCBF responses to ondansetron (relative to saline) revealed no significant group differences between control and cocaine-dependent subjects.
Fig. 2.
Regional cerebral blood flow (rCBF) changes in response to ondansetron relative to saline (p<0.001) in 10 early-onset (top panel) 10 late-onset (middle panel) cocaine-addicted participants and 22 healthy controls (bottom panel). Yellow areas reveal voxels with increased rCBF following ondansetron relative to saline and blue areas reveal voxels with decreased rCBF following ondansetron relative to saline. MNI coordinates (z-axis) noted at the bottom left of each image. L = left. PHP = parahippocampus. HP = hippocampus. ACC = anterior cingulate cortex. ant ins = anterior insula. STN = subthalamic nucleus. See Supplement 1 (healthy controls), Supplement 2 (early-onset), and Supplement 3 (late-onset) for all transverse images.
Early-onset cocaine-dependent subjects showed increased rCBF following ondansetron, relative to saline, in the pregenual anterior cingulate (BA32, BA24a), right parahippocampal gyrus, right subthalamic nucleus (STN), and a region overlapping the left inferior temporal and middle occipital gyri (Fig. 2, top panel; Supplement 2). Decreases in rCBF were observed in the right superior/medial frontal cortex and the right inferior parietal lobule (BA40). Only a single region of increased rCBF was observed in the late-onset cocaine-dependent subjects that overlapped the left superior/medial frontal gyrus and anterior cingulate, a mirror image of the right decrease in the early-onset participants (Fig. 2, middle panel; Supplement 3). Decreased rCBF was observed in a cluster overlapping the right parahippocampal and hippocampal gyri, left anterior insula, and middle/superior temporal gyrus (BA21).
Differences in rCBF following ondansetron between control, early-onset and late-onset
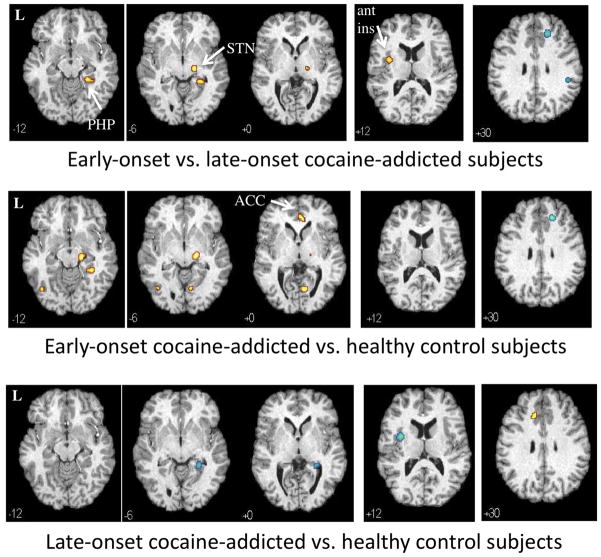
Compared to late-onset subjects, early-onset participants showed increased rCBF in the right parahippocampal gyrus (driven by increased rCBF in the early-onset and decreased rCBF in the late-onset participants), left anterior insula (driven by the late-onset subjects) and right STN (driven by the early-onset subjects) following the administration of ondansetron, relative to saline (Fig. 3, top panel; Supplement 4). Early-onset, relative to late-onset, participants showed decreased rCBF following ondansetron in an area of the right superior frontal gyrus and inferior parietal lobule (BA40) (both driven by the early-onset subjects). To determine if differences in early- and late-onset subjects were influenced by basal differences, rCBF following saline was compared between the two groups. Between-group rCBF differences following saline in the precuneus (−4, −54, 50; 153) (BA7), left postcentral gyrus (−38, −22, 42; 89), and left middle/superior frontal gyrus (−30 24 64; 59) did not appear to influence the differences reflected following ondansetron relative to saline.
Fig. 3.
Differences in regional cerebral blood flow (rCBF) changes in response to ondansetron relative to saline (p<0.001) in 10 early-onset (top panel) vs. 10 late-onset (middle panel) cocaine-addicted participants (top panel), early-onset vs. 22 control (middle panel) and late-onset vs. control (bottom panel) participants. Yellow areas display voxels with greater rCBF response to ondansetron in the first listed group relative to the second; blue areas are voxels with decreased rCBF response in the first listed group relative to the second. MNI coordinates (z-axis) noted at the bottom left of each image. L = left. PHP = parahippocampus. ACC = anterior cingulate cortex. ant ins = anterior insula. STN = subthalamic nucleus. See Supplement 4 (early-onset vs. late-onset), Supplement 5 (early-onset vs. controls) and Supplement 6 (late-onset vs. controls) for all transverse images.
Between-group comparisons of the early-onset and control subjects revealed regions almost entirely driven by the early-onset group, including relative rCBF increases following ondansetron in the right parahippocampal gyrus, right STN, pregenual cingulate (BA32), and left middle occipital gyrus and relative rCBF decreases in the right superior frontal gyrus (Fig. 3, middle panel; Supplement 5). A relative increase in right lingual gyrus appears to have been a result of rCBF increases in the early-onset and rCBF decreases in the control group. Differences between late-onset, relative to control, subjects also were primarily driven by the late-onset group, including relative rCBF increases in area overlapping the left anterior cingulate and rCBF decreases in the right PHP/HP and left anterior insula (driven by the late-onset group) (Fig. 3, bottom panel; Supplement 6).
Parahippocampal sensitivity to age of onset
Contiguous clusters in the right parahippocampal and/or hippocampal gyrus demonstrated an increased rCBF response to ondansetron in early-onset participants and decreased rCBF response to ondansetron in late-onset cocaine-addicted subjects. To explore this further, these overlapping clusters were combined into a single cluster (limited to those voxels that were defined by the PHP and HP), counts/voxel were determined for each participant, and percent difference between ondansetron and saline was determined (Fig. 4). There was a significant group difference between PHP/HP counts/voxel (F = 13.32, p < 0.001). Post-hoc (Tukey) analyses revealed that counts/voxel were lower in late-onset relative to early-onset (p < 0.001) and control (p = 0.001) participants, with no overlap between late- and early-onset subjects. Counts/voxel in early-onset and control subjects did not significantly differ (p = 0.193).
Fig. 4.
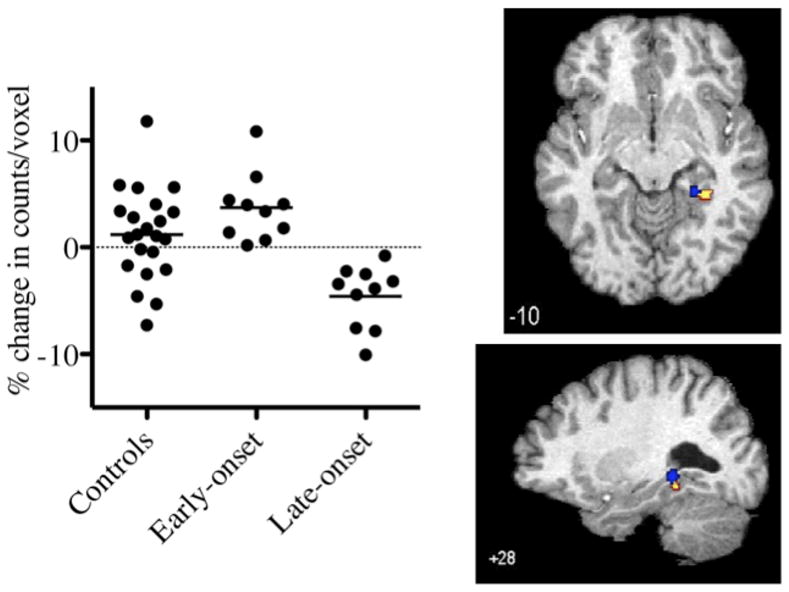
Percent change in counts per voxel following ondansetron, relative to saline, in right parahippocampal (PHP)/hippocampal (HP) gyri region in control and early- and late-cocaine addicted participants (left panel). There was a significant group difference in counts/voxel (F = 13.32, p < 0.001). Right panel displays transverse (top) and sagittal (bottom) image of overlapping clusters. Region encompasses overlapping PHP/HP clusters of increased (yellow cluster) and decreased (blue cluster) rCBF identified in Fig. 1 (upper and middle panel, respectively). MNI coordinates noted at the bottom left of each image.
Subjective Response
Subjective Symptom Inventory
SSI responses to ondansetron vs. saline (ΔSSI) did not significantly differ (p>0.6, FDR corrected) in the control, early-onset, or late-onset groups (Supplement 7). There were no significant group differences in ΔSSI (p>0.28) (Supplement 8).
Brief Symptom Inventory (BSI)
(Supplement 9): Individual symptom and total scores showed minimal changes following either saline or ondansetron infusions. There were no significant within-group differences (p > 0.61, FDR corrected) following ondansetron (post-ondansetron vs. baseline) compared to saline (post-saline vs. baseline) in either the control, early-onset or late-onset groups (Supplement 9). Except for a statistical trend in the Hostility domain (p = 0.051), there were no between-group differences in BSI scores following ondansetron compared to saline (p > 0.39).
Personality Measures
There were significant group differences on four of the seven personality measures [Impulsiveness (N5), Deliberation (C6), Impulsiveness (NS2) and Congruent Second Nature (S5)] and another two showed a statistical trend [Self-discipline (C5) and Purposefulness (S2)] (Table 1). Impulsiveness (N5), Deliberation (C6), and Impulsiveness (NS2) significantly differed (p < 0.05) between control and early-onset subjects but not between control and late-onset subjects.
DISCUSSION
Early-onset cocaine-addicted participants showed a significantly different directional (increased and decreased) and regional pattern of rCBF response to the 5HT3 antagonist ondansetron compared to late-onset cocaine-addicted participants. The observed rCBF differences were relatively discrete and in regions relevant to the addictive process, including the PHP/HP, anterior insula, anterior cingulate and STN. In contrast, the cocaine-addicted population in toto did not demonstrate ondansetron-induced changes in rCBF relative to saline.
Construct of early- and late-onset in alcohol and cocaine addiction
The Type 2, early-onset alcohol-dependent subject, initially proposed by Cloninger et at. (1981), and the somewhat similar Type B later proposed by Babor et al. (1992), were posited to more likely be male, have severe behavioral problems, high genetic and familial loading for alcohol-dependence, and an earlier age of problematic alcohol use than Type 1/Type A alcohol-dependent individuals. Both constructs defined early-onset as the onset of alcohol problems prior to the age of 20–21 years. Although the diagnostic, therapeutic and prognostic utility of the dichotomous Type I/2 and Type A/B remains uncertain (Moss et al., 2007), the early- vs. late-onset distinction has persisted as a potentially useful demarcation. The optimal age that discriminates early- from late-onset alcohol-dependent patients, however, remains controversial, with ages 18 (Dougherty et al., 2004), 20 (Johnson et al., 2000a; Lee and DiClimente, 1985), 22 (Buydens-Branchey et al., 1989), and 25 (Kranzler et al., 2003) years old offered as meaningful cutoffs. In their ondansetron treatment trials, Johnson et al. (2011; 2000b) defined early-onset as problematic drinking prior to 25 years old.
Relatively little work on the early- vs. late-onset dichotomy has been conducted in other substance use disorders, including cocaine addiction. In a trial of ondansetron in cocaine-addicted subjects, Johnson et al. (2006) defined early-onset in cocaine-dependent subjects as first use (as opposed to problematic use) prior to 18 years old, although empirical data supporting this age cut-off has not been published. In the present investigation we used a median split from our participant population of 20 years old. Using the age of first cocaine use to define age of onset (rather than age of problem use) likely offers a reasonable estimation of time to problematic use given the very short delay (~3 years) from the time of first use to dependence in those cocaine users who later develop cocaine dependence (Wagner and Anthony, 2002). The suggestion of heightened impulsivity (as assessed by the NEO-PR-I and TCI) in the early-onset cocaine-addicted, but not the late-onset, subjects is consistent with the reported association of early-onset alcohol-dependence and heightened impulsivity (Bjork et al., 2004), although to our knowledge this association has not previously been explored in cocaine dependence. Whether the findings observed result from pre-morbid differences in the early- and late-onset groups or are a consequence of unique vulnerabilities to cocaine in adolescent, relative to adult, brains (Schepis et al., 2008) remains an area for further exploration. The maturation of the serotonin system during adolescence, particularly in relation to the concurrent development of the dopaminergic system, may also play a role in the onset of drug use (Dawes and Johnson, 2004; Schepis et al., 2008).
Regions of Interest Identified
Early-onset cocaine-addicted participants showed an rCBF increase in the right parahippocampal gyrus following ondansetron whereas the late-onset group showed an rCBF decrease in a nearly identical region. Both the increase and decrease in rCBF was evident when the early- and late-onset subjects, respectively, were compared with control subjects or with each other. Thus, this region – an area with high localization of 5HT3 receptors – appears to distinguish between early-onset, late-onset and healthy control subjects. The right HP/PHP plays a critical role in nonverbal and spatial memory (Aminoff et al., 2007; Bohbot et al., 2000) and the posterior HP/PHP region, in particular, has relevance to addiction. The non-primate dorsal HP, a region considered homologous to the hippocampal tail in the human brain, is required for contextual conditioning during cocaine administration and lesions of the dorsal HP disrupt both the acquisition and expression of cocaine conditioned place preference in the rodent (2006). Interestingly, previous work suggests that Type I alcohol-dependent subjects may exhibit decreased hippocampal volume relative to Type 2 subjects (Laakso et al., 2000).
Other notable areas distinguishing the early- and late-onset groups from each other and/or from control subjects were the anterior insula, pregenual anterior cingulate and STN. The anterior insula is preferentially involved in the evaluative, experiential, or expressive aspects of internally generated emotions (Craig, 2003) and is noted to be active during cocaine craving (Kilts et al., 2001). The pregenual anterior cingulate has been associated with anxiety (Straube et al., 2009), depression (Salvadore et al., 2010), impulsivity (Hinvest et al., 2011) and craving (Rolls and McCabe, 2007). The right STN, which showed increased rCBF in early-onset relative to late-onset and control participants, is implicated in response inhibition (Aron et al., 2007), a region of particular relevance to early-onset cocaine addicted individuals with high impulsivity.
Similar to measures of cerebral glucose or oxygen metabolism with positron emission tomography (PET) or blood-oxygen-level dependent (BOLD) activation with pharmacologic MRI, rCBF provides an integrated measure of including downstream effects, including both inhibitory and excitatory processes. Thus, group differences in rCBF may reflect a combination of local post-synaptic or upstream pre-synaptic responses upon dopaminergic, peptidergic, cholinergic, glutamatergic, GABAergic, and serotonergic receptors (Engleman et al., 2008; Farber et al., 2004). The differential right PHP response in the early- and late-onset subjects may be particularly instructive. 5HT3 antagonists inhibit the serotonergically stimulated release of acetylcholine in the dorsal HP (Faerber et al., 2007). We have recently reported that cocaine-addicted patients, but not healthy controls, showed decreased rCBF in the left posterior HP (similar to the region showing altered rCBF in both cocaine-addicted groups in the present study) in response to cholinergic probes (Adinoff et al., 2010). As a consequence of distinct genetic profiles (Johnson et al., 2011) or unique vulnerabilities to cocaine in the adolescent brain (Schepis et al., 2008), or due to an interaction between the two, differences in either 5HT3 or downstream cholinergic receptors may thereby differentially alter the PHP/HP response in early-and late-onset subjects. The HP region may also respond differently to changes in the 5HT3 receptor, as HP 5HT3 receptors are predominantly postsynaptic whereas they are mostly presynaptic elsewhere in the brain (Miquel et al., 2002).
Clinical Relevance
Preclinical studies reveal that serotonergic systems are highly relevant in the development and persistence of cocaine administration (Kirby et al., 2011). Nevertheless, treatment studies have not shown serotonergic medications to be efficacious in the treatment of stimulant, including cocaine, addiction (Vocci et al., 2005). In fact, the selective serotonin-reuptake inhibitor (SSRI) sertraline has been reported to worsen prognosis in methamphetamine-dependent subjects (Shoptaw et al., 2006), similar to the poorer prognosis observed in early-onset alcohol-dependent subjects treated with SSRIs (Kranzler et al., 1996). As ondansetron has a differential effect upon treatment outcome in alcohol-dependent subjects segregated by age of onset (Johnson et al., 2000a; Kranzler et al., 2003) or serotonergic transporter gene polymorphisms (Johnson et al., 2011), the findings in the present study suggest that a similar approach may be useful in the treatment of cocaine addiction. That is, consideration of clinical characteristics (age of first use), central 5-HT3 or other serotonergic receptor systems, and/or serotonergic transporter polymorphisms may be useful in identifying specific populations for targeted pharmacologic interventions.
Strengths and Limitations
A major strength of our study was the use of age-, sex-, and race-similar cocaine-addicted and control subjects. The early- and late-onset groups were also matched for age, gender, race, and nicotine use. Importantly, these two groups were also matched for recent and lifetime cocaine use, strongly suggesting that the identified rCBF group differences were related to disease onset and not confounded by more extensive cocaine use in the early-onset group. Cocaine-addicted subjects did not have other active drug use disorders (except nicotine) and were studied during a defined period of verified abstinence, thus avoiding the rapid fluctuations in neural activity that occur during the first few days of cocaine abstinence as well as the more gradual changes that may develop with extended abstinence. However, the small number of subjects in the early- and late-onset groups and the absence of an accepted typology in cocaine addiction require that these findings be considered tentative. Menstrual cycles were not controlled due to the several days required to conduct multiple infusions. Nineteen of 23 (9 early-onset and 8 late-onset) of our cocaine-addicted subjects were smokers and all but four of the controls were non-smokers; all four were very light smokers. This confound generally plagues most clinical laboratory research of substance abusers, since most drug-dependent patients smoke and most healthy volunteers without medical or psychological morbidity do not. Finally, it is unknown if the observed differences pre-date cocaine use or reflects differing 5HT3 responses to cocaine-induced increases in dopamine.
Limits of spatial resolution and the ability to only determine relative, not absolute, measures of limbic activation are inherent in our SPECT methodology and camera. Although volumetric reductions have been reported in cocaine-addicted subjects relative to controls, no discrete structural lesions (by MRI) were observed in any of the subjects in this study. However, we cannot rule out the possibility that regions of muted rCBF responses observed in cocaine-addicted subjects resulted from neuronal loss. However, changes in neuronal lesions would be unlikely to account for increased and decreased parahippocampal rCBF in the early- and late-onset groups, respectively. Finally, there are inherent errors in spatial normalization for all functional imaging techniques, thus limiting the precision of our anatomic identifications.
The development of specific 5HT3 imaging agents will be useful in further delineating differences in early- and late-onset addicted subjects. The regions distinguishing the early- and late-onset addicted participants, particularly the posterior HP/PHP region, offer possible targets for future neurobiological and clinical investigations. Optimally, these investigations will clarify the mechanisms associating age of first use with serotonergic receptor systems by concurrently assessing polymorphisms in genes encoding the serotonin transporters/receptors and clinical outcomes.
Supplementary Material
Table 2.
Brain Regions Demonstrating Increased and Decreased (p<0.001) Regional Cerebral Blood Flow (rCBF) Following Ondansetron Compared with Saline in Early-onset and Late-onset Cocaine-dependent Subjects
| Identified ROI | MNI coordinates | kE1 | ||
|---|---|---|---|---|
| x | y | z | ||
| Early-onset | ||||
| Increased rCBF | ||||
| Subthalamic nucleus, right | 18 | −16 | −6 | 94 |
| ACC (BA32) | 4 | 42 | 2 | 66 |
| Inferior temporal gyrus, left | −46 | −72 | −16 | 73 |
| Parahippocampal gyrus, right | 26 | −38 | −12 | 55 |
| Decreased rCBF | ||||
| Superior mid-frontal gyrus, right | 18 | 38 | 30 | 143 |
| Inferior parietal lobule, right | 38 | −34 | 34 | 74 |
| Late-onset Cocaine-Addicted | ||||
| Increased rCBF | ||||
| Anterior cingulate | −18 | 28 | 26 | 93 |
| Decreased rCBF | ||||
| Middle temporal gyrus, left (BA21) | −68 | −30 | 0 | 52 |
| Insula, left (BA13) | −36 | 2 | 10 | 95 |
| Parahippocampal gyrus, right | 26 | −36 | −8 | 65 |
Cluster size
Table 3.
Brain Regions Demonstrating Increased and Decreased (p<0.001) Regional Cerebral Blood Flow (rCBF) Following Ondansetron Compared with Saline in Early-onset vs. Late-onset Cocaine-dependent vs. Healthy Control Participants
| Identified ROI | MNI coordinates | kE1 | ||
|---|---|---|---|---|
| x | y | z | ||
| Early-onset vs. Late-Onset | ||||
| Increased rCBF in Early vs Late | ||||
| Parahippocampal gyrus, right | 26 | −36 | −8 | 114 |
| Subthalamic nucleus, right | 18 | −16 | −6 | 83 |
| Insula, left | −36 | 2 | 12 | 93 |
| Decreased rCBF in Early vs. Late | ||||
| Superior frontal gyrus, right | 18 | 38 | 30 | 163 |
| Inferior parietal lobule, right (BA40) | 48 | −34 | 34 | 64 |
| Early-onset vs. Controls | ||||
| Increased rCBF | ||||
| Subthalamic nucleus, right | 18 | −16 | −6 | 141 |
| Lingual gyrus, right | 8 | −68 | −2 | 87 |
| Anterior cingulate, BA32 | 4 | 42 | 2 | 100 |
| Parahippocampal gyrus, right | 34 | −38 | −12 | 67 |
| Lateral occipital gyri (BA19), left | −44 | −68 | −12 | 62 |
| Decreased rCBF | ||||
| Superior frontal gyrus, right | 18 | 38 | 32 | 83 |
| Late-onset vs. Controls | ||||
| Increased rCBF | ||||
| Anterior cingulate | −16 | 28 | 28 | 85 |
| Decreased rCBF | ||||
| Insula, left | −36 | 4 | 14 | 161 |
| Parahippocampal gyrus, right | 28 | −36 | −4 | 84 |
Cluster size
Acknowledgments
The authors thank the staff of the Substance Abuse Team at the VA North Texas Health Care System, Homeward Bound, Inc., and the Nexus Recovery Center for their support in the screening and recruitment of study subjects. This study was funded by NIH DA11434 and supported by the VA North Texas Health Care System. Ceretec (HMPAO) was generously supplied by GE Healthcare.
Footnotes
Authors Contribution
Dr. Adinoff was responsible for study concept, design and oversight and manuscript preparation. Dr. Devous contributed to study design, manuscript preparation, and supervised the imaging laboratory. Dr. Williams was responsible for subject screening and recruitment, administration of all assessments, and data integrity. Mr. Harris was responsible for obtaining SPECT imaging and image analyses. Ms. Dong performed statistical analysis. Drs. Best contributed to study design and Drs. Best and Zielinski assessed study participants and supervised infusions. All authors critically reviewed the content and approved the final version for publication.
Conflicts of Interest
None of the authors report any relevant conflicts of interest.
Dr. Adinoff receives grant/research support from NIAAA, NIDA, and Department of Veterans Affairs.
Dr. Devous is on the Scientific Advisory Board of AVID Radiopharmaceuticals and receives research support NIA, NIDA, DOE, Alzheimer’s Association and AVID Radiopharmaceuticals.
Dr. Williams, Dr. Best, Mr. Harris, Ms. Yang and Dr. Zielinski report no outside financial interests.
References
- Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, Zielinski T, Cullum M. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology. 2010;35:1485–1499. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Daoud N. New medication treatment for stimulant dependence. 2008. [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Allen JJ, Nadel L. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann N Y Acad Sci. 2000;911:355–368. doi: 10.1111/j.1749-6632.2000.tb06737.x. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey MH, Noumair D. Age of alcoholism onset. I. Relationship to psychopathology. Arch Gen Psychiatry. 1989;46:225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel R. The Temperament and Character Inventory (TCI): a guide to its development and use. Washington University School of Medicine, Department of Psychiatry; St. Louis: 1994. [Google Scholar]
- Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Psychological Assessment Resource, Inc; Odessa, FL: 1992. [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Sites of action of ondansetron to inhibit withdrawal from drugs of abuse. Pharmacol Biochem Behav. 1990;36:97–104. doi: 10.1016/0091-3057(90)90132-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given in the acute withdrawal from a repeated cocaine sensitization dosing regimen reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacology. 2002;27:542–553. doi: 10.1016/S0893-133X(02)00336-6. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Johnson BA. Pharmacotherapeutic trials in adolescent alcohol use disorders: opportunities and challenges. Alcohol Alcohol. 2004;39:166–177. doi: 10.1093/alcalc/agh045. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. The Journal of pharmacology and experimental therapeutics. 2011 doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Tester ML, Marsh DM. Age at first drink relates to behavioral measures of impulsivity: the immediate and delayed memory tasks. Alcohol Clin Exp Res. 2004;28:408–414. doi: 10.1097/01.alc.0000117834.53719.a8. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry. 2011;16:1139–1146. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Fan P, Oz M, Zhang L, Weight FF. Effect of cocaine on the 5-HT3 receptor-mediated ion current in Xenopus oocytes. Brain Res. 1995;673:181–184. doi: 10.1016/0006-8993(94)01316-a. [DOI] [PubMed] [Google Scholar]
- Farber L, Haus U, Spath M, Drechsler S. Physiology and pathophysiology of the 5-HT3 receptor. Scand J Rheumatol Suppl. 2004;119:2–8. [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Schofield PR, Paul RH, Clark CR, Gordon E, Williams LM. Early life stress combined with serotonin 3A receptor and brain-derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biol Psychiatry. 2010;68:818–824. doi: 10.1016/j.biopsych.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Herges S, Taylor DA. Involvement of 5-HT(3) receptors in the nucleus accumbens in the potentiation of cocaine-induced behaviours in the rat. Br J Pharmacol. 2000;131:1294–1302. doi: 10.1038/sj.bjp.0703687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinvest NS, Elliott R, McKie S, Anderson IM. Neural correlates of choice behavior related to impulsivity and venturesomeness. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, Diclemente CC, Li MD. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168:265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Cloninger CR, Roache JD, Bordnick PS, Ruiz P. Age of onset as a discriminator between alcoholic subtypes in a treatment-seeking outpatient population. Am J Addict. 2000a;9:17–27. doi: 10.1080/10550490050172191. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Javors MA, Harrison JM, Elkashef A, Mojsiak J, Li SH, Bloch DA. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84:256–263. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA. 2000b;284:963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- King GR, Xiong Z, Douglass S, Ellinwood EH. Long-term blockade of the expression of cocaine sensitization by ondansetron, a 5-HT(3) receptor antagonist. Eur J Pharmacol. 2000;394:97–101. doi: 10.1016/s0014-2999(99)00926-7. [DOI] [PubMed] [Google Scholar]
- King GR, Xiong Z, Ellinwood EH. Blockade of accumbens 5-HT3 receptor down-regulation by ondansetron administered during continuous cocaine administration. Eur J Pharmacol. 1999;364:79–87. doi: 10.1016/s0014-2999(98)00795-x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27:1150–1155. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Lee GP, DiClimente CC. Age of onset versus duration of problem drinking on the Alcohol Use Inventory. J Stud Alcohol. 1985;46:398–402. doi: 10.15288/jsa.1985.46.398. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Verge D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. The European journal of neuroscience. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci. 2007;26:1067–1076. doi: 10.1111/j.1460-9568.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. Am J Addict. 2008;17:6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, Roll J, Shapiro B, Rotheram-Fuller E, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR. The inventory of drug use consequences (InDUC): test-retest stability and sensitivity to detect change. Psychol Addict Behav. 2002;16:165–168. [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.