Abstract
Virological confirmation of varicella zoster virus (VZV) vasculopathy is provided by presence of virus in cerebral arteries, frequently associated with inflammation. Yet cerebral arteries from normal subjects have never been studied for VZV DNA or antigen. We analyzed 63 human cerebral arteries from 45 subjects for VZV DNA and antigen, control herpes simplex virus (HSV)-1 DNA and antigen and leukocyte-specific CD45 antigen. No cerebral arteries contained VZV or HSV-1 DNA or antigen; 8 arteries from 7 subjects contained leukocytes expressing CD45. Thus, the presence of VZV antigen in cerebral arteries of patients with stroke is likely to be clinically significant.
Keywords: Neuropathology, Herpesvirus, VZV Vasculopathy
Introduction
Varicella zoster virus (VZV) vasculopathy is caused by productive virus infection in cerebral arteries (Doyle et al., 1993; Eidelberg et al., 1986; Fukumoto et al. 1986; Gilden et al., 1996; Berger et al., 2000) where accumulation of myofibroblasts in the arterial intima and loss of smooth muscle cells result in ischemic or hemorrhagic stroke (Nagel et al., 2011). In addition, inflammatory cells associated with viral antigen appear to be important in vascular wall changes (Nagel et al., 2013). However, no systematic investigation has been conducted to determine the frequency of VZV DNA or antigen and leukocytes in cerebral arteries from normal subjects. Herein, we analyzed 63 human cerebral arteries from 45 subjects with no history of transient ischemic attacks or stroke for the presence of VZV DNA and antigen, control HSV-1 DNA and antigen and leukocyte-specific CD45 antigen.
Materials and methods
All arteries obtained at autopsy were de-identified and deemed exempt by the Colorado Multiple Institutional Review Board. Table 1 provides clinical information on all subjects whose arteries were studied, the postmortem interval and the specific artery analyzed. All arteries were obtained within 48 h after death. The 45 subjects consisted of 30 (67%) men and 15 (33%) women with a mean age of 49 years and median age of 51 years (range, 16–89 years). Multiple branches of arteries comprising the Circle of Willis were obtained from subjects 10, 21, 22, 23, and 24; only 1 artery (middle cerebral) was obtained from each of the remaining 40 subjects. Of the 63 cerebral arteries analyzed, 3 (5%) were anterior, 47 (75%) were middle, 6 (10%) were posterior, 2 (3%) were basilar, 4 (6%) were vertebral, and 1 (2%) was from the vertebro-basilar junction.
Table 1.
Clinical features of subjects whose cerebral arteries were analyzed for varicella zoster virus, herpes simplex virus-1 and expression of CD45 antigen
| Subject | Age (years) |
Gender | Cause of death | Postmortem Interval (hours) |
Arterya | CD45/ Locationb |
|---|---|---|---|---|---|---|
| 1 | 31 | M | Alcohol abuse | 18 | MCA | - |
| 2 | 52 | M | Abdominal hemorrhage | 24 | MCA | +/I |
| 3 | 23 | F | Drug overdose | <24 | MCA | - |
| 4 | 45 | M | Suicide | 23 | MCA | - |
| 5 | 21 | M | Drug overdose | <24 | MCA | - |
| 6 | 57 | M | Suicide | 22 | MCA | - |
| 7 | 58 | F | Pulmonary embolism | 17 | MCA | - |
| 8 | 37 | M | Atherosclerosis | 24 | MCA | - |
| 9 | 55 | M | Pulmonary abscess | <24 | MCA | - |
| 10 | 55 | M | Suicide | <24 | MCA R MCA L |
+/A, M - |
| 11 | 16 | F | Suicide | <24 | MCA | - |
| 12 | 89 | M | Cardiac arrest | 24 | MCA | +/A, M |
| 13 | 50 | F | Pulmonary embolism | <24 | MCA | - |
| 14 | 72 | M | Lung cancer | 19 | MCA | - |
| 15 | 71 | M | Cirrhosis | 40 | MCA | - |
| 16 | 55 | F | Respiratory failure | 36 | MCA | - |
| 17 | 75 | F | Metastatic cancer | 14 | MCA | - |
| 18 | 56 | M | Drug overdose | <24 | MCA | - |
| 19 | 70 | M | Lung cancer | 22 | MCA | - |
| 20 | 57 | M | Fall | <24 | MCA | - |
| 21 | 21 | M | Drug overdose | <24 | ACA MCA PCA BA VA |
- - - - - |
| 22 | 44 | F | Drug overdose | 48 | PCA L VA R VA L |
- +/A +/A, M, I |
| 23 | 47 | M | Drug overdose | <24 | ACA L MCA R MCA L PCA R PCA L VA+BA |
- - - - - - |
| 24 | 48 | M | Drug overdose | <24 | ACA MCA R MCA L PCA R PCA L BA VA |
- - - - - - - |
| 25 | 51 | F | Septic shock | 10 | MCA | - |
| 26 | 36 | F | Drug overdose | <24 | MCA | - |
| 27 | 55 | F | Diabetes | 18 | MCA | +/A, M, I |
| 28 | 72 | M | Leukemia | 18 | MCA | - |
| 29 | 62 | M | Suicide | 20 | MCA | +/M |
| 30 | 18 | M | Motor vehicle accident | 30 | MCA | - |
| 31 | 60 | M | Drug overdose | 24 | MCA | - |
| 32 | 56 | F | Drug overdose | <24 | MCA | - |
| 33 | 27 | M | Lipoidosis | <24 | MCA | - |
| 34 | 51 | M | Trauma | <24 | MCA | - |
| 35 | 29 | F | Seizure | <24 | MCA | - |
| 36 | 34 | F | Drug overdose | 8 | MCA | - |
| 37 | 49 | M | Lung cancer | <24 | MCA | - |
| 38 | 52 | M | Suicide | 24 | MCA | - |
| 39 | 55 | M | Suicide | 25 | MCA | - |
| 40 | 53 | M | Suicide | 17 | MCA | +/A |
| 41 | 41 | F | Metastatic breast cancer | 17 | MCA | - |
| 42 | 51 | M | Suicide | <24 | MCA | - |
| 43 | 45 | F | Leukemia | <24 | MCA | - |
| 44 | 48 | M | Drug overdose | <24 | MCA | - |
| 45 | 40 | M | Liver hematoma | <24 | MCA | - |
artery abbreviations: MCA, middle cerebral; ACA, anterior cerebral; PCA, posterior cerebral; VA, vertebral; BA, basilar; R, right; L, left
location abbreviations: I, intima; M, media; A, adventitia
Cerebral arteries were cut into 10-mm pieces, rinsed in phosphate-buffered saline (PBS), and cut further into two 5-mm pieces for PCR and immunohistochemical (IHC) analysis, respectively. DNA extracted from one 5-mm piece was analyzed by quantitative PCR for the presence of VZV DNA using VZV-specific primers (forward 5’-CGAACACGTTCCCCATCAA-3’; reverse 5’- CCCGGCTTTGTTAGTTTTGG-3’ and probe /56-FAM/TCCAGGTTTTAGTTGATACCA/3BHQ_1/) and for HSV-1 DNA using HSV-1-specific primers (forward 5’- TGGTATTGCCCAACACTTTCC-3’; reverse 5’-GCGCCAGGCACACACAT -3’ and probe /56-FAM/CGTGTCGCGTGTGGT/3BHQ_1/); and glyceraldehyde-3-phosphate dehydrogenase (GAPdH)-specific primers (forward 5’-CACATGGCCTCCAAGGAGTAA-3’; reverse 5’- TGAGGGTCTCTGTCTTCCTCT -3’ and probe /5HEX/CTGGACCACCAGCCCCAGCAAG/3IABLFQ/) (Cohrs and Gilden, 2007). Positive and negative controls were provided, respectively, by amplification of serial dilutions of known quantities of VZV DNA and by omission of VZV DNA from the PCR reaction.
The other 5-mm piece of each artery was formalin-fixed and paraffin-embedded (FFPE), sectioned at 5 µm, and baked on non-adhesive, positively charged glass slides for 15 min at 65o C. Sections were deparaffinized in 100% xylene (3 times for 5 min each time) followed by 100% ethanol (3 times for 5 min each time). After sequential dipping in 95%, 70% and 50% ethanol, four consecutive sections from each artery were placed in distilled water, blocked in PBS containing 5% normal rabbit serum (NRS) for 1 h, washed 3 times with PBS, and incubated for 2 h at room temperature with one of the following primary antibodies: (a) 1:1000 dilution of primary rabbit polyclonal anti-VZV 63 antibody (Debrus et al., 1995); (b) 1:1000 dilution of rabbit anti-HSV-1 antibody (Dako, Carpenteria, CA); (c) 1:1000 dilution of rabbit polyclonal anti-CD45 antibody (Abcam, Cambrdige, MA), or (d) NRS. Positive controls for the two antiviral antibodies were provided by cadaveric cerebral arteries infected ex vivo with VZV or HSV-1, respectively, and prepared as FFPE specimens 14 days post-infection. Positive control for CD45 expression was provided by human tonsil obtained at autopsy and processed as for arteries. After incubation with primary antibody, sections were rinsed 3 times with PBS and incubated for 1 h with 1:1000 dilution of biotinylated goat anti-rabbit antibody (Dako) for sections. Sections were then rinsed 3 times in PBS and incubated with prediluted alkaline phosphatase-conjugated streptavidin (Becton-Dickinson, Franklin Lakes, NJ) for 1 h. The color reaction was developed for 2 min using the fresh fuschin substrate system (Dako) in the presence of levamisole (Dako) at a final concentration 24 µg/ml. The presence of VZV, HSV-1 or CD45 antigen (pink staining) was determined using light microscopy.
Results
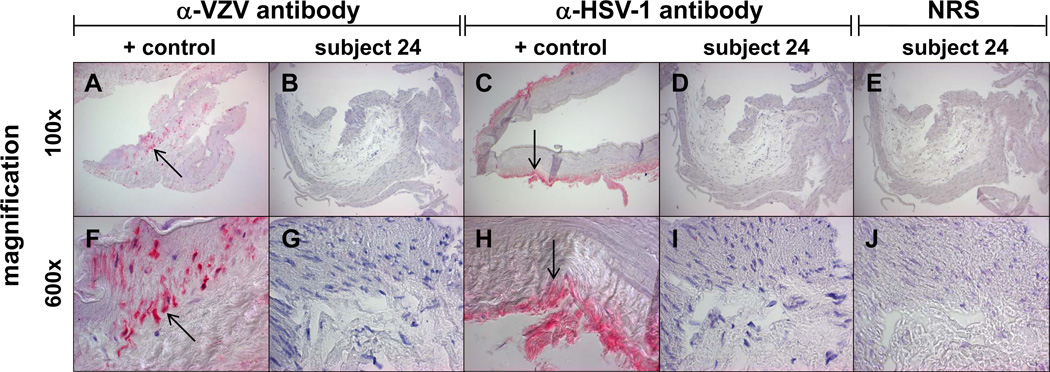
PCR analysis of DNA extracted from all 63 arteries revealed the presence of cellular GAPdH, but not VZV or HSV-1 DNA. IHC analysis (Fig. 1) demonstrated VZV antigen (a, f, arrows) and HSV-1 antigen (c, h, arrows) in cadaveric infected cerebral artery explant cultures, but not in any of 63 human cerebral arteries (b, d, g, i, show representative cerebral arteries from subject 24). No staining was seen in any human cerebral arteries using NRS (e, j).
Fig. 1. Absence of varicella zoster virus (VZV) or herpes simplex virus (HSV-1) antigen in normal human cerebral artery.
Positive control VZV-infected cadaveric cerebral artery at 14 days post-infection contains VZV antigen (a, f; arrow, pink color; x100 and x600, respectively). No VZV antigen was detected in any of 63 arteries from 45 subjects (b, g show representative artery from subject 24; x100 and x600, respectively). Similarly, positive control HSV-1-infected cadaveric cerebral artery at 14 days post-infection shows HSV-1 antigen (c, h; arrow, pink color; x100 and x600, respectively), whereas none of the 63 arteries contained HSV-1 antigen (d, i show representative negative artery from subject 24; x100 and x600, respectively). Staining of arteries with normal rabbit serum (NRS) was negative (e, j; x100 and x600, respectively).
IHC analysis for CD45+ cells (Fig. 2) in cerebral arteries revealed positive staining in control normal human tonsil (a, arrows) and in 8 arteries from 7 subjects (b shows representative artery from subject 2), and no staining with NRS (c). The 7 subjects whose cerebral arteries contained CD45 antigen were comprised of 5 men and 2 women; mean age = 58 years and median age = 55 years; range, 44–89 years. Of these 7 subjects, 3 died by suicide, one from abdominal hemorrhage, one from cardiac arrest, one from drug overdose and one from complications of diabetes. CD45+ cells were present in the adventitia of the middle cerebral artery of subject 40; exclusively in the media of subject 29; exclusively in the intima of subject 2; in both the adventitia and media in subjects 10 and 12; in the adventitia, media and intima of subject 27; and in the multiple arteries examined from subject 22, in the adventitia of the right vertebral artery and in the adventitia, media and intima of the left vertebral artery.
Fig. 2. Some normal human cerebral arteries contain CD45+ leukocytes.
CD45 antigen is seen in positive control tonsils (a) and in 8 cerebral arteries from 7 subjects (b shows representative artery from subject 2; no staining was seen with normal rabbit serum (c). x600
Discussion
Our analysis of 63 cerebral arteries from 45 subjects without symptoms or signs of stroke revealed no VZV or HSV-1 DNA or antigen. In addition, 13% of arteries contained CD45+ leukocytes distributed throughout the adventitia, media and intima. The subjects whose arteries contained CD45+ leukocytes were age 44 years or older, and there was no correlation with a particular disease or cause of death. While the significance of the occasional presence of inflammatory cells in normal cerebral arteries requires further study, the absence of VZV DNA or antigen in normal subjects indicates that the presence of VZV antigen in cerebral arteries of patients with a history of transient ischemic attack or stroke is likely to be significant.
Acknowledgments
This work was supported by Public Health Service grants AG032958 (D.G.) from the National Institutes of Health. We thank Marina Hoffman for editorial review and Lori DePriest for manuscript preparation.
Footnotes
Disclosure The authors have no conflicts of interest to report.
References
- Berger TM, Caduff JH, Gebbers JO. Fatal varicella-zoster virus antigen-positive giant cell arteritis of the central nervous system. Pediatr Infect Dis J. 2000;19:653–656. doi: 10.1097/00006454-200007000-00015. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol. 1995;69:3240–3245. doi: 10.1128/jvi.69.5.3240-3245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle PW, Gibson G, Dolman CL. Herpes zoster ophthalmicus with contralateral hemiplegia: identification of cause. Ann Neurol. 1983;14:84–85. doi: 10.1002/ana.410140115. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Sotrel A, Horoupian DS, Neumann PE, Pumarola-Sune T, Price RW. Thrombotic cerebral vasculopathy associated with herpes zoster. Ann Neurol. 1986;19:7–14. doi: 10.1002/ana.410190103. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kinjo M, Hokamura K, Tanaka K. Subarachnoid hemorrhage and granulomatous angiitis of the basilar artery: demonstration of the varicella-zoster-virus in the basilar artery lesions. Stroke. 1986;17:1024–1028. doi: 10.1161/01.str.17.5.1024. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella-zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology. 2013;80:62–66. doi: 10.1212/WNL.0b013e31827b1ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]