Abstract
Cancer cells are markedly different from normal cells with regards to how their metabolic pathways are utilized to fuel cellular growth and survival. Two basic metabolites that exemplify these differences through increased uptake and altered metabolic usage are glucose and glutamine. These molecules can be catabolized to manufacture many of the building blocks required for active cell growth and proliferation. The alterations in the metabolic pathways necessary to sustain this growth have been linked to therapeutic resistance, a trait that is correlated with poor patient outcomes. By targeting the metabolic pathways that import, catabolize, and synthesize essential cellular components, drug resistant cancer cells can often be resensitized to anti-cancer treatments. The specificity and efficacy of agents directed at the unique aspects of cancer metabolism is expected to be high; and may, when in utilized in combination with more traditional therapeutics, present a pathway to surmount resistance within tumors that no longer respond to current forms of treatment.
Keywords: cancer, metabolism, resistance, chemotherapy
Introduction
One of the distinguishing features of cancer is an escape from the typical regulatory constraints that prevent rapid and uncontrolled cellular division. Many cellular modifications occur within the bioenergetic and metabolic pathways, driven by the energetic needs of a dividing cell. One of the earliest examples of altered metabolism was demonstrated in Otto Warburg’s seminal work, wherein Warburg observed that cancerous tissues use oxygen independent methods for breaking down glucose (1, 2). While the Warburg effect is not yet fully understood, it appears to consistently arise in a wide variety of cancers with diverse genetic backgrounds.
For cells integrated into the vasculature, nutrients are continually available and cancer cells absorb these nutrients at a much higher rate than normal cells (3–5). Thus the metabolic changes exhibited in cancer are a result of factors beyond pure energy production and instead work to support unconstrained cellular growth by generating the cellular resources that a rapidly dividing cell requires (6, 7). Nucleotide triphosphates and amino acids are necessary building blocks for genome replication and protein synthesis that occur as cells grow. Furthermore, growth and division both require expansion of the cellular, nuclear, and mitochondrial membranes which necessitates increased fatty acid synthesis to support the production of the lipid bilayers. In addition to basic building blocks, other components necessary to fuel cellular growth (ATP, NADPH) or protect the growing cell (NADH, NAD+) are regulated through components of the aerobic glycolysis machinery and must have their levels carefully managed in order to ensure that the basic building blocks can be efficiently converted into new biomass (8).
Glucose and glutamine can be broken down into the raw materials necessary to drive tumor growth (6, 9). The metabolic shift and increased glucose catabolism observed in cancer cells results in increased production of the biosynthetic precursors consumed as a cell grows. With upregulated glucose import, a fraction of the glucose taken in will be used in biosynthesis pathways upstream of pyruvate or be converted to pyruvate and enter the TCA cycle to provide precursors for fatty acid and amino acid synthesis while all remaining glucose is converted to lactate and exported from the cell (4). Glutamine catabolism follows a similar pattern: glutamine uptake is enhanced, a portion of the glutamine is catabolized to maintain cellular levels of basic biosynthetic precursors, but the majority of cellular glutamine is converted into lactate or alanine (4, 10, 11). In contrast to the catabolism of glucose, conversion of glutamine to lactate passes through the TCA cycle, which aids in the maintenance of the pool of mitochondrial carbons and NADPH. The remaining glutamine provides carbon and nitrogen sources for amino acid synthesis, and contributes nitrogen in purine and pyrimidine base synthesis (11). The uptake and breakdown of glucose and glutamine represent the central pathway for fueling tumor growth and methods that target these pathways can have significant effects on cellular growth and viability.
While many aspects of tumor metabolism rely on the conserved metabolic pathways commonly employed by rapidly proliferating cells, evidence indicates that there are facets of tumor metabolism that differ from non-tumorigenic proliferating cells. Work investigating the M2 isoform of pyruvate kinase (PKM2) indicates that in cells with low pyruvate kinase activity, as it is in those expressing the PKM2 isoform, pyruvate can be formed from PEP through PGAM1 phosphorylation (12). This alternative pathway allows for a decoupling of ATP production from glucose metabolism, providing a way to prevent the allosteric inhibition of glycolysis pathway enzymes that occurs at high concentrations of ATP while still generating the downstream biomolecules necessary for cellular growth (12). Comparison of the gene expression profiles for oncogenic foci and induced pluripotent stem cells (iPSC) shows that while many pathways are modulated in a similar fashion, such as the upregulation of monosaccharide metabolic genes or downregulation of differentiation related genes, there are still distinct differences (13). The oncogenic foci upregulated a large variety of metabolic clusters - such carbohydrate metabolism, sphingolipid metabolism, membrane lipid metabolism, and organophosphate metabolism – in addition to cellular stress and damage pathways, while the expression profile of the iPSC activated pluriopotency-related gene families (13). Exploiting the cancer-specific upregulation of stress pathways has already been shown to be an effective strategy for the development of highly selective anti-cancer treatments (14). Piperlongumine triggers cell death in cancerous cells but not in rapidly or slowly proliferating normal cells; murine tumor xenografts treated with piperlongumine displayed drastic response with no observed toxicity in normal mice (14). Similar specificity may be achievable through treatments targeting the dysregulated aspects of cancer metabolism.
Work linking dysregulated metabolism to drug resistance indicate that resistance may arise in part because altered metabolism can produce elevated ATP and NADPH levels (15, 16). Common mechanisms for generating chemoresistance are energy intensive and include enhanced DNA repair, misregulation of growth factor signaling, increased drug efflux, higher expression of anti-apoptotic genes or survival signaling pathways (17). Chemotherapies exert cytotoxic effects in part by inducing oxidative damage (18, 19), while increased glucose consumption gives rise to abundant NADPH, which contributes to therapeutic resistance (20, 21). The ability of deregulated metabolic pathways to protect cancer cells makes them attractive targets for improving chemotherapeutics (Supplementary Table 1).
Targeting the resource absorption machinery to potentiate therapeutics
Cancer cells depend on their altered metabolism to generate the necessary resources to fuel their growth and the synthesis of these building blocks is dependent on the catabolism of glucose and glutamine. The uptake of glucose and glutamine is often increased through the upregulation of transporters, such as the GLUT family glucose transporters or the ASCT2 amino acid transporter. These are commonly targeted in an attempt to disrupt the dysregulated metabolism observed in cancer cells.
Glucose transport
The GLUT family proteins facilitate the uptake of extracellular glucose, and are often upregulated in cancer. Directly targeting these transporters is one way to attack tumor growth. In nude mice xenografts and cell culture A549 lung cancer cells display lower GLUT1 expression and glucose uptake when treated with the irreversible GLUT1 inhibitor WZB117, resulting in an inhibition of growth that is synergistic with both cisplatin and paclitaxel (22). Exogenous ATP rescued cellular growth, indicating that the decrease in GLUT1 expression slowed tumor growth by limiting available ATP (22). In vitro WZB117 demonstrates selectivity for cancer cells while studies in animal models demonstrate relatively high efficacy and low toxicity, but treatment also induces transient hyperglycemia and a slight decrease in weight resulting from a loss of fat (22). Renal cell carcinomas lacking functional von Hippel-Lindau (VHL) tumor suppressor genes have been shown to be selectively targeted by an inhibitor of GLUT1, STF-31, while no effect on glycolysis in cells expressing the wild type VHL gene (23). In vivo, STF-31 based inhibition of GLUT1 was able to impede glucose uptake and kill tumor cells with no toxicity for noncancerous cells (23). While inhibition of glucose transporters varies in efficacy based on the tumor type when used alone, it is effective when coupled with other drugs as part of a combinatorial strategy. The glucose transporters are critical for maintenance of multiple myeloma cells, but studies have shown that inhibition of glucose transporters can increase sensitivity to other chemotherapeutics. This can be observed in how the off-target inhibition of the GLUT4 transporter by the HIV protease inhibitor ritonavir increases doxorubicin sensitivity in multiple myeloma cell lines (24). Combinatorial treatment has also been an effective way to overcome chemoresistance. HIF-1α enhances the expression of GLUT1 in hypoxic environments, a condition which frequently occurs in tumors. Hypoxia is well known to contribute to resistance to chemotherapeutics, yet inhibition of GLUT1 via phloretin overcomes this hypoxia-induced resistance when used in combination with daunorubicin (25). While phloretin is a competitive inhibitor for glucose transporters, low concentrations have been shown to be effective on hypoxic cells - a property that could enhance the selectivity of this treatment (25). With data indicating that GLUT3 is upregulated in temozolomide resistant cells, overcoming chemoresistance by targeting glucose transport coupled with treatment with standard chemotherapeutic agents may be an effective strategy against drug-resistant tumors (26).
Glutamine transport
Like glucose transport, upregulation of glutamine transporters is commonly observed in cancer cells (11). For many cancers, disruption of the constant uptake of glutamine can lead to outcomes more drastic than expected for pure amino acid starvation (27–30). In hepatocellular carcinoma, silencing of the glutamine transporter ASCT2 by inducible antisense RNA results in cellular death within 48 hours (31). The importance of glutamine transporters for cellular survival is further demonstrated by the effect of selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, on ER− cell lines and cell lines derived from estrogen insensitive tissues (32). Both tamoxifen and raloxifene can inhibit the glutamine transporter ASCT2 and lower cellular uptake of glutamine, resulting in growth inhibition and apoptosis in cell lines that are estrogen insensitive (32). Finally, inhibition of the SLC6A14 amino acid transporter by α-methyl-DL-tryptophan (α-MT) induces phenotypes associated with amino acid starvation, such as mTOR inhibition and activation of autophagy, but when coupled with an inhibitor of autophagy the combined treatment triggers apoptosis (33). While α-MT is only effective in the subset of cells expressing the SLC6A14 transporter, which is not expressed in all cancer types, it is highly selective for malignant cells, as SLC6A14 appears not to be expressed in non-malignant cells (33). Both glucose and glutamine transporters play a key role in providing cancerous cells with resources and inhibiting them results in slower cell growth or cellular death, even in cells that have been shown to be refractory to other chemotherapeutic treatments.
Targeting the metabolic machinery to potentiate therapeutics
Targeting the resource import machinery that fuels dysregulated metabolism can be an effective strategy for cancer therapeutics. Once the basic resources have entered the cell, a wide variety of components within the biosynthetic cellular machinery are required to synthesize the biological precursors that support continued cellular division. Below we will discuss selected elements within the biosynthetic pathways that have shown promise in sensitizing chemoresistant cells.
Glycolysis
Hexokinase catalyzes the first step in the catabolism of glucose, a key step in the glycolytic pathway. Two small molecule inhibitors, 2-deoxy-D-glucose (2-DG) and 3-bromopyruvate (3-BP), target hexokinase and to disrupt the early steps of glycolysis and have shown promise as chemotherapeutic agents.
2-DG is a glucose analog that cannot be fully processed by the glycolytic enzymes, resulting in an inhibition of glycolysis after it is phosphorylated by hexokinase. We have shown that 2-DG can effectively induce apoptosis in alveolar rhabdomyosarcoma, while hypoxic tumor cells are known to be sensitive to 2-DG treatment (34–36). The use of 2-DG as a solo agent is limited due to the high concentration necessary to compete with glycolytic pathway products, but it has been effective as part of a combinatorial treatment regimen. The chemotherapeutic agents adriamycin and paclitaxel significantly slowed tumor growth and prolonged survival of mice with osteosarcoma and non-small cell lung xenografts, respectively, when mice were co-treated with 2-DG (36). We have also demonstrated that trastuzumab-resistant breast cancer cells can be resensitized to trastuzumab through the addition of 2-DG (37). Finally, Bcl-2 family antagonists are only effective on certain cell varieties, but highly resistant leukemias are sensitized and undergo rapid apoptosis when Bcl-2 antagonist treatment is preceded by glycolysis inhibitor 2-DG (38).
A second small molecule glycolysis inhibitor that has shown promise as a chemotherapeutic agent is the pyruvic acid derivative 3-BP, which also targets hexokinase. Supplying extra ATP exogenously can induce chemoresistance in colon cancer cells, while ATP depleting factors like 3-BP have been shown to help induce sensitivity to cell lines selected for resistance to chemotherapeutic agents such as oxaliplatin and 5-fluorouracil (16). Studies showing that ATP-binding cassette (ABC) transporter based resistance to daunorubicin or mitoxantrone is also highly dependant on cellular ATP levels (39). Daunorubicin or mitoxantrone treatment in vitro (human myeloma, myelogenous leukemia, and liver carcinoma cell lines) or in vivo (murine xenografts) demonstrated suppressed resistance, increased cytotoxicity, and slowed subcutaneous tumor growth when coupled with 3-BP (39). Studies in animal models have demonstrated a lack of in vivo toxicity and an inability to cross the blood brain barrier, but as a competitive inhibitor the concentrations necessary for therapeutic effect as a solo agent may be prohibitive. The success of glycolysis inhibitors such as 2-DG and 3-BP predicts that other inhibitors targeting components in these pathways will also have a high likelihood to produce drug-sensitizing effects.
Pyruvate kinase isoform M2 (PKM2) is preferentially expressed in many cancers and favors glycolysis (12, 40). Inhibitors of PKM2 have shown highly selective anti-tumor activity in animal and human xenografts tumor models (41–45). Recent work also demonstrates that reducing PKM2 activity via CD44 knockdown results in lower cellular glutathione (GSH) levels, decreased glucose uptake, lactate production, and ATP production while increasing ROS levels, while also enhancing cisplatin sensitivity (20). As decreased activity of PKM2 helps overcome hypoxia induced drug resistance, small molecule compounds that modify PKM2 activity - such as TLN-232 (inhibitor) and 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides (activator) - may also be effective in lowering resistance to current chemotherapeutic treatments (41, 46). The TLN-232 inhibitor has exhibited high selectivity at even low doses for cancer cells in vitro and in vivo animal models, and while TLN-232 treatment does trigger a transient weight loss it is well tolerated with no toxicity observed at even high doses (41, 46).
Glutamine catabolism
Glutaminase catalyzes the first step in the catabolism of glutamine, hydrolyzing it into glutamate and ammonia. Inhibition of glutaminase can deprive the cell of biosynthetic precursors needed for rapid growth, evidenced by the reduction of in vitro growth of glioblastoma cells containing an isocitrate dehydrogenase 1 mutation common to many gliomas and acute myelogenous leukemias observed when glutaminase activity was reduced through siRNA or BPTES treatment (30). Glutamine analogs often cause in vivo toxicity, lack specificity, or are generally ineffective; whereas inhibitors of glutaminase -such as BPTES – provide an opportunity to realize the benefits of glutamine deprivation in a way that minimizes the clinical downsides (30). The small molecule inhibitor 968 prevents Rho GTPase mediated cellular transformation by inhibiting glutaminase, an essential component in Rho GTPase mediated transformation, yet elicited no effect on the growth or morphology of normal cells (47). In addition to blocking cellular transformation, 968 also demonstrated the ability to inhibit the in vivo growth of lymphoma murine xenografts (47). Glutaminolysis is also integrated into the amino acid biosynthesis pathways by activating mTORC1 signaling and fueling cellular growth, which also could induce mTORC1-mediated resistance to common chemotherapeutic agents such as cisplatin in malignant AFP-producing gastric carcinomas (48, 49). Inhibition of mTORC1 with the dual PI3K/mTOR inhibitor NVP-BEZ235 can resensitize Jurkat cells with induced vincristine resistance, clearly demonstrating that reducing mTORC1 activity can lead to therapeutic sensitivity in resistant cells (50). NVP-BEZ235 exhibits minimal effects on normal cells in vitro, but demonstrates good oral bioavailability and low toxicity in vivo (50). Inhibiting glutaminolysis is predicted to have a similar sensitizing effect, through the reduction of mTOR signaling and depletion of the cellular pool of available resources. Glutamine is a critical resource for dysregulated cellular growth, and inhibition of glutamine breakdown by targeting glutaminase merits further exploration as an anti-tumorigenic and therapeutic potentiating strategy. The important role of mTOR and PI3K pathways in cancer has been covered exhaustively elsewhere (51–63).
Lactate production and export
Lactate dehydrogenase (LDH) is a key glycolytic enzyme, as it converts pyruvate into lactate and provides a pathway for non-oxidative disposal of the byproducts of glycolysis. Lactic acid is a significant byproduct of cancer cell metabolism and is often exported from the cell to remove excess carbon and maintain cellular NADPH stores (4). Inhibition of MCT1-based lactate export through treatment with alpha-cyano-4-OH-cinnamate (CHC) or lonidamine causes the tumor microenvironment to decrease in pH, with significant levels of cellular death occurring as the pH reaches 6.5, the typical microenvironmental tumor pH (64–66). CHC treatment has been shown to effectively slow the tumor growth rate in murine models of lung adenocarcinoma and colorectal adenocarcinoma xenografts, while Lewis lung carcinoma tumor bearing mice treated with CHC demonstrated greatly increased sensitivity to a single 6 Gy dose of radiation (65, 66). Mild side effects are observed in patients after lonidamine treatment, with approximately half displaying myalgia; similarly animal models indicate that the negative side effects lonidamine are less likely to occur when given orally rather than intravenously. The LDH-A isoform has been shown to be upregulated in response to the overexpression of oncogenes such as HER2 and MYCN (64, 67). Targeting the conversion of pyruvate to lactate has also been demonstrated to be a productive anti-cancer strategy. Glycolytic cell types have shown susceptibility to FX11 based LDHA inhibition, exemplified by inhibition of tumor growth formation in P198 pancreatic xenografts, p493 β-lymphoma xenografts, and pancreatic LZ10.7 cells. Inhibition of LDHA via siRNA or FX11 treatment results in increased mitochondrial function, evidenced by higher oxygen consumption and ROS generation, as well as an increase in necrosis and cellular death. When combined with FK866, a nicotinamide phosphoribosyltransferase inhibitor, tumors undergo regression, whereas FK866 or FX11 alone merely slowed tumor growth (68, 69). Although solubility may require further derivatization of FX11, animals undergoing FX11 treatment did not lose weight or exhibit any symptoms of toxicity (68, 69). Additionally, we have demonstrated that the inhibition of LDHA, either through siRNA or oxamate treatment, can overcome resistance to both paclitaxel and trastuzumab (37, 70).
Citric acid cycle
Metformin is a complex I inhibitor for the mitochondrial respiratory chain that is best known for its use as a treatment for diabetes. A study of the incidence rate of cancer within diabetics linked metformin use to lower incidence rates of cancer and better survival for diabetic patients that developed pancreatic cancer (71–74). In addition to the initial studies linking metformin to better clinical outcomes for diabetic patients with pancreatic cancer, newer work has investigated the ability of metformin to address therapeutic resistance in a variety of cancer types.
Cisplatin resistant ovarian cancer cell lines demonstrated a synergistic response to treatment with metformin and phenethyl isothiocyanate (PEITC), resulting in slower cancer cell growth and induction of cell death for ovarian cancer cells (75). Similarly, pancreatic cancer ductal adenocarcinoma cell lines (SUIT-2 and MIAPaCa-2) exhibiting varying degrees of gemcitabine resistance have been used to test the efficacy of gemcitabine treatment in combination with metformin or metformin and R1507 (a humanized anti-IGF-1R monoclonal antibody). The combination of gemcitabine with metformin or metformin and R1507 induced greater inhibition of cellular proliferation and more apoptosis than solo treatment, whereas gemcitabine treatment alone increased the expression of genes indicative of chemoresistance, such as survivin and XIAP (76). Metformin has also been tested with respect to trastuzumab resistant HER2-amplified breast cancers (77). Selectively targeting more chemoresistant cell populations, such as cancer stem cells (CSCs), may provide a strategy to resensitize a tumor to chemotherapeutic compounds. Metformin has been reported to preferentially target CSC-like cells and is significantly more effective in CD44+/CD24−/low JIMT-1 cells, a cell line derived from a pleural metastasis of a patient resistant to trastuzumab, compared to non-the CD44+/CD24−/low JIMT-1 cell population, in vitro (77). Similarly, xenografts of JIMT1 were resistant to trastuzumab treatment, as no reduction in tumor growth was observed over a seven week course of treatment. However, when treated with metformin alone or trastuzumab in combination with metformin, tumor volumes were reduced two-fold or four-fold respectively, indicating that metformin treatment can help overcome trastuzumab resistance (77).
Pyruvate dehydrogenase kinase (PDK) phosphorylates and inhibits pyruvate dehydrogenase, preventing the formation and entry of acetyl-CoA into the citric acid cycle. Increased expression or activity of PDK isoforms may play a role in the metabolic shift away from mitochondrial oxidation and contributes to drug resistance, as siRNA targeting PDK restores mitochondrial oxidation in cancerous, but not normal, cells (78, 79). The small molecule dichloroacetate (DCA) also inhibits PDK triggering apoptosis in cancerous cells, but requires pharmacologically prohibitive concentrations to be effective, likely due to the decrease in expression of the cellular transporter responsible for DCA uptake in cancer cells (78, 80). Combination therapy with other agents, such as omeprazole or temozolomide, has shown to be more effective than solo treatments (81, 82). This demonstrates that targeting components related to the citric acid cycle can be an effective strategy in the design of new therapeutics.
Fatty acid synthesis
ATP citrate lyase (ACLY) catalyzes the ATP dependant conversion of citrate to acetyl-CoA and oxaloacetae, a key step in fatty acid biosynthesis. As expected, treatment with the ACLY inhibitor SB-204990 reduces cellular acetyl-CoA concentrations (83). In A549 xenografts, ACLY inhibition is cytostatic and induces differentiation, while for pancreatic xenografts SB-204990 treatment resulted in significant growth inhibition. ACLY inhibition results in more severe effects for more glycolytic cells, as ACLY inhibition hinders glucose dependant lipid synthesis, this is expected to make the effects of compounds that inhibit ACLY favor tumor cells over nonglycolytic and vegetative cells, as the latter are less dependant on ACLY activity (83). SB-204990 is orally bioavailable and demonstrates no toxicity in animal models, but may have low in vivo stability (83).
Synthesis of the fatty acids used for lipogenesis requires precursors derived from the citric acid cycle, such as acetyl-CoA or malonyl-CoA. These precursors are then utilized by fatty acid synthase (FASN) enzyme complex to produce components necessary for lipogenesis, such as palmitate. FASN is upregulated in many cancers and correlates with poor patient outcomes such as metastasis. FASN knockdown xenografts had smaller, slower growing tumors with lower tumorigenic potential and less metastasis relative to FASN expressing xenografts (84). FASN inhibitors, such as G28UCM, were able to effectively reduce the size of established xenografts by 20–90%. This decrease in size was accompanied by increased apoptotic cell death and decreased HER2 phosphorylation (85). Most FASN inhibitors (cerulenin, C75, EGCG) are unstable, impotent, or have significant side effects (anorexia and weight loss) in vivo, yet G28UCM treated animals displayed no anorexia or weight loss while G28UCM retained the ability to inhibit FASN activity and reduce tumor size (85). In vitro, G28UCM showed synergistic interactions with trastuzumab, lapatinib, erlotinib and gefitinib; while trastuzumab (AU565TR) or lapatinib (AU565LR) resistant cells retained FASN expression and sensitivity – providing an alternative method for overcoming drug resistance (85).
Conclusion
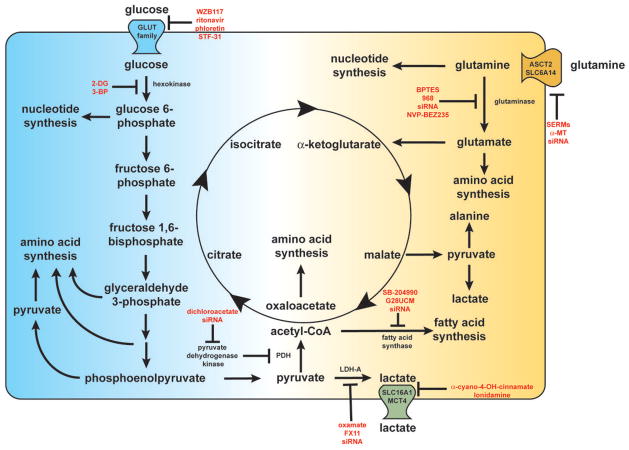
Above we have provided examples of compounds that can effectively re-sensitize cancer cells to therapeutics to which they had demonstrated resistance. The focus of this manuscript is on metabolic inhibitors that target either signaling transduction networks or key metabolic enzymes that are commonly dysregulated in cancer (Figure 1). While both strategies can be effective, directly targeting signal transduction networks often results in compensation through other closely related pathways, whereas the mechanisms allowing a cell to compensate for loss or inhibition of a rate-limiting or pathway initiating enzymes in the essential metabolic pathways are more limited (86).
Figure 1.
A wide range of metabolic components support the cancerous phenotype. Glucose and glutamine are metabolized into critical intermediates necessary for cellular growth, such as amino acids, nucleotides, and fatty acids. The metabolic inhibitors discussed within this manuscript are listed in red near their target of inhibition. Disruption of these metabolic networks can reduce drug resistance and render cancer cells more susceptible to current therapeutic regimens.
Metabolic pathway components, such as those involved in glucose utilization and amino acid biosynthesis, are likely to be good targets. Inhibition of PFKFB3 represses phosphofructokinase 1 (PFK1) by reducing fructose 2,6 bisphosphate (F2,6BP) synthesis. This leads to a decreased glycolytic characteristics evidenced by lower glucose uptake and less production of lactate, ATP, NAD+ and NADH (87). 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) is a small molecule inhibitor of PFKFB3 that has shown selectivity for transformed cells in vitro, and suppresses growth of Lewis lung carcinoma, MDA-MB-231 breast adenocarcinoma, and HL-60 promyelocytic leukemia xenografts (87). Given the observed effects on tumor growth in a variety of cancers as well as the metabolic changes 3PO treatment induces, PFK1 inhibition may produce chemosensitization similar when other glycolytic enzymes are inhibited.
Recent work also demonstrates that for some forms of breast cancer key component of the serine biosynthesis pathway, phosphoglycerate dehydrogenase (PHGDH), is often amplified in cancer resulting in increased activity of the serine biosynthesis pathway (88). Knockdown of PHGDH in breast cancer cell lines exhibiting increased PHGDH expression reduces alpha-ketoglutarate (aKG) levels, inhibits proliferation, and induces cellular death (88). As increased PHGDH is observed in approximately 70% of ER− breast cancers, it is reasonable to expect that treatments disrupting PHGDH function and enzymes related to serine biosynthesis will present an attractive strategy for overcoming resistance to chemotherapeutics (88, 89). Similarly, cellular stress pathways have been shown to be upregulated in cancerous cells (13). New research linking cellular stress to metabolism indicates that components within the ROS and heat shock response pathways might also be target capable of producing highly selective synergistic responses with current chemotherapeutic agents (13, 14, 67, 90, 91).
The link between therapeutic sensitivity and dysregulated cancer cell metabolism is quickly being dissected in a variety of studies concerning a diverse array of cancers and therapeutic agents. It is becoming clear that this may provide a strategy for surmounting the drug resistance that endangers so many cancer patients. We have described a variety of metabolic aspects ranging from resource uptake, breakdown, or biosynthesis that can contribute to or be targeted to overcome therapeutic resistance to an assortment of agents. While no unifying mechanism of how inhibition of metabolic pathways can result in resensitization to chemotherapeutics is apparent, common themes do occur. When cancer cell metabolism is disrupted the presumed resource shortfalls are often not fatal. Many of the metabolic inhibitors or gene knockdowns produced inhibitory effects on the cellular growth rate but do not induce cellular death. Rapid cellular growth appears to have a protective function, possibly due to the fact that rapidly dividing cells have access to sufficient resources to enact energy intensive therapeutic resistance pathways on demand (15, 16). The extra resources available may allow for the synthesis and operation of drug efflux pumps and anti-apoptotic genes such as surviving, and inhibition of the dysregulated metabolism often reduces ATP and other metabolic levels which may prevent efficient activation of resistance pathways (15, 76). Genomic instability frequently observed in cancers also ensures that a population of growing cells is more likely to have the genetic diversity to adapt to the selective pressures induced by chemotherapeutic treatments (92–95). By curtailing or preventing continued cellular growth, metabolic disruption might limit the ability of the tumor to adapt to the chemotherapeutic regimen. Finally, in many cases the disruption caused by inhibiting glycolytic metabolism results in increased cellular stress (often in the form of higher levels of reactive oxygen species) or decreased protective response (such as NADPH levels) which may predispose cells towards apoptosis or limit the ability of the tumor to address further chemotherapeutic insult (14, 20, 68, 69). Many survival pathways have both cytoprotective and cytotoxic functions, and the increase in cellular stress induced by metabolic inhibition may precondition the stress response pathways towards apoptotic outcomes (14). As evidenced by the diverse array of cancers discussed above, this method of attacking therapeutic resistance in cancer is demonstratively effective in a wide variety of cancer types and merits continued investigation.
Supplementary Material
Acknowledgments
Grant Support
Fondo de Investigaciones Sanitarias-ISCIII from Spain (PI10/0104 and RTICC RD06/0020 to C. Muñoz Pinedo); National Institutes of Health grants RO1CA137021 to J. Lu, and RO1CA149646 to M. Tan); The Vincent F. Kilborn, Jr. Cancer Research Foundation (to M. Tan).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 2.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 3.Kilburn DG, Lilly MD, Webb FC. The energetics of mammalian cell growth. J Cell Sci. 1969;4:645–654. doi: 10.1242/jcs.4.3.645. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollig-Fischer A, Dziubinski M, Boyer A, Haddad R, Giroux CN, Ethier SP. HER-2 signaling, acquisition of growth factor independence, and regulation of biological networks associated with cell transformation. Cancer Res. 2010;70:7862–7873. doi: 10.1158/0008-5472.CAN-10-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. Faseb J. 2004;18:1303–1305. doi: 10.1096/fj.03-1001fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 9.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 10.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggs JW, Barrilleaux B, Varlakhanova N, Bush K, Chan V, Knoepfler P. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Fanciulli M, Bruno T, Giovannelli A, Gentile FP, Di Padova M, Rubiu O, Floridi A. Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence of lonidamine. Clin Cancer Res. 2000;6:1590–1597. [PubMed] [Google Scholar]
- 16.Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, Zhang A, Xia X, Brasher H, Widger W, Ellis LM, Weihua Z. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 18.McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky L, McBride WH. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coriat R, Nicco C, Chereau C, Mir O, Alexandre J, Ropert S, Weill B, Chaussade S, Goldwasser F, Batteux F. Sorafenib-Induced Hepatocellular Carcinoma Cell Death Depends on Reactive Oxygen Species Production in vitro and in vivo. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 20.Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, Suematsu M, Saya H. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 21.Tome ME, Frye JB, Coyle DL, Jacobson EL, Samulitis BK, Dvorak K, Dorr RT, Briehl MM. Lymphoma cells with increased anti-oxidant defenses acquire chemoresistance. Exp Ther Med. 2012;3:845–852. doi: 10.3892/etm.2012.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small molecule inhibitor of glucose transporter 1 down-regulates glycolysis, induces cell cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 23.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBrayer SK, Cheng JC, Singhal S, Krett NL, Rosen ST, Shanmugam M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012;119:4686–4697. doi: 10.1182/blood-2011-09-377846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, Zheng X, Sadee W, Sun D. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 26.Le Calve B, Rynkowski M, Le Mercier M, Bruyere C, Lonez C, Gras T, Haibe-Kains B, Bontempi G, Decaestecker C, Ruysschaert JM, Kiss R, Lefranc F. Long-term in vitro treatment of human glioblastoma cells with temozolomide increases resistance in vivo through up-regulation of GLUT transporter and aldo-keto reductase enzyme AKR1C expression. Neoplasia. 2010;12:727–739. doi: 10.1593/neo.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katt WP, Ramachandran S, Erickson JW, Cerione RA. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Mol Cancer Ther. 2012;11:1269–1278. doi: 10.1158/1535-7163.MCT-11-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Mates JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, Dang CV, Riggins GJ. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs BC, Finger RE, Onan MC, Bode BP. ASCT2 silencing regulates mammalian target-of-rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol Cell Physiol. 2007;293:C55–63. doi: 10.1152/ajpcell.00330.2006. [DOI] [PubMed] [Google Scholar]
- 32.Todorova VK, Kaufmann Y, Luo S, Klimberg VS. Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother Pharmacol. 2011;67:285–291. doi: 10.1007/s00280-010-1316-y. [DOI] [PubMed] [Google Scholar]
- 33.Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV, Prasad PD, Thangaraju M, Ganapathy V. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Peinado S, Alcazar-Limones F, Lagares-Tena L, El Mjiyad N, Caro-Maldonado A, Tirado OM, Munoz-Pinedo C. 2-deoxyglucose induces Noxa-dependent apoptosis in alveolar rhabdomyosarcoma. Cancer Res. 2011;71:6796–6806. doi: 10.1158/0008-5472.CAN-11-0759. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ. Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40:5542–5547. doi: 10.1021/bi002426w. [DOI] [PubMed] [Google Scholar]
- 36.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R, Liu B, Fodstad O, Chen J, Wu Y, Price JE, Tan M. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011;71:4585–4597. doi: 10.1158/0008-5472.CAN-11-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meynet O, Beneteau M, Jacquin MA, Pradelli LA, Cornille A, Carles M, Ricci JE. Glycolysis inhibition targets Mcl-1 to restore sensitivity of lymphoma cells to ABT-737-induced apoptosis. Leukemia. 2012;26:1145–1147. doi: 10.1038/leu.2011.327. [DOI] [PubMed] [Google Scholar]
- 39.Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, Fujii S, Kagawa K, Takeuchi K, Sakai A, Ozaki S, Okano K, Nakamura T, Itoh K, Matsumoto T, Abe M. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One. 2011;6:e27222. doi: 10.1371/journal.pone.0027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 41.Keri G, Erchegyi J, Horvath A, Mezo I, Idei M, Vantus T, Balogh A, Vadasz Z, Bokonyi G, Seprodi J, Teplan I, Csuka O, Tejeda M, Gaal D, Szegedi Z, Szende B, Roze C, Kalthoff H, Ullrich A. A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity. Proc Natl Acad Sci U S A. 1996;93:12513–12518. doi: 10.1073/pnas.93.22.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tejeda M, Gaal D, Barna K, Csuka O, Keri G. The antitumor activity of the somatostatin structural derivative (TT-232) on different human tumor xenografts. Anticancer Res. 2003;23:4061–4066. [PubMed] [Google Scholar]
- 43.Tejeda M, Gaal D, Csuka O, Keri G. Growth inhibitory effect of the somatostatin structural derivative (TT-232) on leukemia models. Anticancer Res. 2005;25:325–330. [PubMed] [Google Scholar]
- 44.Tejeda M, Gaal D, Hullan L, Hegymegi-Barakonyi B, Keri G. Evaluation of the antitumor efficacy of the somatostatin structural derivative TT-232 on different tumor models. Anticancer Res. 2006;26:3477–3483. [PubMed] [Google Scholar]
- 45.Tejeda M, Gaal D, Hullan L, Schwab R, Szokoloczi O, Keri G. Antitumor activity of the somatostatin structural derivative (TT-232), against mouse and human melanoma tumor models. Anticancer Res. 2007;27:4015–4019. [PubMed] [Google Scholar]
- 46.Walsh MJ, Brimacombe KR, Veith H, Bougie JM, Daniel T, Leister W, Cantley LC, Israelsen WJ, Vander Heiden MG, Shen M, Auld DS, Thomas CJ, Boxer MB. 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg Med Chem Lett. 2011;21:6322–6327. doi: 10.1016/j.bmcl.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis Activates Rag-mTORC1 Signaling. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 49.Kamata S, Kishimoto T, Kobayashi S, Miyazaki M, Ishikura H. Possible involvement of persistent activity of the mammalian target of rapamycin pathway in the cisplatin resistance of AFP-producing gastric cancer cells. Cancer Biol Ther. 2007;6:1036–1043. doi: 10.4161/cbt.6.7.4253. [DOI] [PubMed] [Google Scholar]
- 50.Chiarini F, Grimaldi C, Ricci F, Tazzari PL, Evangelisti C, Ognibene A, Battistelli M, Falcieri E, Melchionda F, Pession A, Pagliaro P, McCubrey JA, Martelli AM. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against T-cell acute lymphoblastic leukemia. Cancer Res. 2010;70:8097–8107. doi: 10.1158/0008-5472.CAN-10-1814. [DOI] [PubMed] [Google Scholar]
- 51.Busaidy NL, Farooki A, Dowlati A, Perentesis JP, Dancey JE, Doyle LA, Brell JM, Siu LL. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30:2919–2928. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilar E, Perez-Garcia J, Tabernero J. Pushing the envelope in the mTOR pathway: the second generation of inhibitors. Mol Cancer Ther. 2011;10:395–403. doi: 10.1158/1535-7163.MCT-10-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Veelen W, Korsse SE, van de Laar L, Peppelenbosch MP. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30:2289–2303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 55.Martelli AM, Evangelisti C, Chappell W, Abrams SL, Basecke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo V, Ruvolo P, Kempf CR, Steelman LS, McCubrey JA. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 56.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 57.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 58.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dituri F, Mazzocca A, Giannelli G, Antonaci S. PI3K functions in cancer progression, anticancer immunity and immune evasion by tumors. Clin Dev Immunol. 2011;2011:947858. doi: 10.1155/2011/947858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polak R, Buitenhuis M. The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood. 2012;119:911–923. doi: 10.1182/blood-2011-07-366203. [DOI] [PubMed] [Google Scholar]
- 61.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 62.Davies MA. The role of the PI3K-AKT pathway in melanoma. Cancer J. 2012;18:142–147. doi: 10.1097/PPO.0b013e31824d448c. [DOI] [PubMed] [Google Scholar]
- 63.Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012;17:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- 64.Fang J, Quinones QJ, Holman TL, Morowitz MJ, Wang Q, Zhao H, Sivo F, Maris JM, Wahl ML. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol. 2006;70:2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 65.Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frerart F, Gallez B, Ribeiro A, Michiels C, Dewhirst MW, Feron O. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, Fodstad O, Tan M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 68.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 69.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, Ledoux SP, Tan M. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan DK, Miskimins WK. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. J Ovarian Res. 2012;5:19. doi: 10.1186/1757-2215-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawanami T, Takiguchi S, Ikeda N, Funakoshi A. A humanized anti-IGF-1R monoclonal antibody (R1507) and/or metformin enhance gemcitabine-induced apoptosis in pancreatic cancer cells. Oncol Rep. 2012;27:867–872. doi: 10.3892/or.2011.1597. [DOI] [PubMed] [Google Scholar]
- 77.Cufi S, Corominas-Faja B, Vazquez-Martin A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, Martin-Castillo B, Menendez JA. Metformin-induced preferential killing of breast cancer initiating CD44+CD24-/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–398. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 79.Lu CW, Lin SC, Chien CW, Lin SC, Lee CT, Lin BW, Lee JC, Tsai SJ. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am J Pathol. 2011;179:1405–1414. doi: 10.1016/j.ajpath.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Babu E, Ramachandran S, CoothanKandaswamy V, Elangovan S, Prasad PD, Ganapathy V, Thangaraju M. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene. 2011;30:4026–4037. doi: 10.1038/onc.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heshe D, Hoogestraat S, Brauckmann C, Karst U, Boos J, Lanvers-Kaminsky C. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs. Cancer Chemother Pharmacol. 2011;67:647–655. doi: 10.1007/s00280-010-1361-6. [DOI] [PubMed] [Google Scholar]
- 82.Ishiguro T, Ishiguro M, Ishiguro R, Iwai S. Cotreatment with dichloroacetate and omeprazole exhibits a synergistic antiproliferative effect on malignant tumors. Oncol Lett. 2012;3:726–728. doi: 10.3892/ol.2012.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O’Connor K, Morris AJ, Sunkara M, Weiss HL, Lee EY, Evers BM. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72:1504–1517. doi: 10.1158/0008-5472.CAN-11-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puig T, Aguilar H, Cufi S, Oliveras G, Turrado C, Ortega-Gutierrez S, Benhamu B, Lopez-Rodriguez ML, Urruticoechea A, Colomer R. A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res. 2011;13:R131. doi: 10.1186/bcr3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCormick F. Mutant onco-proteins as drug targets: successes, failures, and future prospects. Curr Opin Genet Dev. 2011;21:29–33. doi: 10.1016/j.gde.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 88.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodgkinson A, Chen Y, Eyre-Walker A. The large-scale distribution of somatic mutations in cancer genomes. Hum Mutat. 2012;33:136–143. doi: 10.1002/humu.21616. [DOI] [PubMed] [Google Scholar]
- 95.Hodgkinson A, Eyre-Walker A. Variation in the mutation rate across mammalian genomes. Nat Rev Genet. 2011;12:756–766. doi: 10.1038/nrg3098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.