Abstract
The HIV-1 transgenic (HIV-1Tg) rat shows a deficit in learning to locate a submerged platform in a multiple-trial water maze task compared to transgenic littermate and F344 control rats (Vigorito et al. 2008; Lashomb et al. 2009). Nicotine is known to have neuroprotective effects possibly by minimizing cytotoxic effects of glutamate or by modulating a cholinergic anti-inflammatory pathway. Nicotine also improves performance in a variety of learning tasks by enhancing attention and short-term memory (STM). The purpose of this study was to determine if the learning deficit in HIV-1Tg is ameliorated by repeated nicotine treatment independent of its effects on STM. HIV-1Tg and F344 rats were treated (subcutaneous) with nicotine (0.25mg/kg/injection) or saline twice daily and tested in a single-trial-per-day procedure which precludes the impact of STM on the acquisition of the spatial learning task. HIV-1Tg rats showed a deficit in the acquisition of the task and in the long-term retention for the platform location in a probe test. Nicotine did not ameliorate the deficit in HIV-1Tg rats and slightly worsened performance during acquisition. Analysis of individual differences in performance during the probe test suggested that nicotine improved performance in some F344 rats but not in HIV-1Tg rats. These results indicate that a deficit in the consolidation of long-term memory (LTM) contributes to the acquisition deficit of HIV1-Tg rats. The results, however, do not provide any evidence of the amelioration of the learning deficit observed in this behavioral model at least with the nicotine dose tested.
Keywords: HIV, nicotine, behavior, learning and memory, Morris water maze
Introduction
With the introduction of highly active antiretroviral therapy (HAART), the morbility and mortality in HIV positive patients have been significantly reduced. Although HAART has lessen the incidence of HIV-associated dementia (HAD) (McCutchan et al. 2007; Nath 2010), HIV positive patients continue to suffer from HIV-associated neurocognitive disorders (HAND) and the prevalence of HAD actually increased due to the extended lifespan in HIV positive patients and the resistance to HAART in some patients (Antinori et al. 2007; Gonzalez-Scarano and Martin-Garcia 2005; McArthur et al. 2003; Nath 2010). In addition, recent studies suggest that the greater incidence of drug abuse among HIV-positive patients may exacerbate the progression of HAD as the psychoactive drugs and the products of the HIV-1 virus interact additively or synergistically on common brain regions. Given the prevalence of drug addiction in HIV patients and other factors contributing to disease progression, it becomes a challenge to study disease pathogenesis clinically (Nath 2010).
To better understand the effect of HIV-1 on neurocognitive functioning and the interaction with substances of abuse, we have been studying the HIV-1 Transgenic (HIV-1Tg) rat (Lashomb et al. 2009; Vigorito et al. 2007; Kass et al. 2010; Peng et al. 2010; Liu et al. 2009). The HIV-1 rat was developed at the University of Maryland Biotechnology Institute (UMBI) from F344/NHsd stock and is commercially available. This transgenic rat model is noninfectious and expresses HIV-1 proteins that have been implicated in HAD (Reid et al. 2001). Unlike other rodent models which examine the effects of viral proteins following acute treatment (e.g., gp120, TAT), the HIV-1 viral proteins are genetically expressed in HIV-1Tg resulting in a chronic condition that may be similar to HIV-1-positive patients undergoing HAART (Peng et al. 2010). The availability of a rodent model to investigate HIV-induced neurocognitive dysfunction also permits a more controlled investigation of learning and memory than in clinical populations.
The Morris water maze (MWM) is a widely accepted paradigm for investigating learning and memory in laboratory animals (Morris 1984). In this task the subjects must locate a submerged platform to escape the aversive experience of being placed in water. The MWM takes advantage of rodents’ spatial learning ability and their natural biases in “solving” the water maze. In addition to the visual landmarks that are located outside of the pool (i.e., extramaze cues), the rats can also use static features of the pool (e.g., the pool wall) and other intramaze landmarks when they are made available (Baldi et al. 2003; Sava and Markus 2005). If the platform has been removed in the probe test, rats that rely on environmental cues will show place learning by continuing to search in the location the platform was previously located. This position bias during the probe test is typically interpreted as reflecting the retrieval of a long-term memory (LTM) for the platform location. Rats that learn the water maze task independent of environmental cues (e.g., path integration), however, do not show a place bias (Tamara et al. 2010).
In previous studies we found that HIV-1Tg rats show a deficit in the acquisition of the MWM task (Lashomb et al. 2009; Vigorito et al. 2007). Because HIV-1Tg rats are born with cataracts their visual acuity is impaired relative to transgenic littermate controls and F344 control rats. Thus to compare the performance of HIV-1Tg rats with control rats it was necessary to test the animals under dim red light and replace the typical visual extramaze landmarks with non-visual intramaze landmarks (e.g., tactile and olfactory cues) and an auditory extramaze cue. Under these circumstances HIV-1Tg rats took longer than F344 rats to learn to locate the escape platform efficiently. Once their performance reached asymptote, however, probe tests indicated a position bias (i.e., a place memory) that did not differ from controls. The probe test performance, therefore, confirmed that the HIV-1Tg rats, like the Tg and F344 controls, remembered the place where the platform was located.
Animals may also utilize short-term memory (STM) to complete the task when multiple trials are given per day, especially early in acquisition, as the animals remember the location of the platform on the first trial to locate the escape platform more efficiently on subsequent trials (Baldi et al. 2005). With continued daily training the escape task becomes well learned and the memory for the platform location persists beyond 24 hours. Although our previous studies did not reveal any differences in within-session improvements in performance between the HIV-1Tg and F344 groups, the contribution of possible group differences in STM to the acquisition of the water maze task is uncertain. In the present study we eliminated the possible influence of STM differences between groups by training the rats with only one trial per day. STM lasts 1 to 6 hours (Izquierdo & McGaugh, 200), thus 24 hours between trials is well beyond the limits of STM. In a single-trial-per-day procedure, subjects cannot utilize STM and as such improved performance over days must reflect only improvement in the consolidation of LTM.
The present experiment also investigated the effect of nicotine on performance in the MWM. In human studies, nicotine and other agonists of the nicotinic acetylcholine receptor (nAChRs) have been reported to improve cognitive performance in healthy adults and in different psychiatric populations (Deiana et al. 2011; Levin et al. 2006). In studies with rodents, the results have been mixed with observations of improvement, impairment, or no effect on various measures of learning and memory following acute or chronic systemic treatment (see Deiana et al. 2011 for review). Nicotine effects on rodent spatial learning in the MWM in particular have also been mixed although several studies have reported improvements in acquisition and/or in the probe test particularly in older animals showing age-related performance deficits (Abdulla et al. 1996; Arendash et al. 1995; Attaway et al. 1999; Hernandez and Terry Jr. 2005; Socci et al. 1995). Overall, the most consistent effect of nicotine treatment has been the enhancement of attention and STM, rather than LTM (Hernandez and Terry Jr. 2005; Levin and Torry 1996; Deiana et al. 2011). Thus, with the exception of one study where rats were trained in a one-trial-per-day procedure (Hernandez and Terry Jr. 2005), a possible contribution of nicotine’s STM-enhancing effects on the acquisition of spatial learning cannot be excluded. Restricting MWM training to one trial per day in the present study precluded possible effects of group differences in STM on acquisition of long-term spatial memories.
There is a reason to expect that nicotine may improve learning and memory in HIV-1Tg rats independent of nicotine’s effect on STM. Nicotine has been reported to be involved in protective mechanisms against neuronal damage and in the modulation of immune function. Nicotine seems to protect neurons against cytotoxic effects of glutamate and possibly modulates beta-amyloid to reduce neuronal death (Marin et al. 1994; Shimohama and Kihara 2001; Li et al. 2000; Gutala et al. 2006). Nicotine may also act therapeutically on microglia-mediated inflammation by modulating a cholinergic anti-inflammatory pathway (DeSimone et al. 2005). With respect to HIV-1-infection, gp120 appears to alter cholinergic transmission by competing with acetylcholine for nAChRs on neurons and muscle cells (Bracci et al. 1992; Gonzalez-Lira et al. 2006). Thus nicotine may act as a gp120 antagonist and may be effective in reversing the learning deficit observed in HIV-1Tg rats. Notwithstanding these potential positive effects of nicotine on neuronal functioning, and in light of the fact that drugs of abuse impact more negatively on HIV positive individuals, it is also possible that nicotine may be more disruptive than ameliorating on the behavioral performance of HIV-1Tg rats. In this paper we report the results of the effects of twice daily nicotine treatment on the acquisition and long-term retention of spatial learning in a one-trial per day MWM task in HIV-1 Tg and F344 control rats. The mRNA expression profiling in different brain regions of these animals using RNA deep sequencing technology has also been evaluated and will be reported separately as a molecular study.
Results
Swim speed increased during the first few days of training in all groups (data not shown), F9, 846 = 13.48, p = .000, but there were no differences between strains [ F344: Mean = 0.24 m/sec ± .01, HIV-1Tg: Mean = 0 .23 ± .01], (F1, 94 = 0.63, p > .05) or drug treatment groups [ Nicotine: Mean = .23 m/sec ± .01, Saline: Mean = .24 ± .01], (F1, 94 = 0.63, p > .05) and there was no interaction between these two factors (F1, 94 = 0.14, p > .05). Thus, performance differences between groups were not a result of differences in swim speed, but due to the swimming pattern.
Acquisition
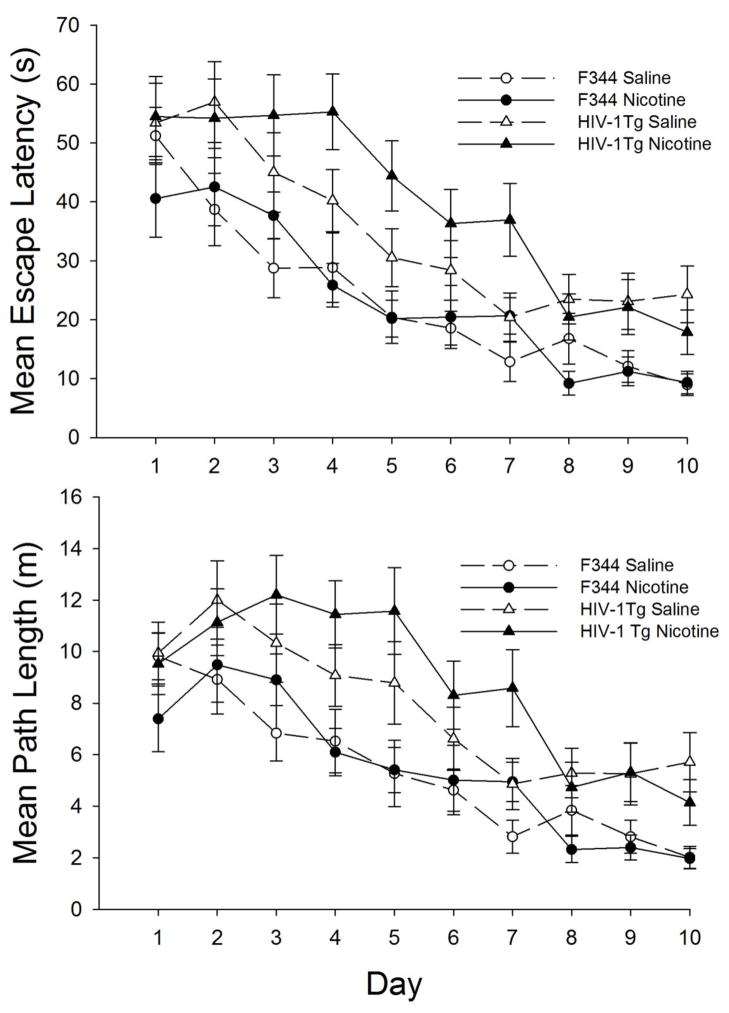
The mean escape latencies (upper panel) and path lengths (lower panel) during the 10 days of water maze training are shown in Figure 1. Analysis of the path-lengths and latencies yielded similar results, thus analysis of the escape latencies are included in the present paper for consistency with our previous reports (Vigorito et al, 2007; Lashomb, 2009) and so that the acquisition and probe data of the present study are in the same units of measurement (secs).
Fig. 1.
Mean escape latencies in seconds (top panel) and mean path lengths in meters (bottom panel) of nicotine-treated (closed symbols) and saline-treated (open symbols) F344 (circles) and HIV-1Tg (triangles) rats during the 10 days of water maze acquisition training
All groups demonstrated acquisition of the water maze task as escape latencies decreased over days, (F9, 846 = 29.66, p = 0.000). However, the HIV-1Tg groups showed consistently longer escape latencies throughout acquisition compared to the F344 control groups (Strain: F1, 94 = 40.10, p = 0.000; Strain x Day interaction, F9, 846 = 0.65, p > 0.05). Although Figure 1 suggests that nicotine-treated HIV-1Tg rats were the worst performing group, the main effect of Drug Treatment(F1, 94 = 1.48, p >0.05) and the interactions of Strain x Drug Treatment, (F1, 94 = 1.42, p > .05) and Strain x Drug Treatment x Day (F9, 846 = 0.77, p > .05) failed to be significant.
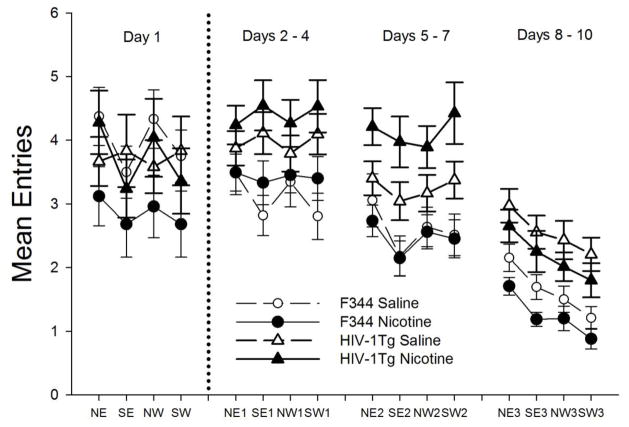
To further investigate group differences during acquisition the number of entries made into the four quadrants during a daily test were examined (Figure 2). The mean entries were averaged over three-day blocks during acquisition days 2 to 10 (The data from the first day of acquisition is shown in the figure but was excluded in the analysis since performance on this first exposure to the water maze could not reflect any learning). An initial Strain (2) x Drug Treatment (2) x Quadrant (4) x Block (3) mixed ANOVA yielded a significant main effect of Strain (F1, 94 = 27/70. p =0.000) and a Strain x Quadrant interaction (F3, 282 = 5.34. p =0 .001) as well as a Strain x Drug Treatment x Block interaction that was just short of significance (F6, 564 = 1.89, p =0 .08). Thus the HIV-1Tg and F344 strains were analyzed with separate ANOVAs. The number of entries into the various quadrants declined over blocks (Figure 2) indicating more efficient performance in the water maze with training, but the nicotine-treated HIV-1Tg rats showed a greater number of entries into all 4 quadrants than the saline-treated HIV-1Tg rats. This effect of nicotine was greatest during the second block of training (F2, 94 = 3.3, p =0.04), and was no longer evident during the third three-day block. By the third block of training the HIV-1Tg rats were showing more entries into the NE quadrant, where the escape platform was located, than into the western quadrants (F6, 282 = 7.45, p =0 .000). The F344 groups also showed improved performance over blocks and proportionately more entries into the NE quadrant as training progressed (F6, 282 = 6.31, p = 0.000). By the third training Block, the nicotine-treated F344 group was showing fewer entries than the saline-control group, but this effect was not significant (F2, 94 = 1.01, p > 0.05).
Fig. 2.
Mean entries of nicotine-treated (closed symbols) and saline-treated (open symbols) F344 (circles) and HIV-1Tg (triangles) rats into the four quadrants (NE, SE, NW, SW) during acquisition plotted as means over three days within each block (Block 1= days 2–4; Block 2= days 5–7; Block 3= days 8–10). The means on the first day of training are also shown but were not included in the statistical analysis since performance on the first day reflects chance discovery of the platform rather than learning.
Probe Test
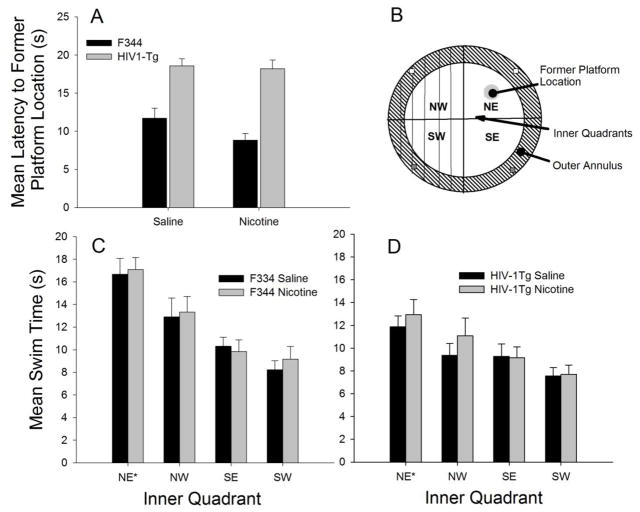
Figure 3a shows that the F344 groups performed a more efficient search path during the probe test that resulted in significantly shorter latencies than the HIV-1Tg groups (F1, 94 = 8.02, p = 0.01). However, neither the main effect of Nicotine Treatment (F1, 94 = 0.32, p >0 .05), nor the Nicotine Treatment x Strain interaction (F1, 94 = 1.75, p >0 .05), was significant.
Fig. 3.
Probe test performance of nicotine-treated and saline-treated F344 and HIV-1Tg rats. (A) Mean latency to the former platform location. (B) Diagram of the modified water maze showing the zones and intra-maze cue locations. Each quadrant consists of an inner area (white) and an outer area (shaded outer annulus). The lines in the NW and SW Quadrants indicate the location of the tactile cues. The small squares in the center of the quadrants along the wall indicate the location of the mint (NW and NE) and vanilla (SW and SE) scented pipette cleaners. The metronome was located near the NW quadrant and is not shown in the diagram. During the acquisition the submerged platform was located in the inner NE quadrant. (C, D) Mean swim time in the four inner quadrants
Both the F344 and HIV-1Tg groups demonstrated a swim bias for the inner NE quadrant (Figure 3c), although the bias was much more prominent in the F344 groups (Strain x Quadrant interaction, F3, 282 = 3.78, p = 0.01). The F344 rats (all ps = .000) and HIV-1Tg rats (all ps ≤ 0.001) showed greater swim time in the inner NE quadrant compared to all other inner quadrants. Moreover, the F344 rats swam more in the inner NE quadrant (F1, 94 = 22.97, p = 0.000), and inner NW quadrant (F1, 94 = 6.64, p = .01), than the HIV-1Tg rats, but they did not differ in terms of time spent swimming in the SE and SW quadrants. Nicotine treatment did not significantly affect swim performance during the probe test.
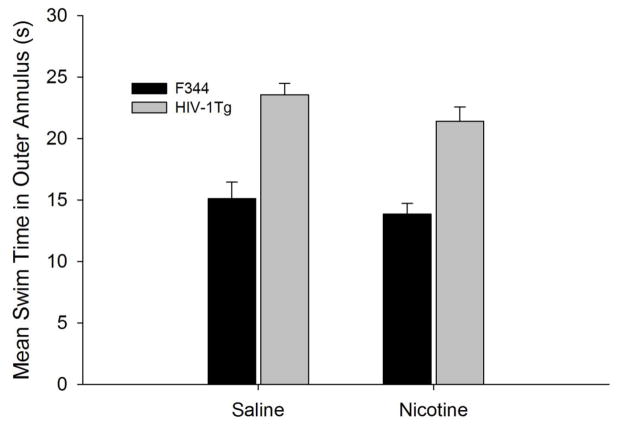
Although the HIV-1Tg rats demonstrated a clear bias for the former location of the platform, their search performance was less effective than the F344 rats. Figure 4 shows that the poorer performance was partly due to swimming in the outer annulus (F1, 94 = 33.87, p =0 .000). As with the other probe test measures, nicotine treatment did not affect swim time in the outer annulus in any group (F1, 94 = 1.54, p > 0.05).
Fig. 4.
Mean swim time of nicotine-treated and saline-treated F344 (black bars) and HIV-1Tg (grey bars) rats in the outer annulus during the probe test
Inspection of the probe latency data yielded considerable between- and within –group variability. To further analyze the individual differences in latency to the former platform location within groups, a K-means cluster analysis was conducted on the four groups separately to subdivide the groups into 3 clusters. Three clusters were chosen on the assumption that there was at least 1 subject in each with extreme latencies and that the remaining subjects can be grouped into two subpopulations that differ in performance on the probe test. Table 1 shows the three clusters classified in the nicotine- and saline-treated HIV-1Tg and F344 groups. The analysis successfully isolated the possible outliers in the F344 groups and the worst-performing HIV-1Tg rats with all but one failing to cross the former escape platform location during the 60 second probe test. The rats assigned to Cluster A were the best performing rats as they reached the platform location in less than 9 seconds. Cluster B included rats that swam to the escape platform location, but less efficiently as the Cluster A rats. Figure 5 shows data from the HIV-1Tg and F344 rats assigned to Cluster A and Cluster B. The Cluster C rats were excluded. A Strain (2) x Drug Treatment (2) x Cluster (2) ANOVA yielded a significant three way interaction (F1, 82 = 8.84, p < 0.01. Additional ANOVAs and t-tests indicated that among the F344 rats, the nicotine-treated Cluster B rats had significantly lower latencies than the saline-treated Cluster B rats (p < .05); the two F344 Cluster B groups did not differ. Nicotine did not have significant differential effects on either cluster of the HIV-1Tg rats.
Table 1.
Group Latencies to Former Platform Location in the Probe Test Based on K-Means Cluster Analysis
| Cluster A
|
Cluster B
|
Cluster C
|
||||
|---|---|---|---|---|---|---|
| Mean | n | Mean | n | Mean | n | |
|
|
|
|
||||
| F344 Nicotine | 4.99 ± 0.5 | 17 | 14.47 ± 0.7 | 7 | 35.20 | 1 |
| F344 Saline | 6.36 ± 0.8 | 18 | 22.50 ± 2.9 | 5 | 53.90 | 1 |
| HIV-1 Tg Nicotine | 6.29 ± 1.0 | 14 | 24.99 ± 2.2 | 8 | 55.6 ± 4.4 | 3 |
| HIV-1 Tg Saline | 7.12 ± 1.0 | 13 | 21.7 ± 2.3 | 8 | 60.00 ± .00 | 3 |
Note. ± S.E.M. shown for all groups except the F344 Cluster C groups which had n = 1.
Fig. 5.
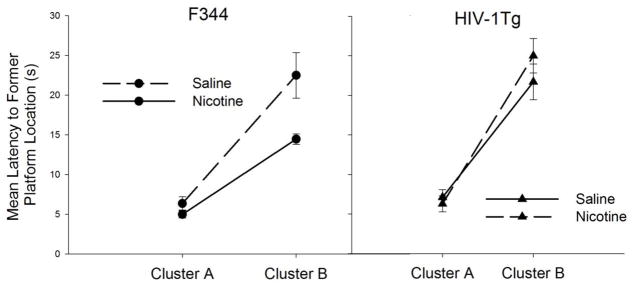
Mean latency to the former platform location in nicotine- and saline-treated F344 and HIV-1Tg group clusters based on K-Means Cluster Analysis
Discussion
Our previous studies demonstrated that HIV-1Tg rats show a consistent deficiency in the acquisition of a multiple-trials-per-day water maze task (Lashomb et al. 2009; Vigorito et al. 2007). The results of the present experiment extend our previous observations by demonstrating a similar acquisition deficit in HIV-1Tg rats tested in a one-trial-per day procedure. In a multiple-trial procedure, the memory of the platform location on a previous trial (i.e. STM) may contribute to improved performance on subsequent trials and therefore with the overall acquisition of the task. Thus, it is possible that the acquisition deficit observed in the HIV-1Tg rats in our previous studies was due at least in part to deficits in the efficacy of STM, rather than, or in addition to, the consolidation of LTM. Although the contribution of STM to the acquisition of the task was eliminated with the one-trial-per day procedure in the present study, a deficit in the HIV-1Tg rats compared to F344 controls was again observed, suggesting that group difference in acquisition was due to a deficit in the consolidation of LTM. Moreover, nicotine disrupted performance of the HIV-1Tg rats during the middle days of acquisition relative to saline-treated HIV-1 Tg rats. This further reduction in performance may reflect a greater susceptibility to disruptive effects on behavior in general when learning is incomplete or already disordered.
Both the F344 and HIV-1Tg showed a place bias for the NE quadrant in the probe test confirming that a search strategy utilizing environmental landmark cues was adopted by the animals and a place memory was learned during acquisition. The stronger place bias of the F344 groups in the probe test also indicated that the F344 rats had a better memory for the platform location than the HIV-1Tg rats. Our previous studies (Lashomb et al. 2009; Vigorito et al. 2007) did not find group differences in place bias in the probe test. But in these prior experiments the rats received more training in acquisition with 4 trials per day for 20 or more total trials – more than double the number of trials in the present experiment. Thus the probe test in the previous studies was conducted after the HIV-1Tg rats “caught up” to the F344 rats in the acquisition phase, whereas in the present study the probe test was conducted while the groups were still showing performance differences.
The effect of nicotine on the acquisition of water maze performance was also investigated based on the hypothesis that the learning and memory deficit in HIV-1Tg rat may be ameliorated through nicotine’s neuroprotective effects (Gutala et al. 2006) or through its modulation of immune function (DeSimone et al. 2005). Because a one-trial-per-day procedure was used in the present experiment, any improved performance could not be attributed to the beneficial effects of nicotine on STM (Hernandez and Terry Jr. 2005; Levin and Torry 1996; Deiana et al 2011). Nevertheless, there was no evidence of improved performance during acquisition of the water maze task in the nicotine-treated HIV-1Tg or F344 groups. Conversely, there was some evidence that nicotine worsened performance in the HIV-1Tg rats but not in the F344 rats. This latter result supports other observations that HIV-1Tg rats show differential sensitivity to drugs of abuse (Liu et al 2009; Kass et al 2010; Midde et al. 2011). Greater sensitivity to a drug, for example, may increase the disruptive effects of the drug on performance when administered during a learning task.
Assessment of group performance in the probe test also failed to find a consistent effect of nicotine on swim latency or memory for the platform as indicated by swim bias times. Thus, nicotine at the dose (0.25 mg/kg) and schedule (twice per day, one week before and during training) used in the present experiment failed to reverse the learning deficit in the HIV-1Tg rats. This negative result was not a result of a floor effect masking a nicotine effect since the HIV-1Tg rats had not yet achieved maximum performance in the task. A limitation of this study is that only one nicotine dose was tested. The dose selected was the highest dose, based on pilot studies, which did not produce performance deficits during acquisition in F344 controls. However, subsequent region-specific mRNA expression profiling in the brains of these rats revealed significant changes in many genes by this nicotine dose (data not shown).
Interestingly, there was some evidence that nicotine reduced the variability in the probe test latencies in the F344 rats. Most of the F344 rats were swimming directly to the platform location with little room for additional improvement in path efficiency; hence, there was little opportunity for shorter escape latencies. Thus, a floor effect may have masked the potential beneficial effects of nicotine. A cluster analysis identified a subpopulation of F344 rats that were not performing at the shortest possible latencies. Among this population, the nicotine-treated rats had shorter latencies than the saline–treated rats, suggesting that nicotine may have improved LTM for the platform location in some F344 rats. A similar analysis yielded no effect of nicotine in the HIV-1Tg rats. Although we did not observe any significant effect of nicotine on HIV-1Tg rats, the results need to be carefully interpreted considering potential limitations of water maze tests such as stress, mixed strategies to complete the tasks, and relatively easy task without high attention demand (Paul, 2009).
Poorer acquisition of the one-trial-per-day water maze task in the HIV transgenic rats suggests that genetically-expressed viral proteins induced a learning deficit when long-term, but not short-term, information about the platform location was available. Although this result confirms that the learning deficit cannot be attributed to deficits in STM, the precise nature of the learning deficits caused by viral proteins is not clear.
Considerable evidence suggests that the hippocampus mediates learning of spatial cues whereas egocentric learning appears to be mediated by the striatum (Cain et al. 2006; Devan et al. 1996; Morris et al.1982) and the prefrontal cortex (Mogensen et al. 2005). The prefrontal cortex and hippocampus in conjunction may additionally be involved in behavioral flexibility (de Bruin et al. 1997). We have also observed impairments in the HIV-1Tg rats when the location of the platform was changed (reversal learning) and when the transgenic rats were required to change their search strategy (Lashomb et al 2009). The persistent presence of HIV viral proteins may result in dysregulation of the neural pathways mediating learning in a water maze task. Both gp120 and tat show greater expression in the prefrontal cortex of HIV-1Tg rats than other brain regions (Peng et al, 2010). Compared to F344 rats, HIV-1Tg rats show lower levels of pCREB and pERK2 in the prefrontal cortex (Midde et al 2011). Alterations in the prefrontal cortex of transgenic rats have also been supported by the finding that HIV-1Tg rats show greater expression of D1 receptors than do F344 controls (Liu et al 2009). These observations suggest that modifications by viral proteins of the prefrontal cortex and related brain areas contribute to deficits in learning and behavior. Elucidating how HIV-1 viral proteins influence the neural pathways mediating learning will help in understanding the cognitive disturbances that are prevalent in HIV positive individuals.
Materials and Methods
Animals
The subjects were 49, male Fischer 344/NHsd (F344) rats and 49, male HIV type 1 (HIV-1) transgenic (Tg) rats purchased from Harlan Co (Indianapolis, IN) that were 8 to 12 weeks of age at the onset of the experiment. Prior to the onset of any experimental manipulations, rats were given two weeks to acclimate to the vivarium. Subjects were double housed in standard, plastic rat cages with Harlan Teklad™1.8″, corn-cob bedding, and were provided food (Harlan Teklad™ Mouse/Rat Laboratory Diet 7102) and water ad libitum. The vivarium was maintained on a 12:12 hour light-dark cycle (7:00am – 7:00pm), and within recommended temperature (22° ± 5° C) and humidity (50% ± 20%) conditions. Rat body weights and intakes were measured daily. All experimental procedures were conducted during the light cycle and in accordance with the Seton Hall University Institutional Animal Care and Use Committee.
Drug preparation and treatment
Nicotine-free base (Sigma-Aldrich Co.; St. Louis, MO) was diluted in 0.9% sterile saline and the pH was adjusted to 7 with 3N HCl. To determine a dose of nicotine treatment several pilot studies were conducted with F344 rats tested in the same MWM procedures used in the present experiment. The pilot studies assessed the effects of nicotine in the range of 0.125 to 1.0 mg/kg administered subcutaneously (s.c.), twice daily. Reduced water maze performance seen at the highest doses was not observed at the lowest doses, thus a dose of 0.25 mg/kg was chosen. Four groups of rats were tested in the present experiment: HIV-1Tg Nicotine (n = 24), HIV-1Tg Saline (n =25), F344 Nicotine (n = 24), and F344 Saline (n = 25). Twice daily (7:00 am and 4:00 pm) sc injections of nicotine (0.25 mg/mL; 1 mL/kg) or saline (1 mL/kg) were administered with 23 gauge/1cc syringes beginning one week before water maze training and continuing until the last day of testing. A fresh stock of nicotine solution was prepared daily.
Modified water maze
The modified Morris water maze (MWM) used in the present experiment has been described elsewhere (Vigorito et al. 2007; LaShomb et al. 2009). In brief, the water maze consisted of a circular pool (130 cm in diameter and 52.5 cm high) made of black plastic and filled with water to a level covering the escape platform surface by 2 cm. The escape platform was painted black and placed in the same fixed location throughout the experiment away from the wall in the center of a quadrant arbitrarily labeled as the NE quadrant. To prevent the use of reflected light from objects in the room as visual landmarks all rats were tested only under dim red-light illumination provided by two red bulbs in metal clamp lamps that were located at opposite ends of the room and pointing upwards. The MWM was modified to provide a combination of intra-maze tactile and olfactory cues and an extra-amaze auditory cue as potential navigational landmarks. The tactile cue covered the entire NW and SW quadrants and consisted of four parallel lines across the top of the pool that dropped numerous strings of nylon fishing lines perpendicular to the surface of the water. As the rats swam through the NW and SW quadrants the nylon fishing lines brushed against them without impeding their swimming ability. Olfactory cues were provided by draping 30 cm pipe cleaners over the side of the pool at the center of each quadrant. The pipe cleaners in the NE and NW quadrants were dipped in mint mouthwash and the pipe cleaners in the SE and SW quadrants were dipped in vanilla extract. Although no single cue served as a beacon for the platform location the rats had the opportunity to learn that the escape platform was located in the area with no tactile cues and a mint odor (i.e., the NE quadrant). In addition a single auditory cue (a metronome) was placed approximately 90 cm away from the wall of NW quadrant and about 20 cm above the top of the pool wall.
Test Procedure
An infrared FireWire camera mounted above the pool was used to capture images of the rats swimming during behavioral testing. These images were analyzed with a comprehensive video-tracking system (Any-maze; Stoelting Co.: Wood Dale, IL) using several of the available measures: (1) escape latency, i.e., time in seconds to reach the escape platform, (2) number of zone entries, (3) total swim time in the zones, (4) average swim speed (meters/second) and (5) path lengths. In addition to the four quadrants serving as zones, an outer annulus was included in the analysis to measure rats’ natural tendency to move along a wall (i.e., thigmotaxis) when searching for an escape (see Figure 3b). The inclusion of the outer annulus permitted the further division of each zone into an inner area and an outer area for a more precise measurement of the search performance during the probe test.
The present study was carried out in three replications with similar numbers of nicotine- and saline-treated HIV-1Tg and F344 rats per replication. All MWM procedures began at 12:00 pm (± 30 min). The rats were tested in a single trial per day for 10 consecutive days. Approximately 45 minutes after all animals completed the training trial on the last day of acquisition the rats were given a 60 second probe test to assess spatial bias.
Acquisition
Acquisition consisted of 10 consecutive days. Rats were familiarized with the escape platform on the first day of acquisition by placing the rat directly on the platform for up to 60 seconds before the trial. If a rat dove back into the water it was gently lifted and placed back on the platform. Familiarization did not take place on the subsequent days. A daily trial consisted of placing the rat in the water facing the pool wall and ended when the rat climbed on the top of the escape platform. The start location of each trial was randomly chosen from eight possible drop points along the perimeter of the pool. The experimenter remained at the drop off point until the trial was completed. When the trial ended the rat was allowed to remain on the escape platform for 15 seconds. If a rat failed to locate the escape platform after 90 seconds, the trial was terminated and the rat placed on the platform for 15 seconds before being removed from the pool.
Probe Test
To asses any spatial bias that may have developed during acquisition of the water maze task a 60-second probe trial was given at least 45 minutes after all of the rats completed the trial on the final day of acquisition. The probe test was similar to an acquisition trial except that the escape platform was absent. The start location for the probe test was randomly chosen for each replication, but was the same for all rats tested within a replication. After 60 seconds, the probe trial was terminated and rats were removed from the pool. Latency to the former location of the platform, time spent in the inner quadrants away from the walls, and time spent in the outer annulus along the walls were analyzed.
Data Analysis
To evaluate the acquisition of the water maze task and probe test performance each dependent measure was analyzed separately with Strain (HIV-1Tg, F344) ) and Drug Treatment (nicotine, saline) as between-subjects factors and Day (10) and Quadrant (4) as within-subjects factors. The mixed factorial ANOVAs were followed by additional ANOVAs and t-tests for analysis of main effects and interactions. The critical p value was by convention set at .05; however p values in the range of .06 and .09 were discussed as marginally significant effects and followed by additional analysis. In addition, to better characterize the differential distribution of scores of the HIV-1Tg and F344 rats, box plots were created for the probe test scores and K Means cluster analysis was used to identify sub-populations within a group. All data were analyzed with SPSS 17.0.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant DA-026356 (SLC & MDL) and DA-016149 (SLC). The authors are grateful to Nicole Anastasides, Daniel Kroner and Marley Kass for assistance with data collection, and to Dr. Tanseli Nesil for providing valuable comments on the paper.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest
Contributor Information
Michael Vigorito, Institute of Neuroimmune Pharmacology, Seton Hall University, 400 South Orange Avenue, South Orange, NJ 07079 USA. Department of Psychology, Seton Hall University, 400 South Orange Avenue, South Orange, NJ 07079 USA.
Junran Cao, Department of Psychiatry and Neurobehavioral Sciences, 1670 Discovery Drive, Suite 110, University of Virginia, Charlottesville, VA 22911 USA.
Ming D. Li, Department of Psychiatry and Neurobehavioral Sciences, 1670 Discovery Drive, Suite 110, University of Virginia, Charlottesville, VA 22911 USA
Sulie L. Chang, Email: sulie.chang@shu.edu, Institute of Neuroimmune Pharmacology, Seton Hall University, 400 South Orange Avenue, South Orange, NJ 07079 USA. Department of Biological Sciences, Seton Hall University, 400 South Orange Avenue, South Orange, NJ 07079 USA
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, et al. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology (Berl) 1996;124:323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Sanberg PR, Sengstock GJ. Nicotine enhances the learning and memory of aged rats. Pharmacol Biochem Behav. 1995;52:517–523. doi: 10.1016/0091-3057(95)00119-h. [DOI] [PubMed] [Google Scholar]
- Attaway CM, Compton DM, Turner MD. The effects of nicotine on learning and memory: a neuropsychological assessment in young and senescent Fischer 344 rats. Physiol Behav. 1999;67:421–431. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Baldi E, Efoudebe M, Lorenzini CA, Bucherelli C. Spatial navigation in the Morris water maze: Working and long lasting reference memories. Neuroscience Letters. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Task solving by procedural strategies in the Morris water maze. Physiology and Behavior. 2003;78:785–793. doi: 10.1016/s0031-9384(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Bracci L, Lozzi L, Rustici M, Neri P. Binding of HIV-1 gp120 to the nicotinic receptor. FEBS Letters. 1992;311:115–118. doi: 10.1016/0014-5793(92)81380-5. [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: A dissociation between brain mechanisms for learning how versus learning where to navigate. Behavioural Brain Research. 2006;170:241–256. doi: 10.1016/j.bbr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- de Bruin JPC, Swinkels WAM, de Brabander JM. Response learning of rats in a Morris water maze: Involvement of the medial prefrontal cortex. Behavioural Brain Research. 1997;85:47–55. doi: 10.1016/s0166-4328(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behavioural Brain Research. 2011;221:389–411. doi: 10.1016/j.bbr.2010.11.036. [DOI] [PubMed] [Google Scholar]
- DeSimone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of Hippocampal and Striatal Contributions to Spatial Navigation in the Water Maze. Neurobiology of Learning and Memory. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lira B, Rueda-Orozco PE, Galicia O, Montes-Rodriguez CJ, Guzman K, Guevara-Martinez M, et al. Nicotine prevents HIVgp120-caused electrophysiological and motor disturbances in rats. Neurosci Lett. 2006;394:136–139. doi: 10.1016/j.neulet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The Neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gutala R, Wang J, Hwang YY, Haq R, Li MD. Nicotine modulates expression of amyloid precursor protein and amyloid precursor-like protein 2 in mouse brain and in SH-SY5Y neuroblastoma cells. Brain Res. 2006;1093:12–19. doi: 10.1016/j.brainres.2006.03.100. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Terry AV., Jr Repeated nicotine exposure in rats: effects on memory function, cholinergic markers and nerve growth factor. Neuroscience. 2005;130:997–1012. doi: 10.1016/j.neuroscience.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, McGaugh JL. Behavioural pharmacology and its contribution to the molecular basis of memory consolidation. Behavioural Pharmacology. 2000;11:517–534. doi: 10.1097/00008877-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2010;5:566–573. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Matta SG, Blaner WS, Sharp BM. Nicotine enhances the biosynthesis and secretion of transthyretin from the choroid plexus in rats: implications for beta-amyloid formation. J Neurosci. 2000;20:1318–1323. doi: 10.1523/JNEUROSCI.20-04-01318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–316. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- Marin P, Maus M, Desagher S, Glowinski J, Premont J. Nicotine protects cultured striatal neurones against N-methyl-D-aspartate receptor-mediated neurotoxicity. Neuroreport. 1994;5:1977–1980. doi: 10.1097/00001756-199410000-00035. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Wu JW, Robertson K, Koletar SL, Ellis RJ, Cohn S, et al. HIV suppression by HAART preserves cognitive function in advanced, immune-reconstituted AIDS patients. AIDS. 2007;21:1109–1117. doi: 10.1097/QAD.0b013e3280ef6acd. [DOI] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–597. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J, Moustgaard A, Khan U, Wortwein G, Nielsen KS. Egocentric spatial orientation in a water maze by rats subjected to transection of the fimbria-fornix and/or ablation of the prefrontal cortex. Brain Research Bulletin. 2005;65:41–58. doi: 10.1016/j.brainresbull.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Paul CM, Magda G, Abel S. Spatial Memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009;203:151–164. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava S, Markus EJ. Intramaze cue utilization in the water maze: effects of sex and estrous cycle in rats. Horm Behav. 2005;48:23–33. doi: 10.1016/j.yhbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Kihara T. Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol Psychiatry. 2001;49:233–239. doi: 10.1016/s0006-3223(00)01100-8. [DOI] [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiol Aging. 1995;16:857–860. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Tamara C, Timberlake W, Leffel J. Rats (Rattus norvegicus) in a water maze learn both an egocentric trajectory and landmarks. J Comp Psychol. 2010;124:302–316. doi: 10.1037/a0019458. [DOI] [PubMed] [Google Scholar]
- Vigorito M, Lashomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]