Abstract
Purpose
The proteasome consists of chymotrypsin-like (CT-L), trypsin-like, and caspase-like subunits that cleave substrates preferentially by amino acid sequence. Proteasomes mediate degradation of regulatory proteins of the p53, Bcl-2 and nuclear factor-κB (NF-κB) families that are aberrantly active in chronic lymphocytic leukemia (CLL). CLL remains an incurable disease, and new treatments are especially needed in the relapsed/refractory setting. We therefore investigated the effects of the proteasome inhibitor carfilzomib (CFZ) in CLL cells.
Experimental Design
Tumor cells from CLL patients were assayed in vitro using immunoblotting, real-time polymerase chain reaction and electrophoretic mobility shift assays. Additionally, a p53 dominant-negative construct was generated in a human B-cell line.
Results
Unlike bortezomib, CFZ potently induces apoptosis in CLL patient cells in the presence of human serum. CLL cells have significantly lower basal CT-L activity compared to normal B and T cells, although activity is inhibited similarly in T cells vs. CLL. and the cytotoxicity of CFZ correlates with baseline CT-L activity. Co-culture of CLL cells on stroma protected from CFZ-mediated cytotoxicity; however, PI3K inhibition significantly diminished this stromal protection. CFZ-mediated cytotoxicity in leukemic B-cells is caspase-dependent and occurs irrespective of p53 status. In CLL cells, CFZ promotes atypical activation of NF-κB evidenced by loss of cytoplasmic IkBα, phosphorylation of IκBα and increased p50/p65 DNA binding, without subsequent increases in canonical NF-κB target gene transcription.
Conclusions
Together, these data provide new mechanistic insights into the activity of CFZ in CLL and support Phase I investigation of CFZ in this disease.
Keywords: proteasome, carfilzomib, bortezomib, p53, NF-kB
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is characterized by the growth and accumulation of malignant B-lymphocytes in the blood, bone marrow, lymph nodes, and spleen. Despite the introduction of new therapies, most CLL patients eventually relapse and succumb to their disease. Identification of new strategies for relapsed and refractory CLL is essential to make a positive impact on patient survival.
Protein homeostasis is critical for the processes that govern cell survival, and the multi-catalytic 26S proteasome regulates the turnover of key proteins in these processes. Proteasomes consist of a catalytic 20S core capped by regulatory 19S subunits1. There are two forms of the 20S core: c20S and i20S. The former is ubiquitiously expressed in all cells, and the latter specifically in hematopoietic cells and cells exposed to inflammatory cytokines2. The active site in the c20S and i20S core has 6 N-terminal threonine proteases consisting of chymotrypsin-like (CT-L), trypsin-like (T-L) and caspase-like (C-L) subunits3, 4. Because selective inhibition of the CT-L site has a minor effect on total cellular protein turnover, proteasome inhibitors that block only the CT-L subunit are considered optimal5. Proteasomes mediate degradation of a variety of key regulatory factors including cell cycle control proteins (e.g. cyclins6, p217 and p278), p539, p53 target proteins Puma, Noxa, and Bax of the Bcl-2 family10, and the inhibitor of NF-κB (IκB)11. Imbalanced expression of Bcl-2 family proteins, constitutive NF-κB activation, and variable p53 function are hallmarks of CLL cells12–14.
Bortezomib (BTZ, Velcade©), is a proteasome inhibitor approved for the treatment of multiple myeloma and mantle cell lymphoma15. Concentrations of BTZ that produce an anti-tumor response in vitro inhibit activities of the CT-L and C-L subunits of the proteasome2. In spite of a high degree of cytotoxicity in vitro in CLL cells, BTZ failed to produce objective responses in CLL patients in a phase II clinical trial at the achieved doses16. The lack of BTZ efficacy in vivo has been attributed to the inactivation of its boronate moiety by dietary flavonoids in human plasma17. Carfilzomib (CFZ, PR-171) is a novel proteasome inhibitor that specifically and irreversibly inhibits the CT-L activity of the proteasome18. Unlike BTZ, CFZ has minimal activity against off-target enzymes including serine proteases, while at the same time inhibiting the CT-L subunit of the proteasome more potently19–21. Importantly, CFZ lacks the boronate moiety of BTZ that is potentially responsible for that agent’s inactivity in CLL patients. Here, we investigate the effects of CFZ on CLL patient cells. This work demonstrates that CFZ irreversibly inhibits the CT-L activity, has potent activity in CLL including cases with del(17p13.1), and promotes an atypical activation of NF-κB that may lack the classical pro-survival effect of this pathway.
MATERIALS AND METHODS
Reagents
Carfilzomib (CFZ) was provided by Onyx Pharmaceuticals (South San Francisco, CA). Boc-D-FMK (Enzyme Systems Products, Aurora, OH) was used at 100 micromolar (µM). Bortezomib (BTZ) was obtained from Millennium Pharmaceuticals Inc. (Cambridge, MA), and TNF from R&D Systems (Minneapolis, MN). CD40L was purchased from PeproTech (Rocky Hill, NJ). 2-fluoro-ara-A (active metabolite of fludarabine), G418, doxycycline and puromycin were purchased from Sigma (St. Louis, MO). CpG DSP3022 was purchased from Eurofins/Operon (Huntsville, AL).
Cells and cell lines
Blood was obtained from patients following written, informed consent under a protocol approved by the Institutional Review Board of The Ohio State University. All patients examined had immunophenotypically defined CLL as outlined by IWCLL criteria23 and were newly diagnosed or without treatment for a minimum of 30 days at time of collection. The occurrence of del(17p13.1) was determined in CLL patient samples by fluorescence in situ hybridization as described24, and in each positive case at least 30% of cells showed this deletion. Normal cells were obtained from partial leukocyte preparations from the American Red Cross. B- or T-lymphocytes and CLL cells were negatively selected using RosetteSep reagents (StemCell Technologies, Vancouver, BC). The HS-5-GFP stromal cell line was provided by Dr. Beverly Torok-Storb (Fred Hutchinson Cancer Research Center)25. 293 cells were obtained from ATCC (Manassas VA) and 697 lymphoblastic cells were obtained from DSMZ (Braunschweig, Germany). Cells were incubated at 37°C and 5% CO2 in AIM-V medium (Invitrogen, Carlsbad, CA) or in RPMI 1640 with 10% fetal bovine serum (FBS) or 10% human serum (HS) supplemented with penicillin, streptomycin, and L-glutamine (Sigma).
Viability Assays
Cell viability was monitored by flow cytometry using annexin V/propidium iodide (PI) (BD Biosciences, San Jose, CA) on a FC500 instrument (Beckman Coulter, Brea, CA). CellTiter 96 (MTS) assays were performed to monitor growth inhibition per manufacturer’s instructions (Promega, Madison WI). LIVE/DEAD (Invitrogen) staining was performed to monitor cytotoxicity with drug treatments using the manufacturer’s instructions.
Immunoblot Analyses
Nuclear and cytoplasmic lysates were prepared with NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL). Antibody to polyADP-ribose polymerase (PARP) was from EMD Biosciences (La Jolla, CA), p21 (OP64) and p53 (OP43) from Calbiochem (Philadelphia, PA), p27 (88264) from Abcam (Cambridge, MA), and pIκBα from Cell Signaling Technologies (Danvers, MA). Remaining antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Bands were quantified on an AlphaImager system (Proteinsimple, Santa Clara CA).
Electrophoretic mobility shift assay
A probe containing an NF-κB consensus binding site (5’ AGTTGAGGGGACTTTCCCAGGC 3’; Santa Cruz Biotechnology) was 32P-labeled using the Nick Translation System (Invitrogen). Five µg nuclear protein was incubated 30 minutes at room temperature in binding buffer (10 mM Tris-HCl, pH 7.5, 1.0 mM ethylenediaminetetraacetic acid (EDTA), 4% Ficoll, 1.0 mM dithiothreitol, 75 mM KCl) plus 250 ng poly dI-dC (Sigma). Complexes were separated on polyacrylamide gels in Tris-borate EDTA buffer (89 mM Tris-base, 89 mM boric acid, 2.0 mM EDTA), dried, and autoradiographed. For supershift experiments, antibodies to p65, p50 or c-Rel (Santa Cruz Biotechnology) were incubated with nuclear extract for 10 minutes before addition of probe.
Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RNA was extracted using TRIzol (Invitrogen). cDNA was prepared with SuperScript First-Strand Synthesis System (Invitrogen). Real-time RT-PCR was performed on an ABI 7900 (Applied Biosystems, Foster City, CA) using TaqMan Universal Master Mix, primers, and labeled probes (Applied Biosystems) and TBP as an endogenous control. Mean threshold cycle (Ct) values were calculated by PRISM software (Applied Biosystems) to determine fold differences according to manufacturer’s instructions.
Chymotrypsin-like (CT-L) activity assay
Whole cell extracts without protease inhibitors were analyzed using a CT-L specific 20S proteasome assay kit (Chemicon, Temecula, CA) following the manufacturer's instructions. The assay is based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate. Free AMC was measured using a 380/460 nm fluorometer filter set. Relative activity was standardized by protein concentration, determined by BCA assay.
Gamma irradiation
Cells were irradiated using 5 or 8 Gy as indicated. RNA and lysates were collected from a subset of cells after 4 hr, and viability was determined in remaining cells at 24 hr by annexin V/PI or LIVE/DEAD staining.
Retroviral vectors and generation of cell line with inducible dominant-negative (DN) p53 activity
The retroviral construct pRetroX-Tight-Puro (pRetro) (Clontech, Mountain View, CA) was used to stably transfect 697 cells with the p53 DN system. Mutations in the p53 DNA-binding domain at codon 273 (Arg->His, p53DN818) and codon 281 (Asp->Glu, p53DN843) were selected to generate two different p53DN constructs using the QuikChange mutagenesis kit (Stratagene, Wilmington, DE). DNA was sequenced to confirm mutations. Using primer sequences provided by Clontech, the presence and orientation of p53DN818 and p53DN843 sequences in the pRetro vector were similarly confirmed. Retrovirus particles were produced by co-transfecting plasmid DNA and ecotropic helper plasmids (pVSV and pGPZ) into the 293 cell line using calcium phosphate precipitation. The inducible Tet activator 697 cell line (pRetrox-tet-on) was established according to the manufacturer’s protocol (Clontech). 697pTet-on cells were infected with retrovirus by culturing for 10 hr in conditioned media with 8 µg/ml polybrene. Cells were then washed and incubated 48 hr before selection with 1µg/ml puromycin and 100 µg/ml G418.
Statistics
For all experiments, linear mixed effects models were used to account for dependencies among the data. From the models, estimated differences between experimental conditions were calculated along with 95% confidence intervals (CI). Log transformations of the data were applied when necessary to stabilize variances. Data from Real time RT-PCR experiments were first normalized to internal controls, and then linear mixed models were applied to the log-transformed fold changes. All analyses were performed using SAS/STAT software, v9.2 (SAS Institute, Cary, NC).
RESULTS
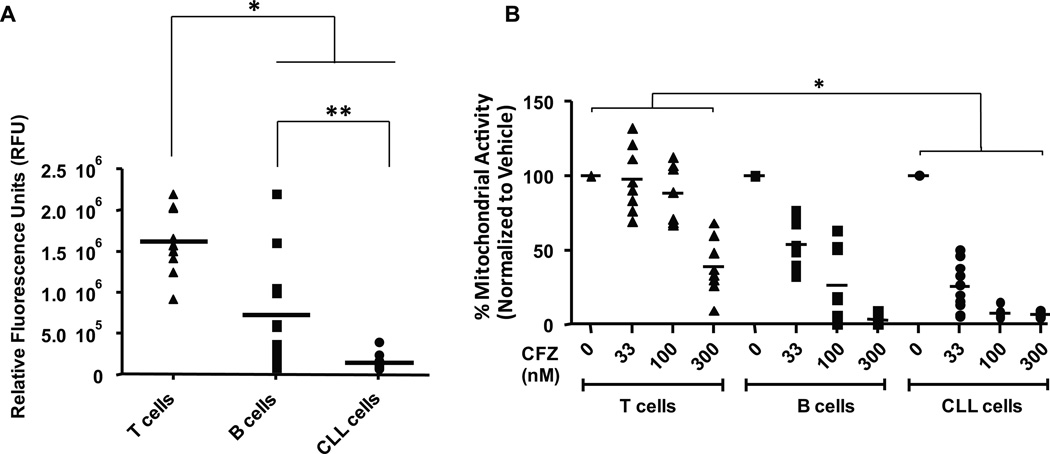
T cells express increased chymotrypsin-like (CT-L) proteasome activity and decreased sensitivity to CFZ compared to B cells
A common disadvantage of current therapies for CLL is the negative impact on normal lymphocytes, especially T-cells, that leaves patients at increased risk of opportunistic infection. To address the impact of CT-L inhibition on CLL and normal cell types, we first measured the basal levels of CT-L activity in CLL cells compared to B- and T-cells from healthy volunteers. Interestingly, normal T-cells showed significantly higher CT-L activity compared to normal B-cells and CLL cells (p<0.0001 for both). CT-L activity in normal B cells was also significantly higher than in CLL cells (p=0.0003) (Figure 1A). We next tested the relative cytotoxicity of CFZ in these cell types. Because CFZ exhibits a short in vivo half-life (approximately 30 minutes26, 27), cells in all experiments were incubated with CFZ for 1 hr, washed, and further cultured in fresh media for the indicated times to more closely mimic in vivo exposure. Concentrations used were based on pharmacokinetics data from a Phase I clinical trial in CLL now underway at OSU. In this trial, the maximum concentration achieved was 0.876 ± 0.099 µg/mL (1.22 ± 0.138 µM) for the 45 mg/m2 dose (unpublished results). This is similar to concentrations previously reported to be clinically achievable in patients with hematologic malignancies27. As shown in Figure 1B, normal B cells were significantly more sensitive to CFZ than normal T cells at 48 hr using 100 and 300 nM concentrations (p<0.0001 for both). This finding is consistent with the elevated CT-L activity observed in T-cells relative to B cells.
Figure 1. T cells express more chymotrypsin-like (CT-L) proteasome activity than B cells.
(A) Basal CT-L activity in T cells (n=10) is higher than B cells (n=11) from healthy donors and in primary CLL cells (n=10) (*p<0.0001 for both). CT-L activity in B cells was also higher than in CLL cells (**p=0.0003). Results are displayed in relative fluorescence units (RFU). (B) B cells and T cells from healthy donors (n=8 each) and CLL patient samples (n=10) were incubated in serum-free AIM-V media with various concentrations of CFZ for 1 hr. Mitochondrial activity as a surrogate for cell viability was determined by MTS assay at 48 hr, and is shown relative to time-matched untreated controls. Horizontal lines represent the mean. CFZ across all doses is more cytotoxic to CLL cells than T cells (*p<0.0001). CFZ is also more cytotoxic to CLL cells than to B cells at 33 and 100 nM (p<0.0001 for both) but is not different at 300 nM.
Carfilzomib irreversibly inhibits the CT-L subunit and promotes apoptosis in CLL cells
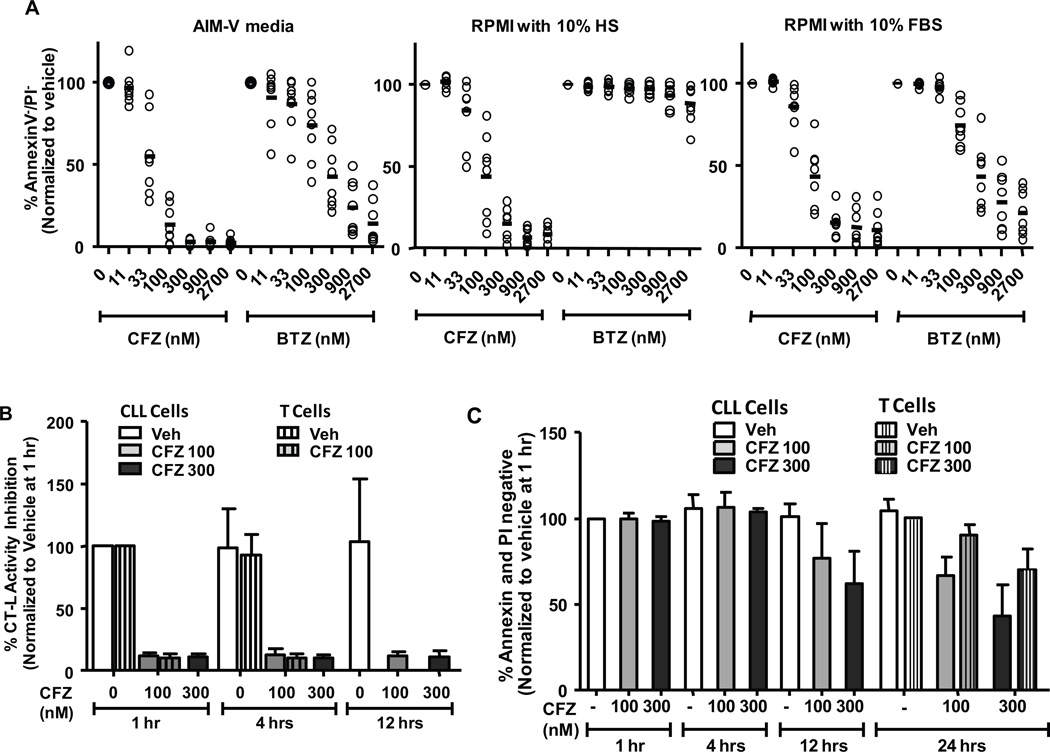
Because of the lower CT-L activity in CLL cells, we hypothesized that the CT-L-specific inhibitor CFZ would show particular efficacy in these cells. We therefore tested the cytotoxicity of CFZ in CLL patient samples. As BTZ was previously shown to be inactivated by human plasma components17, we also included this agent and tested in the presence of three different media: serum-free AIM-V medium, RPMI 1640 with 10% human serum (HS), and RPMI 1640 with 10% fetal bovine serum (FBS). In serum-free or FBS-containing media, CFZ was more cytotoxic to CLL cells than BTZ (approximate LC50 in AIM-V and FBS: CFZ = 40 and 80 nM; BTZ = 200 and 170 nM, respectively). In media containing HS, however, CFZ maintained efficacy but BTZ was largely inactive (approximate LC50 of CFZ in HS = 80 nM) (Figure 2A). To more closely approximate in vivo conditions, media with HS was used for the remaining work. Next, CT-L activity was assessed in CLL patient cells and in normal T cells after total incubations of 1, 4, or 12 hrs (Figure 2B). Viability was also determined by PI flow cytometry (Figure 2C). CFZ rapidly (within 1 hr) suppressed the CT-L proteasome activity both in CLL samples and in T cells. This was followed by a time-dependent increase in apoptosis (as determined by annexin positivity) and cytotoxicity (as determined by PI uptake) that was greater in CLL vs. T cells. Despite drug washout, CT-L inhibition was not reversible within the 12 hr period following CFZ treatment. Proteasome activity was not determined at later time points due to the high degree of CFZ-induced cell death.
Figure 2. Carfilzomib irreversibly inhibits the CT-L subunit and promotes selective apoptosis in CLL cells cultured in human serum.
(A) CLL cells (n=8) were treated with various concentrations of CFZ or BTZ for 1 hr in media as indicated. Viability was determined by annexin V/PI flow cytometry at 24 hr. Horizontal lines represent the mean. (B) CLL cells or normal T cells (n=6 each) were incubated with CFZ in RPMI with 10% HS for 1 hr and cells were harvested at several time points to determine CT-L activity. (C) Viability of cells from B was determined by annexin/PI flow cytometry. Due to cell number limitations, T cells (striped bars) were investigated for viability at 24 hr only.
Stromal protection of CFZ-treated CLL cells is diminished by a pan-PI3K inhibitor
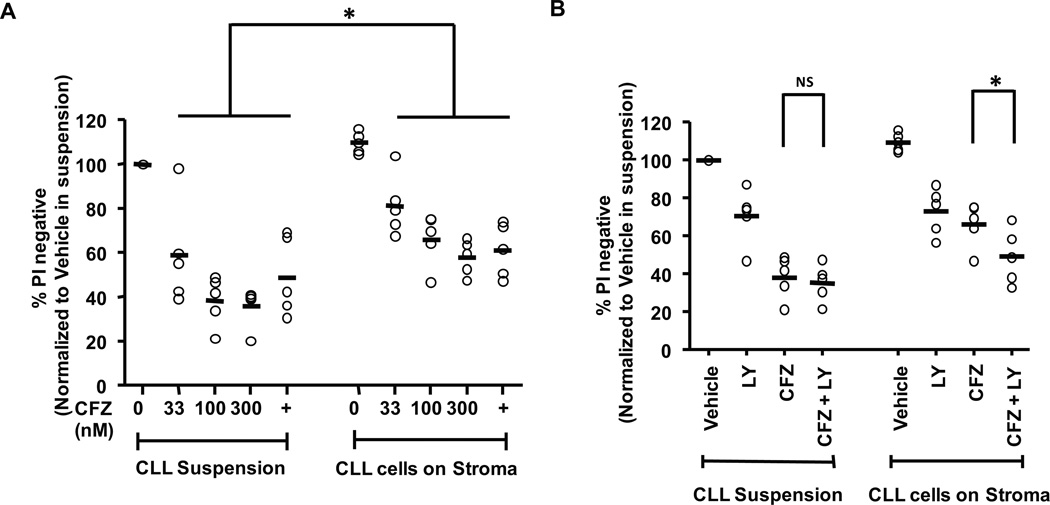
CLL cells have dysregulated apoptosis in vivo, but in vitro they rapidly undergo spontaneous apoptosis due to the lack of external signals from the microenvironment provided by soluble factors and contact with a variety of cells in the bone marrow and lymph nodes28. We therefore sought to determine the impact of microenvironment factors on CFZ-mediated cytotoxicity using a previously established stromal cell line, HS-5-GFP29. CFZ did not substantially affect viability of HS-5-GFP stromal cells at 48 hr at concentrations up to 300 nM (data not shown). To test the protective effects of stroma, CLL cells were incubated with CFZ or vehicle for 1 hr and transferred to plates with or without HS-5-GFP cells. Using flow cytometry, GFP-negative CLL cells were distinguished from GFP-positive HS-5 cells and cytotoxicity was assessed by PI uptake. CFZ caused significantly more cytotoxicity in CLL cells alone as compared to CLL cells on stroma, although CLL cells on stroma remained sensitive to CFZ at the tested concentrations (p<0.0001) (Figure 3A). The importance of the PI3K signaling pathway to the survival of mature B cells, and specifically co-culture of CLL on stromal cells, has been established29. Therefore, we sought to determine if the stromal cell protection of CLL cells was mediated by PI3K signaling. As shown in Figure 3B, the addition of the pan-PI3K inhibitor LY294002 significantly diminished the stromal-cell mediated protection of CFZ treated CLL cells (p=0.0083). These data indicate that CFZ can mediate cytotoxicity in CLL cells despite the protective effect of stromal cells, and that stromal cell protection from CFZ-mediated cytotoxicity can be diminished by PI3K inhibition.
Figure 3. Stromal protection of CLL cells treated with CFZ is reversed by pan-PI3K inhibitor.
(A) CLL cells (n=5) were incubated with various concentrations of CFZ for 1 hr, washed, and transferred to plates with or without HS-5-GFP stromal cells. Dinaciclib (indicated by +) was included as a positive control52. Viability of GFP negative (CLL) cells were determined by PI uptake at 48 hrs and the differences were significant (p<0.0001). (B) Addition of 25 µM pan PI-3K inhibitor LY294002 to 100 nM CFZ treated CLL cells (n=5) decreased stromal protection as determined by PI uptake at 48 hrs (*p=0.0083). Horizontal lines represent the mean.
Cytotoxicity induced by carfilzomib in CLL cells is caspase-dependent
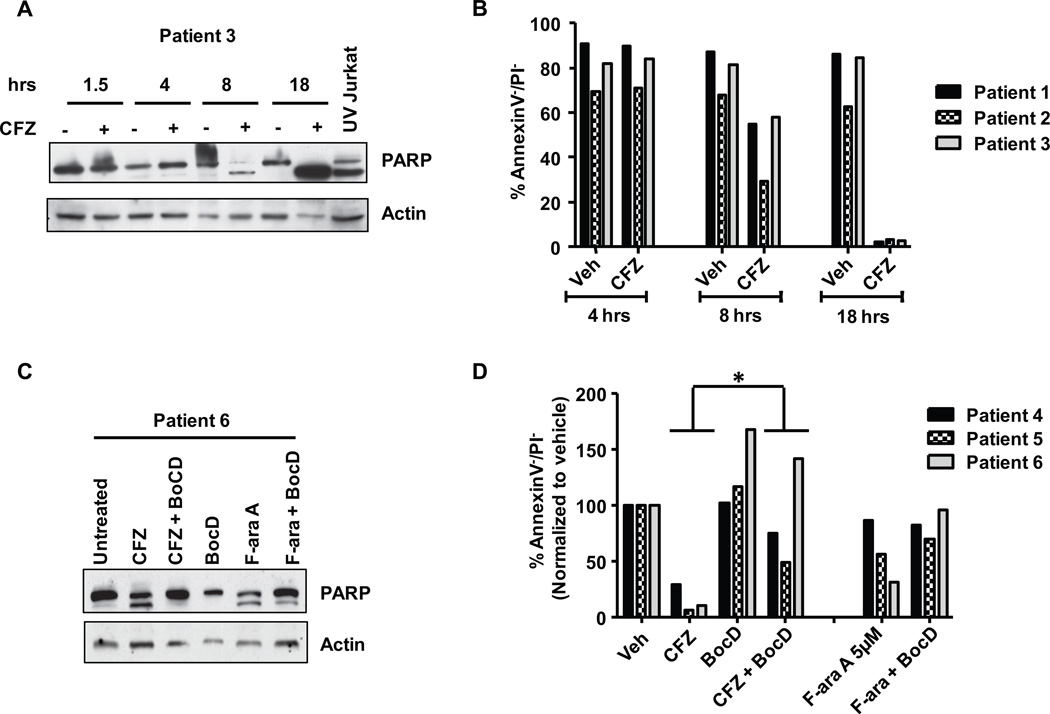
CFZ has been reported to mediate caspase-dependent apoptosis in mature B-cell malignancies such as Waldenstrom's macroglobulinemia30 and multiple myeloma31. We sought to determine if similar death pathways were used in less-differentiated malignant B-cells. CFZ caused a time-dependent cleavage (Figure 4A) of the caspase substrate polyADP ribose polymerase (PARP) and cytotoxicity (Figure 4B) that was rescued by the pan-caspase inhibitor Boc-D-fmk (Figure 4C). The cytotoxic effect of CFZ on CLL cells was also diminished with the addition of Boc-D-fmk (Figure 4D; p=0.0003). These data indicate that the primary mechanism of the cytotoxicity by CFZ in primary CLL cells is caspase-dependent.
Figure 4. CFZ-mediated cytotoxicity in CLL cells is caspase-dependent.
(A) CLL samples (n=3) were treated with CFZ 350 nM for 1 hr. Lysates were collected at 1.5, 4, 8, and 18 hrs, and cleavage of PARP was assessed by immunoblot. UV Jurkat was included as positive control to assess PARP cleavage products. A representative immunoblot is shown. (B) Viability of cells from A was determined by annexin V/PI at 4, 8 and 18 hrs. (C) CLL cells (n=3) were incubated with or without CFZ and pan-caspase inhibitor 100 µM Boc-D-fmk (BocD) for 18 hr. PARP cleavage was assessed by immunoblot. A representative immunoblot is shown. (D) Viability of cells from C was determined by annexin/PI at 24 hr. Differences in cytotoxicity of CFZ with the addition of BocD were significant (p=0.0003). F-ara A (active metabolite of fludarabine) was included as a positive control for PARP cleavage and caspase-dependent cytotoxicity.
Carfilzomib is cytotoxic to cells independent of p53
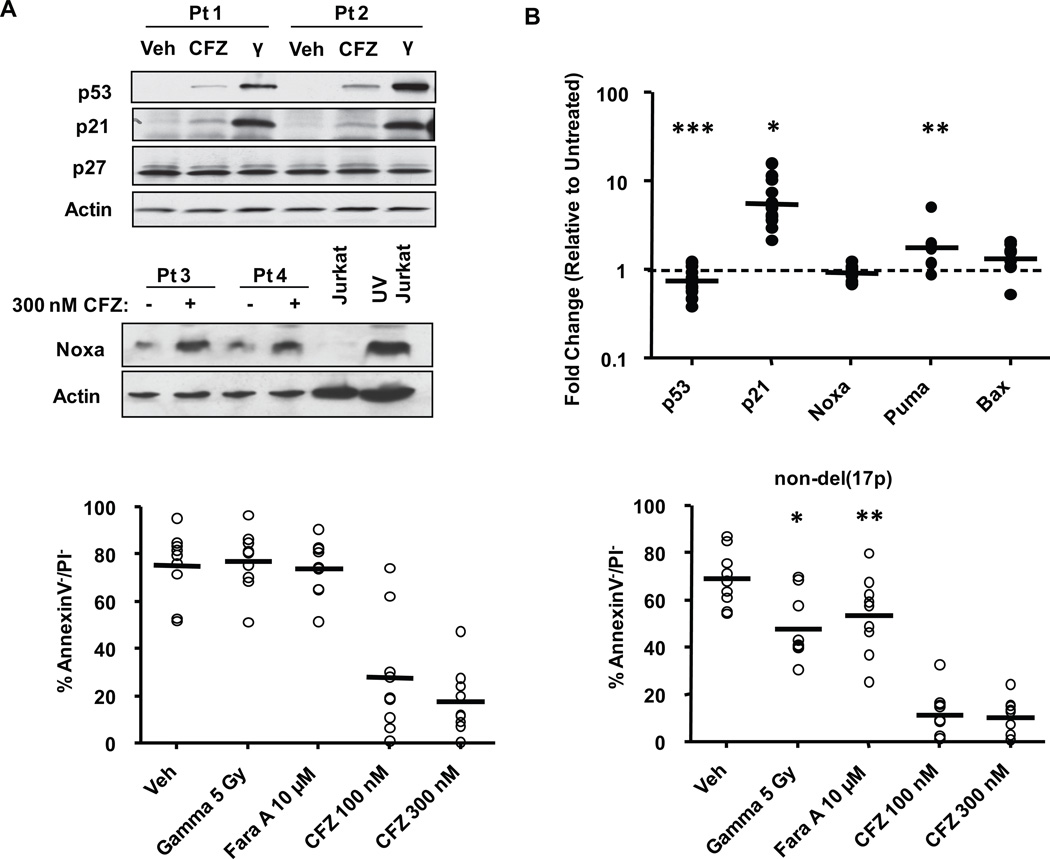
Mutations or deletions of p53 are far more common in advanced cases of CLL and are associated with resistance to alkylating agents, disease progression in 1 or 2 years, and overall significantly poorer outcome32. The proteasome regulates the turnover of p53 as well as several key p53 targets. We therefore sought to determine the effects of proteasome inhibition on p53 and its targets. CLL cells incubated with CFZ were analyzed by immunoblot or real-time RT-PCR. Gamma irradiation was included as a positive control representing a classical p53-inducing treatment. As shown in Figure 5A, CFZ treatment caused only modest accumulation of p53 and p21 proteins compared to irradiated cells, and p27 was unchanged. Puma and Bax proteins were similarly unchanged (data not shown). Significant increases in p21 transcription were noted in all patient samples following CFZ treatment (average fold change of 5.78, p<0.0001), and Puma transcription was slightly increased (average fold change of 1.97, p=0.0323) (Figure 5B). However, p53 was found to be down-regulated by an average of 0.75 fold (p= 0.0193), and no significant changes were observed in the transcription of Noxa (p=0.2232) or Bax (p=0.1531). These findings show that CFZ does not induce a typical p53 response in CLL cells, and suggests that CFZ-mediated p21 induction may be due to an indirect mechanism rather than direct p53 activity. As was shown previously with BTZ33, we observed an increase in Noxa protein (Figure 5A), but not mRNA, with CFZ treatment.
Figure 5. Carfilzomib is cytotoxic to CLL cells independent of p53.
CLL cells were treated with CFZ (300 nM) for 1 hr and whole cell lysates were collected at 8 hr. Gamma irradiation (5 Gy) was included as a positive control for p53-dependent treatment. Untreated and UV-irradiated Jurkat cells were included as negative and positive controls for expression of Noxa protein. Changes in p53 and its targets were assessed by (A) immunoblot (n=6) and (B) real-time RT-PCR (n=6–14). CFZ treatment induced p21 (*p<0.0001) and PUMA (**p=0.0323) and significantly down regulated p53 by 0.75 fold (***p=0.0193) compared to vehicle. Horizontal lines represent the geometric mean. (C) CLL samples from patients with or without del(17p13.1) (n=9) were incubated with 100 and 300 nM of CFZ for 1 hr. No significant differences were observed (p=0.3924). 5 Gy Gamma irradiation and fludarabine (Fara A) were included as controls for p53-dependent treatments and were more cytotoxic in non-del(17p13.1) patients than del(17p13.1) patients (*p<0.0001,** p=0.0002). Viability was determined by annexin V/PI flow cytometry at 24 hr. Horizontal lines represent the mean.
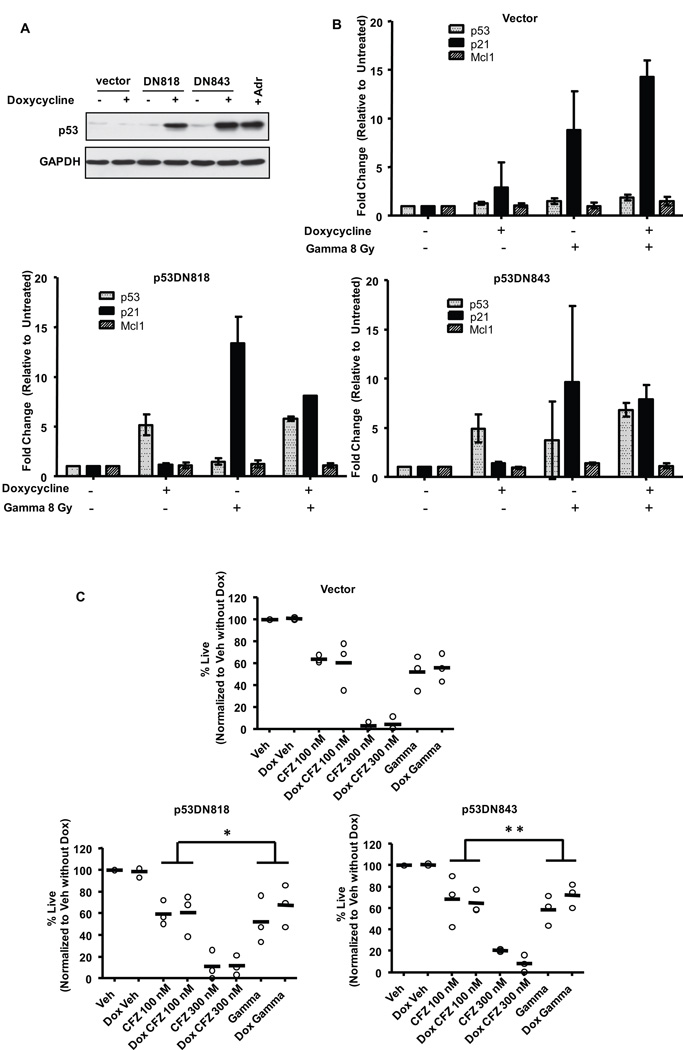
Next, we analyzed CLL samples from patients with or without del(17p13.1), the chromosomal site of the p53 gene (Figure 5C). CFZ was similarly cytotoxic to patient cells irrespective of del(17p13.1) status (p=0.3924). In contrast, significant differences in cytotoxicity were observed between patient samples with and without this deletion for both gamma irradiation and fludarabine treatments (p<0.0001 and p=0.0002, respectively). As p53 is only one of the genes deleted on del(17p13.1), we next sought to test the cytotoxicity of CFZ in cells with wild-type vs. defective p53 activity. In CLL, a transition mutation in the p53 DNA binding domain at codon 273 that converts Arg to His has been described as a gain-of-function dominant negative mutation34. Additionally, mutations at codons 281 (Asp→Glu) and 273 (Arg→His) are found in certain leukemias35. Doxycycline-inducible p53DN constructs representing these mutations were transfected into 697 cells, an acute B-lymphoblastic leukemia cell line that has wild-type p53 and responds to CFZ treatment similarly to CLL cells (Supplementary Figure 1B). As shown in Figure 6A, p53 protein was induced in cell lines carrying the p53DN constructs, but not the empty vector, as early as 18 hrs after doxycycline addition. Induction of p53DN activity increased p53 transcription by more than 5-fold in both p53DN818 and p53DN843 cells but not in empty-vector transfected cells (p<0.0001) (Figure 6B). Addition of doxycycline also caused a significant reduction in cytotoxicity (Figure 6C) with gamma irradiation, but not with CFZ treatment, in the cells with p53DN activity (p=0.0378 and p=0.0243 for p53DN818 and p53DN843, respectively). Furthermore, in gamma-irradiated cells, while doxycyline caused a 1.5-fold upregulation of p21 transcription in empty-vector control cells, p21 was reduced (0.7-fold) in doxycycline-treated p53DN cells (Figure 6B). Although these changes did not reach statistical significance, they further support the functional activity of the p53DN constructs in the 697 cells. Cumulatively, these experiments indicate that cytotoxicity of CFZ is not dependent on functional p53 activity in leukemic cells.
Figure 6. Carfilzomib is similarly cytotoxic in cells expressing dominant negative p53 mutants.
The 697 acute B-lymphoblast leukemia cell line was transfected with an empty vector or with either of 2 different inducible p53 dominant negative constructs. After 18 hr induction with doxycycline (n=3): (A) cells were harvested for lysates to determine expression of p53 protein. Adriamycin (Adr) treated 697 cells were included as a positive control for p53 protein expression. (B) Cells were gamma-irradiated (8 Gy) and RNA was collected at 4 hr. Changes in p53 and p21 were detected by real-time RT-PCR. Mcl-1 is not a p53-target and was included as a negative control. Doxycycline induced p53 (p<0.0001) in p53DN cells. (C) Cells were gamma-irradiated (8 Gy) or treated with CFZ for 1 hr. At 24 hr, viability was determined by LIVE/DEAD staining. In p53DN cells the difference in cytotoxicity in doxycycline-induced and uninduced cells following gamma treatment was significantly different than with CFZ treatment (*p=0.0378 and **p=0.0243 for p53DN818 and p53DN843, respectively). Horizontal lines represent the mean.
Carfilzomib induces an atypical NF-κB response in CLL cells
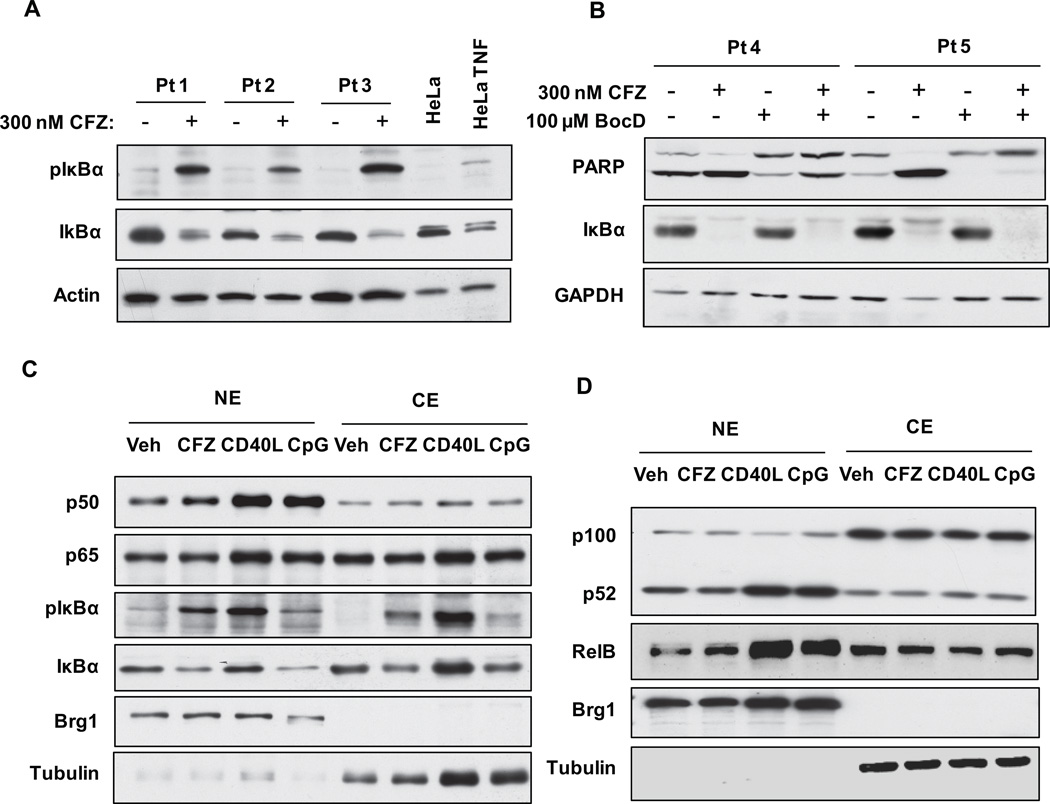
CLL cells are characterized by constitutive activation of NF-κB as indicated by high levels of nuclear NF-κB, p50 and p65 (RelA) proteins. In cutaneous T-cell lymphoma, BTZ was reported to inhibit the NF-κB pathway by preventing the degradation of the phosphorylated form of IκBα, thus preventing the nuclear translocation of p50 and p65 and further activation of NF-κB targets36. More recently it was shown in multiple myeloma cell lines that IκBα can be degraded by proteasome-independent mechanisms following BTZ treatment to allow for translocation and activation of NF-κB subunits37. Thus, NF-kB response to proteasome inhibition may vary by cell type or other factors not yet identified. To determine the effects of CFZ on NF-κB activity in CLL, CLL cells were incubated with CFZ for 1 hr and examined by immunoblot. As shown in Figures 7 A-C, IκBα protein decreases in both whole cell extracts as well as nuclear and cytoplasmic fractions. However, phosphorylation of IκBα (p-IκBα) increased in all fractions. To determine if this decrease in IκBα was due to caspase activity as was previously reported38, CLL cells were incubated with CFZ in the presence or absence of Boc-D-fmk (Figure 7B). Although Boc-D-fmk rescued cells from CFZ-mediated PARP cleavage and apoptosis, it did not prevent degradation of IκBα. Antibodies recognizing epitopes in both the N and C-terminal region of IκBα produced identical results (data not shown). It also was reported that IκBα is a substrate of calpains,37 but similar to caspase inhibitors, calpain inhibitors did not prevent degradation of IκBα (data not shown). As proteasome inhibitors were previously shown to induce or inhibit both the canonical and non-canonical NF-κB pathways36, 37, nuclear and cytoplasmic fractions of CFZ-treated CLL cells were probed for canonical (Figure 7C) and non-canonical (Figure 7D) proteins. No significant changes were observed in the nuclear or cytoplasmic levels of NF-κB subunits p50, p65, p52 or p100 in the CFZ treated samples. As controls, CpG and CD40L increased expression of both canonical and non-canonical proteins in the nuclear fraction.
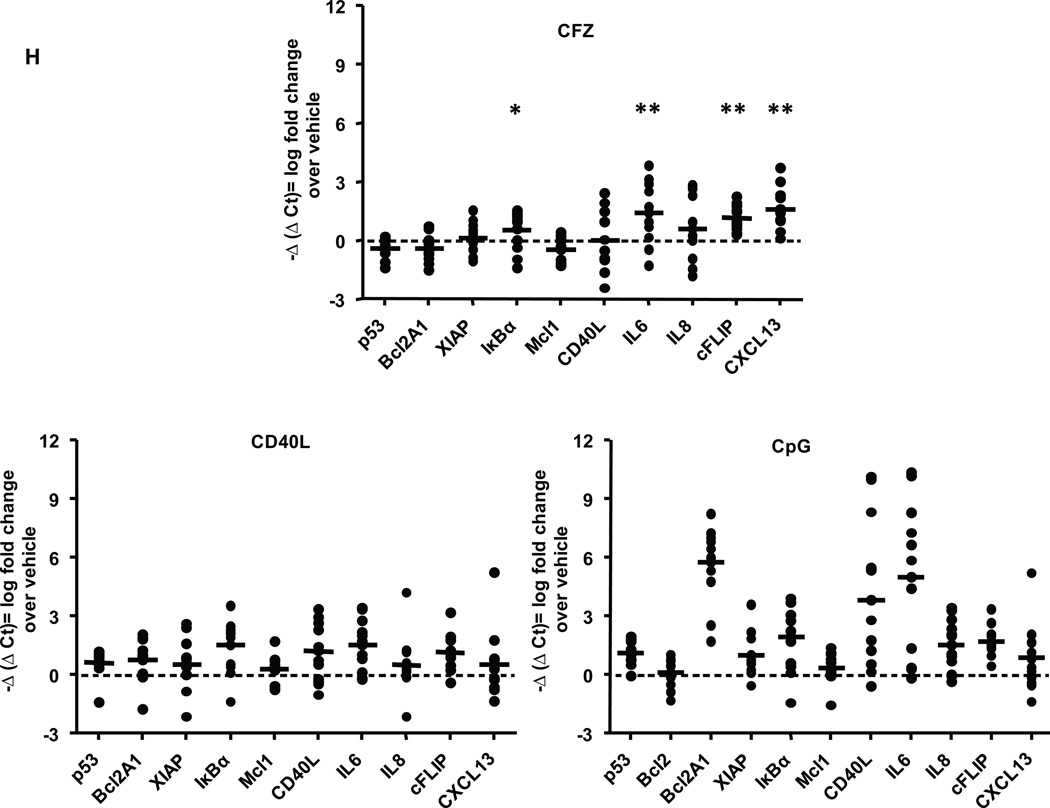
Figure 7. CFZ induces a defective NF-κB response.
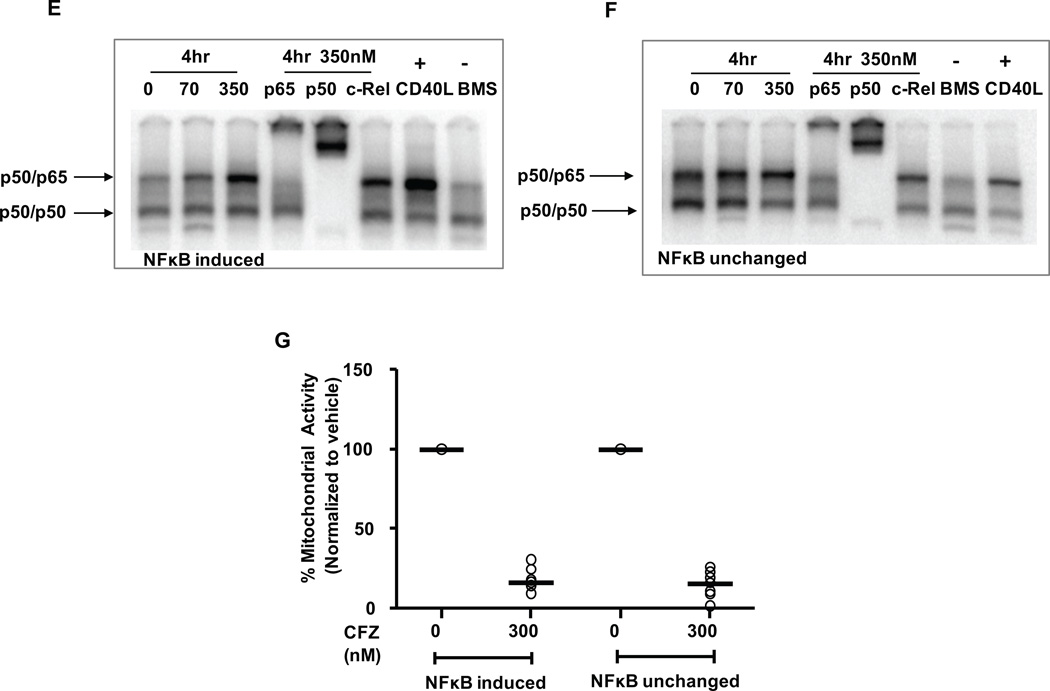
(A) CLL cells (n=6) were incubated 1 hr without or with 300 nM CFZ, and lysates were collected at 8 hr. IκBα and phosphorylated IκBα (pIκBα) were assessed by immunoblot. Untreated and TNF-treated HeLa cells were included as negative and positive controls for pIκBα protein expression, respectively. (B) CLL cells (n=6) were treated as in A, with or without 100 µM Boc-D-fmk. Lysates were isolated at 18 hr and analyzed by immunoblot. CLL cells (n=6) were incubated with 0, 70 and 350 nM CFZ for 1 hr or 500 ng/ml CD40L for 4 hr. Nuclear (NE) and cytosolic (CE) extracts were collected at 4 hr and analyzed by immunoblot for NF-kB (C) canonical pathway and (D) non-canonical pathway proteins. EMSAs were run using NE from cells treated with 350 nM CFZ (1 hr treatment, 4 hr incubation) or 500 ng/ml CD40L (4 hr) (n=14), using a probe containing a consensus NF-kB binding site. Representative EMSAs from CFZ-treated NE that displayed (E) increased NF-kB binding and (F) no changes are shown. CD40L and BMS-345541 (IKK inhibitor) treated NE were included as positive and negative controls for p50/p65 binding, respectively. (G). Viability of all samples analyzed by EMSA was determined by MTS at 48 hr. (H) CLL cells (n=12) were treated with 300 nM CFZ (1 hr), 1.7 µM CpG (4 hr) and 500 ng/ml CD40L (4 hr), and RNA was collected at 8 hrs from CFZ treated samples. mRNA expression was analyzed using real-time RT-PCR. CFZ treatment significantly induced expression of IκBα (*) by 1.5 fold, and IL-6, c-FLIP and CXCL-13 (** all 3) by greater than 2 fold. Horizontal lines represent the mean.
To determine whether nuclear translocation of pIκBα affects NF-κB activity, NF-κB DNA binding was measured in nuclear extracts prepared from CLL cells treated with CFZ or CD40L. The constitutive NF-κB (p50/p65) DNA binding activity in CLL cells was increased with CFZ treatment in approximately 50% of the samples, while there was no change in the remainder (Figures 7E and 7F, respectively). Importantly, CFZ was similarly cytotoxic to all CLL samples tested (Figure 7G). These results indicate that the impact of CFZ on NF-κB is not relevant to its cytotoxic activity in CLL. Finally, to determine whether the increased NF-κB activity observed by EMSA resulted in changes in gene expression, we analyzed several known NF-κB targets by real-time RT-PCR (Figure 7H). CFZ induced a subset of these targets, including IκBα, IL-6, c-FLIP, and CXCL13, but with substantial inter-patient variability (average fold changes for target genes are indicated in Supplementary Table 1). Notably, CFZ did not induce the transcription of several classical NF-κB targets such as Bcl2A1, XIAP, Mcl-1 and p53. Thus, our results show that CFZ induces rather than inhibits NF-κB in CLL patient cells, but that this response is atypical and does not contribute to the CFZ induced cytotoxicity or resistance to this therapy.
DISCUSSION
CFZ represents a new class of irreversible proteasome inhibitors that specifically target the CT-L subunit. It is currently in clinical trials for B-cell malignancies, but its mechanism of cell death is poorly understood. Here, we demonstrate that CLL cells have a low level of CT-L proteasome activity relative to normal B- and T-lymphocytes. We hypothesized that an irreversible inhibitor of the CT-L subunit would be most effective in cells having the least baseline activity, and used this to justify the examination of CFZ in CLL. In fact, we show that a short (1 hr) exposure of 100 nM CFZ is more cytotoxic to CLL cells compared to normal lymphocytes, even though this concentration similarly inhibits the CT-L subunit in CLL and normal T cells. This finding indicates that the differential sensitivity of CLL versus T cells to CFZ is not directly related to the extent of CT-L inhibition. Unlike BTZ, the cytotoxicity of CFZ is not diminished in media with human serum. Importantly, our studies indicate that CFZ induces cytotoxicity irrespective of del(17p13.1) status or p53 function. Stromal cells partially protected CLL cells from CFZ-mediated cytotoxicity, although this protective effect was decreased by the addition of a pan-PI3K inhibitor. Finally, we show that CFZ mediates an atypical NF-κB activation that does not associate with transcriptional induction of classical NF-kB targets or with CLL cell death. As previously reported with other proteasome inhibitors33, Noxa induction is observed with CFZ treatment concurrent with the onset of apoptosis, suggesting that Noxa-mediated caspase induction is the mechanism of action of CFZ in CLL.
To our knowledge, this is the first study demonstrating the reduced CT-L activity in primary CLL cells versus normal B- and T-cells. Although activity of proteasome inhibitors is well-documented, the mechanism by which these agents preferentially target malignant cells is yet to be elucidated. We speculate that differences in CT-L activity explain the observed therapeutic window between CLL tumor cells and normal lymphocytes. This hypothesis could be further tested by comparing the levels of inhibition of the CT-L activities in the constitutive (c20S) and immunoproteasome (i20S). We observed that levels of CT-L activity inhibition did not necessarily correlate with CFZ cytotoxicity. One potential explanation is differing levels of inhibition of the CT-L activity in c20S and i20S by CFZ, although this remains to be tested.
The tumor suppressor protein p53 induces apoptosis under conditions of cellular stress or DNA damage. Although p53 is functional in most CLL patients at the time of diagnosis, the gene becomes mutated or deleted in at least one allele in approximately 40% of patients with advanced CLL24, 39, corresponding with significantly poorer outcome32. Thus, agents that work independently of p53 are of significant interest in the development of therapies for drug-resistant CLL. Proteasome inhibitors have been shown to induce apoptosis in both p53-independent40 and p53-dependent manners41. Here, we demonstrate that CFZ induces cytotoxicity irrespective of del(17p13.1) status or the expression of a dominant negative p53 protein. Given the poor outcome and lack of effective therapies for del(17p13.1) CLL patients, these findings suggest CFZ therapy may be effective in this disease subtype.
Recent advances in CLL biology demonstrate the importance of both internal and external (microenvironment) factors in the survival and drug resistance of CLL tumor cells. The PI3K and NF-κB pathways are key regulators of differentiation and survival in B-cells and are induced by microenvironmental factors42, 43. CLL cells cultured with stromal cells were less sensitive to CFZ compared to CLL cells in suspension. This protective effect has been linked to the PI3K pathway29, 44, and our data show that PI3K inhibition indeed reverses this protection. Further work is necessary to determine the specific PI3K isoform responsible for this effect, but our data suggest that combination therapy of CFZ with PI3K inhibitors may provide added benefit.
Although the mechanism of proteasome inhibitor-mediated cell death is unclear, these agents have commonly been reported to be inhibitors of the NF-κB pathway36, 37. This notion is attributed to the observation that the NF-κB inhibitory molecule IκBα is a proteasome substrate, and that proteasome inhibitors should prevent its degradation. More recent studies indicate that proteasome inhibitors can either inhibit or induce NF-κB, again through interaction with IκBα36, 37. However, these reports are conflicting and effects may vary by cell type. Our data in CLL demonstrate that the NF-κB pathway is in fact activated by CFZ as evidenced by degradation of total IκBα, accumulation of phosphorylated IκBα in nuclear and cytoplasmic fractions, and enhanced binding of NF-κB subunits to NF-κB consensus sites. However, NF-κB activation does not correlate with CLL cell death. This result is similar to what was reported in multiple myeloma cell lines using BTZ37, although our studies in CLL primary cells extend these observations to demonstrate that a classical NF-κB gene induction pattern is not observed. Therefore, while the inhibition of proteasome activity is not inhibitory to the NF-κB pathway per se, it induces an atypical response that does not result in the ultimate transcription of canonical NF-κB targets and thus is unlikely to provide a pro-survival effect.
To characterize the mechanism of CFZ-mediated cell death, we assessed the effects of CFZ on the Bcl-2 family of proteins and subsequent caspase activation. We found that inhibition of caspase activity caused a significant reduction in cytotoxicity of CFZ. Additionally, of the Bcl-2 family of proteins, only Noxa was consistently upregulated in all CFZ-treated samples. This effect was recently shown to induce caspase activity in CLL cells, further supporting a caspase-dependent pattern of cytotoxicity33. Proteasome inhibitors can also cause cytotoxicity due to endoplasmic reticulum (ER) stress by blocking the degradation of regulatory and misfolded proteins33, 45. However in our studies CFZ did not cause changes in conventional ER stress markers, including splicing of XBP1 or mRNA or protein levels of CHOP, GRP78 or pEIF2α (data not shown). Thus we concluded that in primary CLL cells, CFZ-mediated cytotoxicity was caused by a caspase-dependent pathway and not via ER stress.
To date, BTZ is the only proteasome inhibitor approved for treatment of malignant blood disorders and is now being used in front-line therapy for multiple myeloma46. In spite of a high degree of cytotoxicity in vitro, BTZ failed to produce any objective responses in CLL patients in a phase II clinical trial16. Its failure in vivo was hypothesized to be due to the inactivation of the boronate moiety in BTZ by flavonoids in human serum17, which is consistent with our observation that BTZ lacks activity against CLL cells in media containing human serum. Despite the initial clinical success of BTZ in hematological disorders, significant populations of patients remain refractory to treatment. Furthermore, toxicities with BTZ treatment such as peripheral neuropathy and thrombocytopenia have increased the intervals between dosing, allowing recovery of the proteasome function47, 48. This has spurred development of a new class of proteasome inhibitors that lack these toxicities. CFZ is being investigated through clinical trials in newly diagnosed, relapsed or refractory multiple myeloma, and has seen promising activity both as a single agent and in combination with immunomodulators49–51.
In summary, our study indicates that CFZ represents a promising, p53-independent therapeutic for CLL and provides the rationale for development of CFZ in this disease. Based on these data, we have initiated a phase I dose escalation study of this agent in relapsed and refractory CLL.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Standard therapies for chronic lymphocytic leukemia (CLL) are often ineffective in p53-mutated cases and detrimental to existing T cells, leaving patients at risk of opportunistic infections. A major focus of therapeutic development has been the investigation of agents that are cytotoxic to B cells independent of p53 but have minimal effects on T cells. Proteasome inhibitors have known activity in multiple myeloma, but to date have not shown clinical efficacy in CLL. This manuscript demonstrates B-cell selective efficacy of the proteasome inhibitor carfilzomib that is independent of p53, and provides new mechanistic insights to its mode of action in CLL patient cells. This work supports an ongoing Phase I clinical trial of carfilzomib in CLL.
ACKNOWLEDGMENTS
CFZ was provided for these studies by Onyx Pharmaceuticals, South San Francisco CA. The HS-5-GFP stromal cell line was kindly provided by Dr. Beverly Torok-Storb, Fred Hutchinson Cancer Research Center, Seattle WA. We thank the members of our laboratory for helpful comments and the many patients who donated blood for our studies.
Research support was received from The Leukemia and Lymphoma Society and the National Cancer Institute (P50 CA140158, 1K12 CA133250). Mr. and Mrs. Michael Thomas, The Harry Mangurian Foundation and The D. Warren Brown Foundation also supported this work.
Footnotes
Supplementary information is available at the journal's website.
AUTHORSHIP CONTRIBUTIONS
SVG: Designed, conducted and analyzed experiments and wrote the paper; EH: Designed, conducted and analyzed experiments; YL: Designed, conducted and analyzed experiments; RL: designed experiments and generated key reagents; EJS, TLC, MED, JAW: designed and conducted experiments; AL, DJ: performed statistical analyses; JCB, DML: conceived the work, designed experiments, analyzed data, and wrote the paper. All authors provided final approval of the manuscript.
CONFLICT OF INTEREST DISCLOSURE
No authors have conflicts of interest to report.
REFERENCES
- 1.Peters JM, Franke WW, Kleinschmidt JA. Distinct 19-S and 20-S Subcomplexes of the 26-S Proteasome and Their Distribution in the Nucleus and the Cytoplasm. Journal of Biological Chemistry. 1994;269:7709–7718. [PubMed] [Google Scholar]
- 2.Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 3.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 4.Myung J, Kim KB, Crews CM. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. Journal of Biological Chemistry. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 6.Yew PR. Ubiquitin-mediated proteolysis of vertebrate G1- and S-phase regulators. J Cell Physiol. 2001;187:1–10. doi: 10.1002/1097-4652(2001)9999:9999<1::AID-JCP1049>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Blagosklonny MV, Wu GS, Omura S, El-Deiry WS. Proteasome-Dependent Regulation of p21WAF1/CIP1Expression. Biochemical and Biophysical Research Communications. 1996;227:564–569. doi: 10.1006/bbrc.1996.1546. [DOI] [PubMed] [Google Scholar]
- 8.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer. 2009;9:371–377. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: a classical view in evolution. Cell Death Differ. 2007;14:3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZJ. Ubiquitin signalling in the NF-[kappa]B pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertlein E, Byrd JC. Signalling to drug resistance in CLL. Best Pract Res Clin Haematol. 2010;23:121–131. doi: 10.1016/j.beha.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114:441–449. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 16.Faderl S, Rai K, Gribben J, Byrd JC, Flinn IW, O'Brien S, et al. Phase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemia. Cancer. 2006;107:916–924. doi: 10.1002/cncr.22097. [DOI] [PubMed] [Google Scholar]
- 17.Liu FT, Agrawal SG, Movasaghi Z, Wyatt PB, Rehman IU, Gribben JG, et al. Dietary flavonoids inhibit the anticancer effects of the proteasome inhibitor bortezomib. Blood. 2008;112:3835–3846. doi: 10.1182/blood-2008-04-150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groll M, Kim KB, Kairies N, Huber R, Crews CM. Crystal structure of epoxomicin : 20S proteasome reveals a molecular basis for selectivity of alpha ',beta '-epoxyketone proteasome inhibitors. Journal of the American Chemical Society. 2000;122:1237–1238. [Google Scholar]
- 19.Adams J, Behnke M, Chen SW, Cruickshank AA, Dick LR, Grenier L, et al. Potent and selective inhibitors of the proteasome: Dipeptidyl boronic acids. Bioorganic & Medicinal Chemistry Letters. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 20.Demo SD, Buchholz TJ, Laidig GJ, Parlati F, Shenk KD, Smyth MS, et al. Biochemical and cellular characterization of the novel proteasome inhibitor PR-171. Blood. 2005;106:455a–455a. [Google Scholar]
- 21.Dorsey BD, Iqbal M, Chatterjee S, Menta E, Bernardini R, Bernareggi A, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51:1068–1072. doi: 10.1021/jm7010589. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Nishioka Y, Reich CF, Pisetsky DS, Lipsky PE. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J Clin Invest. 1996;98:1119–1129. doi: 10.1172/JCI118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103:3278–3281. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 25.Graf L, Iwata M, Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a. Blood. 2002;100:1509–1511. doi: 10.1182/blood-2002-03-0844. [DOI] [PubMed] [Google Scholar]
- 26.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 27.O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 29.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco A, Aujay M, Morgan B, Azab AK, Maiso P, Liu Y, et al. Carfilzomib-dependent selective inhibition of the chymotrypsin-like activity of the proteasome leads to antitumor activity in Waldenstrom's Macroglobulinemia. Clin Cancer Res. 2011;17:1753–1764. doi: 10.1158/1078-0432.CCR-10-2130. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zenz T, Krober A, Scherer K, Habe S, Buhler A, Benner A, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112:3322–3329. doi: 10.1182/blood-2008-04-154070. [DOI] [PubMed] [Google Scholar]
- 33.Baou M, Kohlhaas SL, Butterworth M, Vogler M, Dinsdale D, Walewska R, et al. Role of NOXA and its ubiquitination in proteasome inhibitor-induced apoptosis in chronic lymphocytic leukemia cells. Haematologica-the Hematology Journal. 2010;95:1510–1518. doi: 10.3324/haematol.2010.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trbusek M, Smardova J, Malcikova J, Sebejova L, Dobes P, Svitakova M, et al. Missense Mutations Located in Structural p53 DNA-Binding Motifs Are Associated With Extremely Poor Survival in Chronic Lymphocytic Leukemia. J Clin Oncol. 2011;29:2703–2708. doi: 10.1200/JCO.2011.34.7872. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara W, Arai Y, Kasai F, Fujiwara Y, Haruta M, Hosaka R, et al. Association of Germline or Somatic TP53 Missense Mutation with Oncogene Amplification in Tumors Developed in Patients with Li-Fraumeni or Li-Fraumeni-like Syndrome. Genes Chromosomes & Cancer. 2011;50:535–545. doi: 10.1002/gcc.20878. [DOI] [PubMed] [Google Scholar]
- 36.Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC, et al. Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB--dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9:183–194. doi: 10.1158/1541-7786.MCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravi R, Bedi A, Fuchs EJ. CD95 (Fas)-induced caspase-mediated proteolysis of NF-kappaB. Cancer Res. 1998;58:882–886. [PubMed] [Google Scholar]
- 39.Zenz T, Vollmer D, Trbusek M, Smardova J, Benner A, Soussi T, et al. TP53 mutation profile in chronic lymphocytic leukemia: evidence for a disease specific profile from a comprehensive analysis of 268 mutations. Leukemia. 2010;24:2072–2079. doi: 10.1038/leu.2010.208. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 41.Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 42.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 44.Niedermeier M, Hennessy BT, Knight ZA, Henneberg M, Hu J, Kurtova AV, et al. Isoform-selective phosphoinositide 3'-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113:5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruschak AM, Slassi M, Kay LE, Schimmer AD. Novel proteasome inhibitors to overcome bortezomib resistance. J Natl Cancer Inst. 2011;103:1007–1017. doi: 10.1093/jnci/djr160. [DOI] [PubMed] [Google Scholar]
- 47.Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 48.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 49.Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158:739–748. doi: 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson AJ, Yeh Y-Y, Smith LL, Wagner AJ, Hessler J, Gupta S, et al. The Novel Cyclin-Dependent Kinase Inhibitor Dinaciclib (SCH727965) Promotes Apoptosis and Abrogates Microenvironmental Cytokine Protection in Chronic Lymphocytic Leukemia Cells. Leukemia. 2012 doi: 10.1038/leu.2012.144. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.