Abstract
Gas-phase intra-molecular crosslinking of protein ubiquitin cations has been demonstrated via ion/ion reactions with anions of a homobifunctional N-hydroxysulfosuccinimide (sulfo-NHS) ester reagent. The ion/ion reaction between multiply-protonated ubiquitin and crosslinker monoanions produces a stable, charge reduced complex. Covalent crosslinking is indicated by the consecutive loss of two molecules of sulfo-NHS under ion trap collisional activation conditions. Covalent modification is verified by the presence of covalently crosslinked sequence ions produced by ion-trap collision-induced dissociation of the ion generated from the losses of sulfo-NHS. Analysis of the crosslinked sequence fragments allows for the localization of crosslinked primary amines, enabling proximity mapping of the gas-phase 3-D structures. The presence of two unprotonated reactive sites within the distance constraint of the crosslinker is required for successful crosslinking. The ability to covalently crosslink is therefore sensitive to protein charge state. As the charge state increases, fewer reactive sites are available and protein structure is more likely to become extended due to intramolecular electrostatic repulsion. At high charge states, the reagent shows little evidence for covalent crosslinking but does show evidence for ‘electrostatic crosslinking’ in that the binding of the sulfonate groups to the protein is sufficiently strong that backbone cleavages are favored over reagent detachment under ion trap collisional activation conditions.
Keywords: quadrupole/time-of-flight, crosslinking, top-down proteomics, ubiquitin, gas-phase structure
Introduction
The introduction of electrospray ionization (ESI) [1,2] and matrix assisted laser desorption ionization (MALDI) [3,4] provided robust means for generating gaseous ions of proteins and, under some conditions, protein complexes. Since then, various forms of mass spectrometry have been applied to the study of protein primary, secondary, tertiary, and quaternary structure. Primary structure characterization is generally approached by collisional, photon-based, and electron-based activation methods to induce dissociation of the peptide/protein backbone. For example, collision-induced dissociation (CID) [5-7], surface-induced dissociation (SID) [8-12], electron capture dissociation (ECD) [13-16] and electron transfer dissociation (ETD) [17-20], infrared multiphoton dissociation (IRMPD) [21-24], UV photodissociation (UVPD) [25-29], and blackbody infrared radiative dissociation (BIRD) [30,31] have all been used to probe the primary structures of peptides and proteins by generating sequence-related fragment ions.
The study of higher order structure of gas-phase peptides, proteins, and protein complexes (viz. secondary, tertiary, and quaternary structures), a challenging problem for mass spectrometry, is also an active area of research.[32] A few model systems have been widely used in the development of mass spectrometry and related techniques for generating higher order structure information. One such system is the regulatory protein ubiquitin (8.6 kDa). Ubiquitin is a convenient model for 3-D structure analysis due to its relatively small size and its potential conformational diversity due to a lack of disulfide bond linkages. Gaseous ubiquitin ions have been studied by a variety of approaches, many of which provide charge state-resolved information. Ion mobility spectrometry (IMS), for example, has been used to measure collision cross sections for charge states [M+5H]5+ to [M+15H]15+ generated from solution conditions conducive to the native state (N) as well as from solution conditions consistent with the partially unfolded A-state; viz. pure water and a mixture of 49.5% methanol/49.5% water/1% acetic acid, respectively.[33,34] IMS studies have illustrated that immediately after ionization, lower charge states tend to have relatively compact gas-phase structures, while higher charge states tend to be more extended. Time-resolved IMS has shown that once entering the gas-phase, ubiquitin charge states will often unfold to a stable intermediate and either refold to the more compact structure or a more extended structure.[35-43] Multiple gas-phase conformations have also been observed for each charge state, including 5 to 10 conformer distributions for the [M+7H]7+ charge state.[44] Along with low field IMS, field asymmetric IMS (FAIMS), [45] hydrogen-deuterium exchange (H-D exchange), [46-48] ECD, [49-53] and site-specific radical directed dissociation [54] have been used to characterize ubiquitin’s gas-phase structures.
Crosslinking reagents have been developed for peptide and protein proximity (intramolecular) and contact (intermolecular) mapping.[55-57] Many of these reagents are available commercially and have been developed to be specific for particular amino acid residues.[58] For example, homobifunctional N-hydroxysuccinimide (NHS) esters have been used to link primary amines (e.g. lysine side-chains and N-termini).[58] Cleavable crosslinkers have also been developed that are selective for certain chemical reactions and gas-phase dissociation methods.[58] Briefly, these strategies react whole proteins with crosslinking reagents in solution before enzymatic digest and introduction into a mass spectrometer, often following separation with liquid chromatography.[59] Once in the gas-phase, the crosslinked product constituent peptides are isolated and fragmented with mass analysis of the fragmentation products. Mass shifts in the sequence fragments due to the covalent addition of the crosslinker allow for the identification of crosslinking sites. Since crosslinkers have distance constraints and can bind specifically to certain chemical functional groups, inferences can be made about the 3-D structures of the protein in solution.[59] However, the sheer amount of data produced by these experiments makes interpretation difficult, and software algorithms have been developed to help identify crosslinks.[60] Other issues with this “bottom-up” approach include isobaric interferences and missed enzymatic cleavages next to crosslinker-modified amino acid residues.[55] “Top-down” proteomics has been developed to purify whole proteins in the gas-phase, measure the molecular mass, and use dissociative chemistries to analyze sequence fragments of various proteins, including ubiquitin, up to and above 200 kDa.[61,62] The top-down strategy has been applied to proteins crosslinked in solution such as ubiquitin [63-65] and bovine rhodopsin.[66] Top-down crosslinking of ubiquitin with NHS esters provided evidence of crosslinking, and thus proximity, of lysines 48-63 and lysines 6-11.[63]
We have recently demonstrated the formation of covalent linkages to polypeptide ions in the gas-phase via reactions between ions of opposite polarity. The first of these involved the ion/ion reaction between peptide cations with unprotonated primary amines and anions with an aldehyde group to form Schiff bases.[67,68] We have since demonstrated that sulfo-NHS esters can be used to generate amide linkages to unprotonated primary amines[69] and guanidine groups[70], covalently tagging them with SS-biotin, acetate, and the fluorophore aminomethylcoumarin acetate (AMCA). Recently, in proof-of-concept experiments, peptide ions have been intermolecularly and intramolecularly crosslinked via ion/ion reactions involving peptide cations and homobifunctional sulfo-NHS crosslinker anions.[71] In this work, we demonstrate the gas-phase intramolecular cross-linking of the protein ubiquitin with homobifunctional sulfo-NHS esters. Crosslinked products are dissociated by ion-trap CID to determine crosslinked sequence fragments in a top-down experiment. These results demonstrate that the top-down proximal mapping approach via crosslinking of proteins in solution can be extended to gaseous proteins via ion/ion reactions.
Experimental
Materials
Ubiquitin from bovine erythrocytes was purchased from Sigma-Aldrich (St. Louis, MO, USA). The crosslinking reagent ethylene glycol bis(sulfosuccinimidylsuccinate) (sulfo-EGS) (Scheme 1) was purchased as the sodium salt from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Amberlite cation exchange resin was purchased from Supelco (Bellefonte, PA, USA). The crosslinking reagent was subjected to cation exchange to replace the sodium counterions with protons. Glacial acetic acid and methanol were purchased from Mallinckrodt (Phillipsburg, NJ, USA). Ubiquitin solutions were made 20 μM in 50/50/1 water/methanol/acetic acid (vol/vol/vol). The reagent was dissolved in 50/50 water/methanol at a concentration of approximately 2 mM.
Scheme 1.

Sulfo-EGS reagent anion, 16.1 Å linker arm.
Mass Spectrometry
Reactions were performed on a modified QqTOF adopted for mutual storage ion/ion reactions with resolving power of approximately 8000 FWHM and mass measurement accuracy between 10 and 20 ppm.[72] (QStar XL, AB Sciex, Concord, ON, CA). Reagent and analyte ions were sequentially injected via a homebuilt pulsed nano-electrospray (nESI) source.[73] Gas-phase modification of peptide cations by NHS esters has been previously described.[69,71] The method has been modified for use on the QqTOF instrument. Briefly, ubiquitin cations and reagent anions were sequentially transferred to q2 after each was isolated by Q1. The mutually stored ions reacted to produce long lived complexes. The products were transferred to Q1 for isolation.[74] After isolation, product ions were transferred back to q2 for ion-trap CID. When a covalent reaction occurred, a dominant loss of neutral sulfo-NHS was observed. The CID product was transferred back to Q1 for isolation and then returned to q2 for ion-trap CID. A second loss neutral sulfo-NHS indicates intramolecular crosslinking. The covalently modified product ions were isolated in Q1 and activated by ion-trap CID in q2. Ion-trap CID was performed at 115 kHz with an amplitude of 3.9 Vp-p/charge over a period of 100 ms. The pressure in q2 was roughly 6 mTorr. The fragments generated in q2 were then transferred into the TOF for mass analysis.
Results and Discussion
The homobifunctional NHS crosslinker used here (Scheme 1) is available commercially in sulfonate form, which improves reagent solubility. In the context of the ion/ion reaction experiment, the sulfonate functionality is of further significance in that it facilitates anion formation via electrospray and, of critical importance, engages in strong electrostatic interactions with protonated sites that lead to long-lived complex formation.[69] The ion/ion complex takes on the form [M+nH+*](n−1)+, where n refers to the number of protons in the isolated charge state and * represents the crosslinker. Two criteria must be met for successful crosslinking within the complex: 1) at least two reactive sites (i.e, unprotonated primary amines or guanidine groups) must be present, and 2) the reactive sites must be within the distance constraint of the linker. Covalent crosslinking is indicated when, upon ion-trap CID, the complex loses two neutral sulfo-NHS molecules. Structural characterization of the crosslinked protein ion approached via gas-phase dissociation, which, for these experiments, was effected by ion-trap CID. The mass-analyzed fragment ions are assigned to particular sequence ions, where an increase in mass equal to the linker arm is evidence of a crosslinked fragment. The crosslinked sequence fragments are complimentary to uncrosslinked sequence fragments because the ion/ion reaction product containing only one crosslink was isolated for the subsequent ion-trap CID experiments. No mass shifts due to cleavage of the crosslinker were observed. By this method, crosslinks can be localized to varying degrees, depending upon the information that can be derived from the product ion spectrum.
Crosslinking of the [M+7H]7+ ubiquitin ion
The crosslinking experiment is illustrated in Figure 1, which summarizes the gas-phase crosslinking of the [M+7H]7+ charge state of ubiquitin with [sulfo-EGS-H]−. The mass spectrum (Figure 1(a)) generated from nESI of a solution of 49.5/49.5/1 (water/methanol/acetic acid) shows a bimodal charge state distribution. After sequential isolation and accumulation of the [M+7H]7+ and [sulfo-EGS-H]1− ions in q2, mutual storage of the ions resulted in the spectrum of Figure 1(b), showing the addition of up to two [sulfo-EGS-H]1− ions to the protein ion. Ion-trap CID of the mass selected [M+6H+sulfo-EGS]6+ ion (Figure 1(c)) leads predominantly to the loss of a single molecule of sulfo-NHS. Collisional activation of the first generation product that arises from loss of sulfo-NHS leads to the facile loss of a second molecule of sulfo-NHS with small contributions from cleavages of peptides bonds (Figure 1(d)). The product from the loss of two sulfo-NHS molecules represents the intramolecularly crosslinked protein. The results of Figures 1(c) and 1(d) strongly suggest that a large fraction of the [M+7H]7+ ions underwent crosslinking. The [M+5H+2sulfo-EGS]5+ ion dissociates to form two covalent links under the same CID conditions (data not shown).
Figure 1.
Crosslinking [ubiquitin +7H]7+ with [sulfo-EGS-H]−. (a) nESI mass spectrum ubiquitin. (b) Product ion spectrum of the ion/ion reaction between [ubiquitin +7H]7+ and [sulfo-EGS-H]−. (c) CID product ion spectrum of the [M+6H+sulfo-EGS]6+ ion. (d) CID product ion spectrum of the [M+6H+sulfo-EGS - sulfo-NHS]6+ ion (i.e. ions indicated as –sulfo-NHS) from (c).
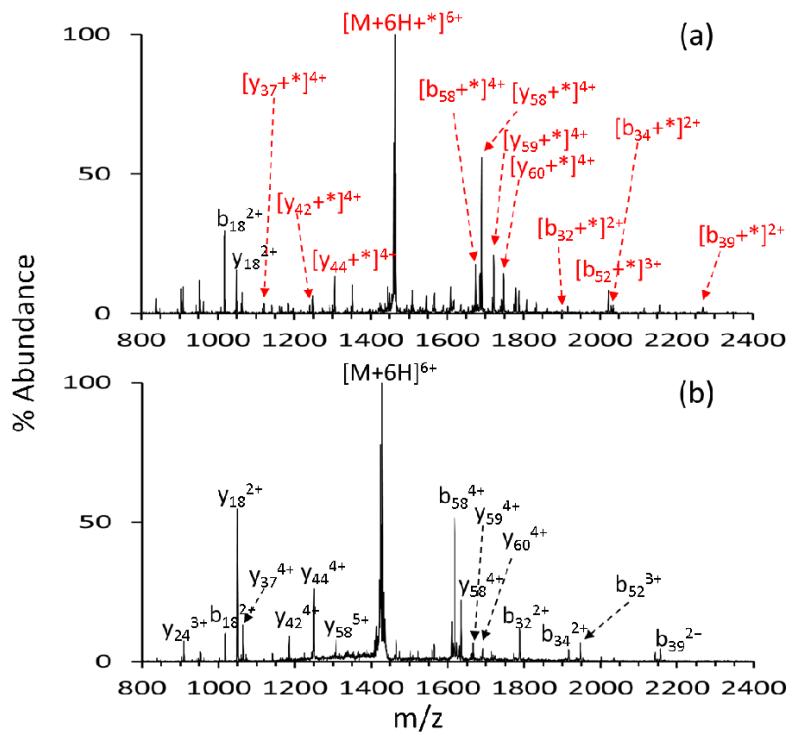
The [M+6H+*]6+ ion (i.e., the ion generated in Figure 1(d) by loss of two sulfo-NHS molecules from the [M+7H+sulfo-EGS]6+ complex) and the unmodified [M+6H]6+ ion were each subjected to ion trap CID and the product ion spectra are compared in Figure 2. The product ion spectrum generated via CID of the [M+6H+*]6+ is given by Figure 2(a), with the asterisk indicating the loss of two sulfo-NHS moieties from the ubiquitin/sulfo-EGS ion/ion reaction complex (i.e. gas-phase covalently crosslinked ubiquitin). The product ion spectrum contains both crosslinked sequence ions and unmodified sequence ions. The crosslinked sequence ions contain both of the two crosslinked residues and the intact linker. This is illustrated by Scheme 2(a) for the crosslinked y37 ion (y37*). Unmodified sequence ions do not contain the crosslinker (Scheme 2(b)). The 7+ charge state of ubiquitin, which has a total of 13 basics sites (1 N-terminus, 7 lysine residues, 1 histidine residue, and 4 arginine residues) and is therefore expected to have up to 5 reactive sites, (histidine is not reactive to sulfo-NHS ester chemistry). Therefore, the addition of one crosslinker to the population of 7+ ubiquitin ions likely yields a product ion population comprised of a mixture of structures crosslinked in several locations. Crosslinking sites must be determined by MS/MS experiments since each of the crosslinked gas-phase structures is an isomer. Some ions in Figure 2(a), such as the y374+ ion, appear in both crosslinked and unmodified forms. This observation reflects the presence of a mixture of structures. For instance, the unmodified y374+ ion results from one or more structures crosslinked N-terminal to Q40 whereas the modified y374+ ion results from one or more structures crosslinked C-terminal to D39. Peak assignments were facilitated for Figure 2(a) by recording the product ion spectrum of the unmodified ubiquitin 6+ charge state under the same CID conditions (Figure 2(b)). Peak assignments for Figure 2(b) were verified by proton transfer reactions for spectral simplification (see, for example, data reported in [72] for ubiquitin).
Figure 2.
Unimolecular dissociation to determine crosslinked sites. +* indicates a covalently attached crosslinker. (a) CID of −2sulfo-NHS from [M+7H+sulfo-EGS-H]6+. (b) CID of [M+6H]6+.
Scheme 2.
(a) Cartoon of a crosslinked y374+ sequence ion. (b) An unmodified y374+ ion. (c) A “hanging: crosslink. (d) An electrostatic crosslink.
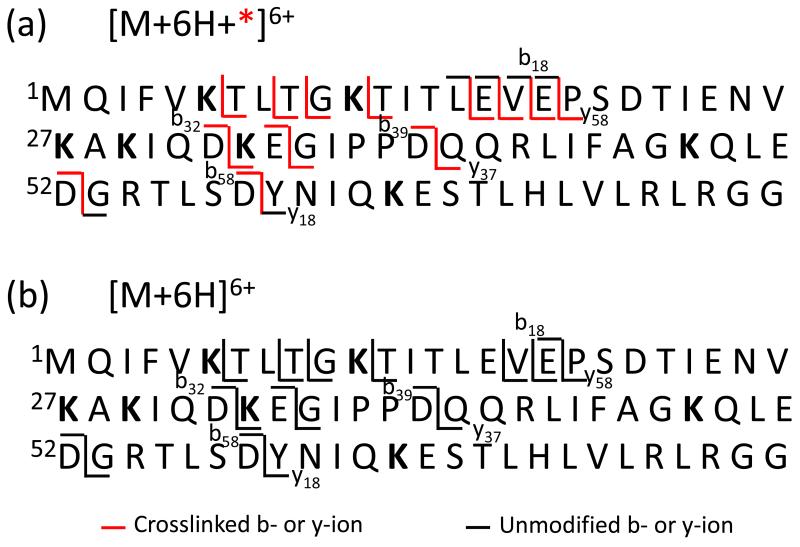
Scheme 3 summarizes the major amide bond cleavages observed in the data of Figure 2 with red lines indicating fragments for which the crosslink was present in at least some of the ions (e.g., a red line is used for the y37 fragments because a crosslink was noted for some of them) (Scheme 3(a)) and black lines indicating fragments for which no evidence for the presence of the crosslink was noted. To facilitate assignment of likely crosslinking sites, it is useful to generate information regarding the locations of the potential reactive sites. An indication of which sites are potentially reactive can be obtained by examining the reactivity of a mono-functional reagent (i.e., a reagent without the same distance constraint as the crosslinking reagent). Figure 3 shows the CID products from sulfo-NHS loss from [M+7H+sulfo-NHS-AMCA]6+. Neither the b18 nor b17 ions show any evidence for covalent attachment of AMCA, which provides evidence that the primary amines contained in these fragments (viz., the N-terminus, K6, and K11) are unreactive towards the NHS-ester chemistry. Likewise, all of the complementary y58 ions are modified (i.e., they appear as y58+AMCA ions). This behavior is fully consistent with the crosslinking data in that there are no b18-ions with the crosslinker nor are there any smaller b-ions with the crosslink. The lack of evidence for crosslinking between the N-terminus, K6, and K11 is therefore likely to be attributable to a lack of reactivity for these sites, rather than a failure of these sites to reside within the distance constraint of the linker. On the other hand, an abundant y18+AMCA product, roughly equal in abundance to the unmodified y18 ion, is observed in Figure 3. No y18 ions with the crosslinker are noted in the data of Figure 2. This indicates that there is one reactive site within the first 18 residues from the C-terminus but probably not two reactive sites. An MS3 experiment involving the (y18+AMCA)2+ ion (see Supplemental Figure S-1(a)) indicates that all of the modified fragment ions contain K63 whereas none of the unmodified product ions contains K63. This indicates that the only lysine in this region of the protein is reactive to sulfo-NHS ester chemistry for the 7+ charge state and that R72 and R74 residues are unreactive, presumably because they are solvating some of the excess charge. Similarly, the modified y374+ ion was isolated and subjected to ion trap CID (Supplemental Figure S-1(b)). The product ion spectrum includes the unmodified b6-ion and its complementary modified y31-ion, and not vice versa. However, the b11-ion appears in the modified form, indicating that one of the residues between A46 and L50 is reactive. The only residue with a nucleophilic side-chain in this region is K48. The MS3 data for the (y18+AMCA)2+ and (y37+AMCA)4+ ions cannot provide definitive information regarding the reactivity of R54. Hence, some crosslinking with R54 cannot be precluded, although reactivity of R54 need not be invoked to rationalize any of the experimental data. The most likely scenario is that the crosslinking in the (y37+*)4+ ion in Figure 2 takes place between K48 and K63.
Scheme 3.
Summary of major amide bond cleavages from the crosslinked (a) and unmodified (b) 6+ ions of ubiquitin.
Figure 3.
(a) CID of [M+7H + sulfo-NHS-AMCA – sulfo-NHS]6+. +AMCA indicates the covalent attachment of AMCA to the fragment ion. (b) Structure of sulfo-NHS-AMCA.
With the information available regarding the reactive sites generated from the data of Figure 3 and the crosslinking data of Figure 2, it is also possible to narrow down possibilities for the crosslinking site(s) N-terminal to the y37 fragment. As mentioned above, examination of the fragmentation data of Figure 2 reveals no crosslinked fragments N-terminal to b18, indicating that there are no crosslinks between the N-terminus, K6, and K11. The smallest N-terminal fragment that contains a crosslink is the (b32+*)2+ ion. Given the lack of reactivity for the N-terminus, K6, and K11 noted above, the most likely scenario is crosslinking between K27 and K29, as these are the only other residues with nucleophilic side-chains in the b32 fragment. A crosslink between K27 or K29 and one of the primary amines N-terminal to these residues is unlikely, based on their lack of reactivity with the mono-functional reagent.
Further evidence that crosslinks between K27 or K29 and any of the N-terminal primary amines of ubiquitin are relatively unimportant can be drawn from the effect of crosslinking on the relative abundances of products in Figure 2. For example, the two major cleavage processes for the [M+6H]6+ ion yield the b58/y18 (cleavage at D58-Y59) and b18/y58 (cleavage at E18-P19) complementary pairs. In the absence of crosslinking, the summed abundances of the b58/y18 ions is 3.0 times greater than the summed abundances of the b18/y58 ions (Figure 2(b)). A similar abundance ratio of 2.9 is also observed in the data with the AMCA modification (Figure 3) when the modified and unmodified versions of the same b- and y-ions are summed together (i.e., the summed abundances of b58, (b58+AMCA), y18, and (y18+AMCA) ions versus the summed abundances of the (y58+AMCA) and b18 ions (there are no (b18+AMCA and y58 ions, as mentioned above)). However, in the crosslinked ions (Figure 2(a)), the abundance ratio is 0.45. The main change is the dramatic decrease in the appearance of D58-Y59 cleavage products (i.e., the (b58+*) and y18 ions) in the crosslinked protein data. This observation is fully consistent with the assigned crosslinks. The D58-Y59 amide linkage is present in the loop generated by the K48-K63 crosslink which would be expected to inhibit observation of cleavage at this site because two bonds must be cleaved. The (b584++*) and y18+ ions that are observed in Figure 2(a) are expected to arise from the components of the precursor ion population that do not encompass the D58-Y59 residues within the loop generated by a crosslink (e.g., a K27-K29 crosslink). The change in relative contributions of cleavages of the E18-P19 and D58-Y59 sites in the unmodified versus crosslinked species is therefore consistent with the interpretation of the absence of crosslinks that inhibit observation of E18-P19 cleavage products whereas a major fraction of the modified proteins are crosslinked between K28 and K63, which reduces contributions from products arising from D58-Y59 cleavage.
A key question associated with gas-phase crosslinking via the sulfo-NHS crosslinker anions described here is the extent to which conformational changes might be driven either by the exothermicity of the ion/ion reaction itself or via the collisional activation steps used to drive off the neutral sulfo-NHS groups. Such changes could influence the crosslinking patterns, for example. Of particular relevance to this issue are the time-scales associated with the reactions and with any conformational changes that might occur. For example, if the covalent linkages take place in the long-lived complex prior to the collisional activation steps, any conformational changes driven by ion trap collisional activation would take place after crosslinking. In this scenario, crosslinking occurs before ion heating via collisional activation and the sulfo-NHS products are simply attached via strong electrostatic interactions. Collisional activation is needed simply to detach sulfo-NHS groups from the attachment sites on the protein. The evidence for this scenario is the observation of spontaneous loss of the NHS group when NHS-ester reagents with fixed positive charges on the R group are reacted with polypeptide anions [69] and when 4-sulfobenzoate NHS anions (i.e., the sulfo-group is present on the R-group rather than on the NHS ring) are reacted with polypeptide cations. Figure 4, for example, compares the results of ion/ion reactions of doubly protonated ARAKARA with deprotonated 4-sulfobenzoate NHS (Figure 4(a)) and with deprotonated benzoate sulfo-NHS (Figure 4(b)). The isomeric reagent anions differ in the position of the sulfonate group. When the sulfonate group is present on the NHS ring, the two major ion/ion reaction products arise from proton transfer to give the [M+H]+ ion and the complex arising from the attachment of the (apparently) intact reagent to the peptide analyte ion (see Figure 4(b)). Upon collisional activation of the complex, loss of sulfo-NHS to yield the covalently modified peptide and loss of the intact reagent to yield protonated ARAKARA are the dominant processes (see Figure 4(c)). When the sulfonate group is present on the R-group, as is the case with 4-sulfobenzoate NHS, proton transfer is observed but essentially no attachment of the intact complex to the peptide is noted (see Figure 4(a)). Rather, an abundant product corresponding to attachment of the reagent with subsequent loss of neutral NHS is observed. This indicates that covalent linkage of sulfobenzoate to the peptide takes place without requiring a collisional activation step. Collectively, the results of Figure 4 suggest that the crosslinking observed in this work can take place prior to the collisional activation steps. If so, collisional activation steps serve simply to detach the residual electrostatically bound sulfo-NHS products. In any case, at this stage in the study of gas-phase crosslinking few definitive conclusions can be drawn regarding how well the gas-phase crosslinking experiment samples the three dimensional structure(s) of the ambient temperature analyte ion population. It is clear, however, that the cross-linking noted in these experiments is hardly random. Based on the number of primary amine groups in the protein, there are 28 possible crosslinks. The CID data of Figures 2 and 3 rule out all but 10 (See Supplemental Table 1), with two, the K27-K29 and K48-K63 crosslinks, being indicated in the product ion spectra. The latter crosslink was also the major crosslink identified via top-down tandem mass spectrometry applied to ubiquitin subjected to NHS-ester crosslinking in solution using the same spacer as that used here.[63] (Based on the lack of evidence in any of the CID data for reaction at arginine and the relatively high basicity of the guanidine side-chain, it is likely that the arginine residues in the 7+ charge state are engaged in solvating the excess charge.)
Figure 4.
a) Post-ion/ion reaction spectrum from the reaction of doubly protonated ARAKARA with deprotonated 4-sulfobenzoate NHS (SBN, structure indicated). b) Post-ion/ion reaction spectrum from the reaction of doubly protonated ARAKARA with deprotonated benzoate sulfo-NHS (BSN, structure indicated). c) Product ion spectrum from the ion trap collisional activation of the [M+BSN+H]+ complex generated in the experiment leading to b).
Reactions with high charge states of ubiquitin
As the ubiquitin charge state increases, crosslinking reactivity is expected to decrease due to the reduction is the number of reactive sites (i.e., fewer unprotonated primary amines and guanidine side-chains) and the tendency for protein unfolding due to intramolecular electrostatic repulsion. [38] As expected, evidence for covalent crosslinking decreases with an increase in ubiquitin charge state. The trend in behavior is illustrated with the data of Figure 5, which shows the ion trap CID product ion spectra derived from the ion/ion complexes formed by reacting [sulfo-EGS-H]− with the [M+8H]8+ (Figure 5(a)), [M+10H]10+ (Figure 5(b)), and [M+12H]12+ (Figure 5(c)) ubiquitin precursor ions. In contrast with the results for the [M+7H+sulfo-EGS-H]6+ complex (see Figure 1(c)), significant contributions from backbone cleavages are apparent in the data for the higher charge state complexes and many of the products include the intact sulfo-EGS reagent. The fact that the intact reagent is retained suggests that both sulfo groups are engaged in relatively strong electrostatic interactions with the polypeptide. Ion trap CID of the ion generated by sulfo-NHS loss from the [M+8H+sulfo-EGS-H]7+ complex showed an abundant loss of a second sulfo-NHS molecule (data not shown), which suggests that some of the precursor ion underwent crosslinking. However, significant contributions from backbone cleavage, with many products showing a hanging crosslinker (i.e., covalent attachment at one end only), were also observed (Scheme 2(c)). A hanging crosslink is defined as a sequence fragment with a singly reacted linker (i.e., a fragment ion with the addition of the sulfo-EGS reagent less one sulfo-NHS group). All previous work with gas-phase sulfo-NHS ester reactions involving a single sulfo-NHS group has shown that upon CID loss of sulfo-NHS is a signature of covalent reaction whereas detachment of the intact reagent is a signature that no covalent reaction occurred. In the case of the whole protein, as in Figure 1(c), the facile loss of two sulfo-NHS groups is the signature for crosslinking.[69] Essentially no [M+7H]7+ is observed in the product ion spectrum of Figure 5(a). The [M+8H]8+ precursor, therefore, yielded evidence for a mixture of behaviors that included crosslinking, covalent attachment at one end of the linker, and electrostatic interaction of both ends of the cross-linker with the protein without covalent reaction. This behavior stands in contrast with the [M+7H]7+ charge state, which both showed crosslinking to be the dominant process. The [M+6H]6+ ubiquitin ion was the lowest charge state examined with the crosslinker and its behavior closely mirrored that of the [M+7H]7+ charge state (data not shown).
Figure 5.
Ion trap CID spectra of the ion/ion reaction complexes generated from the attachment of [sulfo-EGS-H]− to (a) [M+8H]8+, (b) [M+10H]10+, and (c) [M+12H]12+ precursor ions of ubiquitin.
At the higher precursor charge states included in Figure 5, evidence for covalent reaction is largely absent. That is, loss of sulfo-NHS is not observed. Rather, backbone cleavages with many of the products retaining the intact sulfo-EGS reagent make major contributions to the spectra (see Figures 5(b) and 5(c)). The [M+12H]12+ precursor is almost “fully charged” and may have no unprotonated reactive sites whereas the [M+10H]10+ ion might be expected to be more reactive, although solvation of charge by multiple basic sites might affect the reactivity. In any case, the higher charge states show little evidence for covalent modification but attachment of the reagent and its retention under CID conditions might be regarded as a form of non-covalent or electrostatic crosslinking (e.g. y58+sulfo-EGS), illustrated by Scheme 2(d). The possible connection between the product ion spectra and the proximities of particular side-chains, however, is less clear with electrostatic crosslinking relative to covalent crosslinking. For example, the electrostatic binding takes place at protonation sites whereas covalent binding occurs at neutral primary amines. The selectivity of the crosslinking, therefore, might be expected to differ between the two cases. Furthermore, under collisional activation conditions scrambling of the attachment sites is expected to be much more likely with the electrostatic interaction. A different kind of structural probe that has a minimal impact on non-covalent interactions, such as electron capture dissociation or electron transfer dissociation, may be better suited to characterization of the protein structure with the electrostatically bound reagent.
CONCLUSIONS
Intramolecular crosslinking of intact protein cations in the gas-phase has been demonstrated using a homobifunctional crosslinking reagent anion with subsequent top-down tandem mass spectrometry. The reaction of a multiply protonated protein with monoanions of a sulfo-NHS crosslinking reagent results in the formation of a long-lived complex that, upon subsequent CID steps, can form covalent crosslinks between reactive sites (i.e., unprotonated primary amines and quanidine groups) located within distance constraint of the linker. For the charge states examined here, no clear evidence for reaction at arginine residues was apparent. A covalent crosslink is indicated when the complex loses two sulfo-NHS groups. Subsequent CID can be used to localize crosslinks. The extent to which particular crosslinks can be ruled in or out depends on the information content of the product ions that can be produced. For the ubiquitin charge states of [M+7H]7+ and [M+6H]6+, 18 of 28 possible crosslinking combinations could be ruled out and strong evidence was noted for crosslinks between K27 and K29 and between K48 and K63 on the basis of ion trap CID. These results indicate that gas-phase intramolecular covalent crosslinking of protein ions is not a random process and that there is specificity in the sites that are crosslinked. This chemistry may therefore provide information that complements the information provided by other approaches, such as ion mobility, H/D exchange, etc. Backbone dissociation following the loss of only one sulfo-NHS indicated the presence of “hanging” crosslinkers, which arises when only one side of the crosslinker undergoes covalent reaction. This situation was noted to be most prevalent for the [M+8H]8+ precursor ion. At higher charge states, minimal evidence for any covalent reaction was noted, as reflected by minimal loss of sulfo-NHS. This reflects a lesser number of reactive sites as well as a lower likelihood that two reactive sites will be within the distance constraint of the linker. However, backbone cleavage was found to be favored over detachment of the neutralized reagent upon ion trap CID, which suggests a form of non-covalent crosslinking based on the strong electrostatic interactions of the sulfonate groups of the reagent with the protein. Further studies regarding the nature of these non-covalently crosslinked ions are warranted to determine if they can provide useful structural information.
Supplementary Material
ACKNOWLEDGMENTS
This research was sponsored by the National Institutes of Health under Grant GM 45372.
References
- 1.Whitehouse CM, Dreyer RN, Yamashita M, Fenn JB. Electrospray interface for liquid chromatographs and mass spectrometers. Anal. Chem. 1985;57:675–679. doi: 10.1021/ac00280a023. [DOI] [PubMed] [Google Scholar]
- 2.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 3.Karas M, Bachmann D, Bahr U, Hillenkamp F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Processes. 1987;78:53–68. [Google Scholar]
- 4.Karas M, Hillenkamp FH. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988;60:2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- 5.McLuckey SA. Principles of collisional activation in analytical mass spectrometry. J. Am. Soc. Mass Spectrom. 1992;3:599–614. doi: 10.1016/1044-0305(92)85001-Z. [DOI] [PubMed] [Google Scholar]
- 6.Jennings KR. The changing impact of the collision-induced decomposition of ions on mass spectrometry. Int. J. Mass Spectrom. 2000;200:479–493. [Google Scholar]
- 7.Shukla AK, Futrell JH. Tandem mass spectrometry: dissociation of ions by collisional activation. J. Mass Spectrom. 2000;35:1069–1090. doi: 10.1002/1096-9888(200009)35:9<1069::AID-JMS54>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Cooks RG, Ast T, Mabud Md. A. Collisions of polyatomic ions with surfaces. Int. J. Mass Spectrom. Ion Processes. 1990;100:209–265. [Google Scholar]
- 9.Chorush RA, Little DP, Beu SC, Wood TD, McLafferty FW. Surface-induced dissociation of multiply-protonated proteins. Anal. Chem. 1995;67:1042–1046. doi: 10.1021/ac00102a004. [DOI] [PubMed] [Google Scholar]
- 10.Dongré AR, Somogyi Á, Wysocki VH. Surface-induced dissociation: An effective tool to probe structure, energetics and fragmentation mechanisms of protonated peptides. J. Mass Spectrom. 1996;31:339–350. doi: 10.1002/(SICI)1096-9888(199604)31:4<339::AID-JMS322>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Laskin J, Denisov EV, Shukla AK, Barlow SE, Futrell JH. Surface-induced dissociation in a Fourier transform ion cyclotron resonance mass spectrometer: Instrument design and evaluation. Anal. Chem. 2002;74:3255–3261. doi: 10.1021/ac025514q. [DOI] [PubMed] [Google Scholar]
- 12.Sleno L, Volmer DA. Ion activation methods for tandem mass spectrometry. J. Mass Spectrom. 2004;39:1091–1112. doi: 10.1002/jms.703. [DOI] [PubMed] [Google Scholar]
- 13.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]
- 14.Zubarev RA, Kruger NA, Fridricksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW. Electron capture dissociation of gaseous multiply-charged proteins is favored at disulfide bonds and other sites of high hydrogen atom affinity. J. Am. Chem. Soc. 1999;121:2857–2862. [Google Scholar]
- 15.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron Capture Dissociation for Structural Characterization of Multiply Charged Protein Cations. Anal. Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 16.Zubarev RA. Reactions of polypeptide ions with electrons in the gas phase. Mass Spectrom. Rev. 2003;22:57–77. doi: 10.1002/mas.10042. [DOI] [PubMed] [Google Scholar]
- 17.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Hunt DF. Anion dependence in the partitioning between proton and electron transfer in ion/ion reactions. Int. J. Mass Spectrom. 2004;236:33–42. [Google Scholar]
- 19.Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Electron Transfer Ion/Ion Reactions in a Three-Dimensional Quadrupole Ion Trap: Reactions of Doubly and Triply Protonated Peptides with SO2*- Anal. Chem. 2005;77:1831–1839. doi: 10.1021/ac0483872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunawardena HP, He M, Chrisman PA, Pitteri SJ, Hogan JM, Hodges BD, McLuckey SA. Electron transfer versus proton transfer in gas-phase ion-ion reactions of polyprotonated peptides. J. Am. Chem. Soc. 2005;127:12627–12639. doi: 10.1021/ja0526057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Hart WJ. Studies of ion structures by photodissociation. Int. J. Mass Spectrom. 1992;118/119:617–663. [Google Scholar]
- 22.Uechi GT, Dunbar RC. The kinetics of infrared laser photodissociation of n-butylbenzene ions at low pressure. J. Chem. Phys. 1992;96:8897–8905. [Google Scholar]
- 23.Little DP, Speir JP, Senko MW, O’Connor PB, McLafferty FW. Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal. Chem. 1994;66:2809–2815. doi: 10.1021/ac00090a004. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Hendrickson CL, Emmett MR, Marshall AG. Identification of intact proteins in mixtures by alternated capillary liquid chromatography electrospray ionization and LC ESI infrared multiphoton dissociation Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 1999;71:4397–4402. doi: 10.1021/ac990011e. [DOI] [PubMed] [Google Scholar]
- 25.Crowe MC, Brodbelt JS. Differentiation of phosphorylated and unphosphorylated peptides by high performance liquid chromatography/electrospray ionization/infrared multiphoton dissociation in a quadrupole ion trap. Anal. Chem. 2005;77:5726–5734. doi: 10.1021/ac0509410. [DOI] [PubMed] [Google Scholar]
- 26.Oh JY, Moon JH, Lee YH, Hyung S-W, Lee S-W, Kim MS. Photodissociation tandem mass spectrometry at 266 nm of an aliphatic peptide derivatized with phenyl isothiocyanate and 4-sulfophenyl isothiocyanate. Rapid Commun. Mass Spectrom. 2005;19:1283–1288. doi: 10.1002/rcm.1922. [DOI] [PubMed] [Google Scholar]
- 27.Thompson MS, Cui W, Reilly JP. Factors that impact the vacuum ultraviolet photofragmentation of peptide ions. J. Am. Soc. Mass Spectrom. 2007;18:1439–1452. doi: 10.1016/j.jasms.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JJ, Brodbelt JS. MS/MS simplification by 355 nm ultraviolet photodissociation of chromophore-derivatized peptides in a quadrupole ion trap. Anal. Chem. 2007;79:7883–7892. doi: 10.1021/ac071241t. [DOI] [PubMed] [Google Scholar]
- 29.Reilly JP. Ultraviolet photofragmentation of biomolecular ions. Mass Spectrom. Rev. 2009;28:425–447. doi: 10.1002/mas.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnier PD, Price WD, Jockusch RA, Williams ER. Blackbody infrared radiative dissociation of bradykinin and its analogues: energetics, dynamics, and evidence for salt-bridge structures in the gas phase. J. Am. Chem. Soc. 1996;118:7178–7189. doi: 10.1021/ja9609157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunbar RC. BIRD (blackbody infrared radiative dissociation): Evolution, principles, and applications. Mass Spectrom.Rev. 2004;23:127–58. doi: 10.1002/mas.10074. [DOI] [PubMed] [Google Scholar]
- 32.Kaltashov IA, Eyles SJ. Mass Spectrometry in Biophysics. Wiley-Interscience; Hoboken, NJ: 2005. ISBN 0-471-45602-0. [Google Scholar]
- 33.Stockman BJ, Euvrard A, Scahill TA. Heteronuclear three-dimensional NMR spectroscopy of a partially denatured protein: The A-state of human ubiquitin. J. Biomol. NMR. 1993;3:285–296. doi: 10.1007/BF00212515. [DOI] [PubMed] [Google Scholar]
- 34.Brutscher B, Brüschweiler R, Ernst RR. Backbone dynamics and structural characterization of the partially folded A state of ubiquitin by 1H, 13C, and 15N nuclear magnetic resonance spectroscopy. Biochem. 1997;36:13043–13053. doi: 10.1021/bi971538t. [DOI] [PubMed] [Google Scholar]
- 35.Koeniger SL, Merenbloom SI, Sevugarajan S, Clemmer DE. Transfer of structural elements from compact to extended states in unsolvated ubiquitin. J. Am. Chem. Soc. 2006;128:11713–11719. doi: 10.1021/ja062137g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyttenbach T, Bowers MT. Structural stability from solution to the gas phase: native solution structure of ubiquitin survives analysis in a solvent-free ion mobility-mass spectrometry environment. J. Phys. Chem. B. 2011;115:12266–12275. doi: 10.1021/jp206867a. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, Pierson NA, Valentine SJ, Clemmer DE. Conformation types of ubiquitin [M+8H]8+ ions from water:methanol solutions: evidence for the N and A states in aqueous solution. J. Phys. Chem. B. 2012;116:3344–3352. doi: 10.1021/jp210797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myung S, Badman ER, Lee YJ, Clemmer DE. Structural transitions of electrosprayed ubiquitin ions stored in an ion trap over 10 ms to 30 s. J. Phys. Chem. A. 2002;106:9976–9982. [Google Scholar]
- 39.Li J, Taraszka JA, Counterman AE, Clemmer DE. Influence of solvent composition and capillary temperature on the conformations of electrosprayed ions: unfolding of compact ubiquitin conformers from pseudonative and denatured solutions. Int. J. Mass Spectrom. 1999;185/186/187:37–47. [Google Scholar]
- 40.Valentine SJ, Counterman AE, Clemmer DE. Conformer-dependent proton-transfer reactions of ubiquitin ions. J. Am. Soc. Mass Spectrom. 1997;8:954–961. [Google Scholar]
- 41.Segev E, Wyttenbach T, Bowers MT, Gerber RB. Conformational evolution of ubiquitin ions in electrospray mass spectrometry: molecular dynamics simulations at gradually increasing temperatures. Phys. Chem. Chem. Phys. 2008;10:3077–3082. doi: 10.1039/b718610j. [DOI] [PubMed] [Google Scholar]
- 42.Koeniger SL, Clemmer DE. Resolution and structural transitions of elongated states of ubiquitin. J. Am. Soc. Mass Spectrom. 2007;18:322–331. doi: 10.1016/j.jasms.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Badman ER, Hoaglund-Hyzer CS, Clemmer DE. Dissociation of different conformations of ubiquitin ions. J. Am. Soc. Mass Spectrom. 2002;13:719–723. doi: 10.1016/S1044-0305(02)00374-4. [DOI] [PubMed] [Google Scholar]
- 44.Koeniger SL, Merenbloom SI, Clemmer DE. Evidence for many resolvable structures within conformation types of electrosprayed ubiquitin ions. J. Phys. Chem. B. 2006;110:7017–7021. doi: 10.1021/jp056165h. [DOI] [PubMed] [Google Scholar]
- 45.Shvartsburg AA, Li F, Tang K, Smith RD. Characterizing the structures and folding of free proteins using 2-D gas-phase separations: Observation of multiple unfolded conformers. Anal. Chem. 2006;78:3304–3315. doi: 10.1021/ac060283z. [DOI] [PubMed] [Google Scholar]
- 46.Freitas MA, Hendrickson CL, Emmet MR, Marshall AG. Gas-phase bovine ubiquitin cation conformations resolved by gas-phase hydrogen/deuterium exchange rate and extent. Int. J. Mass Spectrom. 1999;185/186/187:565–575. [Google Scholar]
- 47.Evans SE, Lueck N, Marzluff EM. Gas phase hydrogen/deuterium exchange of proteins in an ion trap mass spectrometer. Int. J. Mass Spectrom. 2003;222:175–187. [Google Scholar]
- 48.Hoerner JK, Xiao H, Kaltashov IA. Structural and dynamic characteristics of a partially folded state of ubiquitin revealed by hydrogen exchange mass spectrometry. Biochem. 2005;44:11286–11294. doi: 10.1021/bi0509548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 50.Breuker K, Oh H, Horn DM, Cerda BA, McLafferty FW. Detailed unfolding and folding of gaseous ubiquitin ions characterized by electron capture dissociation. J. Am. Chem. Soc. 2002;124:6407–6420. doi: 10.1021/ja012267j. [DOI] [PubMed] [Google Scholar]
- 51.Breuker K, Oh H, Cerda BA, Horn DM, McLafferty FW. Hydrogen atom loss in electron-capture dissociation: A Fourier transform-ion cyclotron resonance study with single isotopomeric ubiquitin ions. Eur. J. Mass Spectrom. 2002;8:177–180. [Google Scholar]
- 52.Oh H, Breuker K, Sze SK, Ge Y, Carpenter BK, McLafferty FW. Secondary and tertiary structures of gaseous protein ions characterized by electron capture dissociation mass spectrometry and photofragment spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15863–15868. doi: 10.1073/pnas.212643599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skinner OS, McLafferty FW, Breuker K. How ubiquitin unfolds after transfer into the gas phase. J. Am. Soc. Mass Spectrom. 2012;23:1011–1014. doi: 10.1007/s13361-012-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ly T, Julian RR. Elucidating the tertiary structure of protein ions in vacuo with site specific photoinitiated radical reactions. J. Am. Chem. Soc. 2010;132:8602–8609. doi: 10.1021/ja910665d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinz A. Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions. Mass Spectrom. Rev. 2006;25:663–682. doi: 10.1002/mas.20082. [DOI] [PubMed] [Google Scholar]
- 56.Chakravarti B, Lewis SJ, Chakravarti DN, Raval A. Three dimensional structures of proteins and protein complexes from chemical cross-linking and mass spectrometry: a biochemical and computational overview. Curr. Proteom. 2006;3:1–21. [Google Scholar]
- 57.Singh P, Panchaud A, Goodlett DR. Chemical cross-linking and mass spectrometry as a low-resolution protein structure determination technique. Anal. Chem. 2010;82:2636–2642. doi: 10.1021/ac1000724. [DOI] [PubMed] [Google Scholar]
- 58.Hermanson GT. Bioconjugate Techniques. 2nd ed. Academic Press; San Diego: 2008. pp. 234–275. [Google Scholar]
- 59.Dihazi GH, Sinz A. Mapping low-resolution three-dimensional protein structures using chemical cross-linking and Fourier transform ion-cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17:2005–2014. doi: 10.1002/rcm.1144. [DOI] [PubMed] [Google Scholar]
- 60.ExPASy Crosslinker Mapping Tools. [accessed May 7, 2012]. http://expasy.org/tools/ [Google Scholar]
- 61.Reid GE, McLuckey SA. “Top down” protein characterization by tandem mass spectrometry. J. Mass Spectrom. 2002;37:663–675. doi: 10.1002/jms.346. [DOI] [PubMed] [Google Scholar]
- 62.Han X, Jin M, Breuker K, McLafferty FW. Extending top-down mass spectrometry to proteins with masses greater than 200 kilodaltons. Science. 2006;314:109–112. doi: 10.1126/science.1128868. [DOI] [PubMed] [Google Scholar]
- 63.Kruppa GH, Schoeniger J, Young MM. A top down approach to protein structural studies using chemical cross-linking and Fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 2003;17:155–162. doi: 10.1002/rcm.885. [DOI] [PubMed] [Google Scholar]
- 64.Novak P, Young MM, Schoeniger JS, Kruppa GH. A top-down approach to protein structure studies using chemical cross-linking and Fourier transform mass spectrometry. Eur. J. Mass. Spectrom. 2003;9:623–631. doi: 10.1255/ejms.590. [DOI] [PubMed] [Google Scholar]
- 65.Ly T, Liu Z, Pujanauski BG, Sarpong R, Julian RR. Surveying ubiquitin structure by noncovalent attachment of distance constrained bis(crown) ethers. Anal. Chem. 2008;80:5059–5064. doi: 10.1021/ac800177s. [DOI] [PubMed] [Google Scholar]
- 66.Novak P, Haskins WE, Ayson MJ, Jacobsen RB, Schoeniger JS, Leavell MD, Young MM, Kruppa GH. Unambiguous assignment of intramolecular chemical cross-links in modified mammalian membrane proteins by Fourier transform-tandem mass spectrometry. Anal Chem. 2005;77:5101–5108. doi: 10.1021/ac040194r. [DOI] [PubMed] [Google Scholar]
- 67.Han H, McLuckey SA. Selective covalent bond formation in polypeptide ions via gas-phase ion/ion reaction chemistry. J. Am. Chem. Soc. 2009;131:12884–12885. doi: 10.1021/ja904812d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassell KM, Stutzman JR, McLuckey SA. Gas-phase bioconjugation of peptides via ion/ion charge inversion: Schiff base formation on the conversion of cations to anions. Anal. Chem. 2010;82:1594–1597. doi: 10.1021/ac902732v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mentinova M, McLuckey SA. Covalent modification of gaseous peptide ions with n-hydroxysuccinimide ester reagent ions. J. Am. Chem. Soc. 2010;132:18248–18257. doi: 10.1021/ja107286p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGee WM, Mentinova M, McLuckey SA. Gas-phase conjugation to arginine residues in polypeptide ions via n-hydroxysuccinimide ester-based reagent ions. J. Am. Chem. Soc. 2012;134:11412–11414. doi: 10.1021/ja304778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mentinova M, McLuckey SA. Intra- and inter-molecular cross-linking of peptide ions in the gas phase: Reagents and conditions. J. Am. Soc. Mass Spectrom. 2011;22:912–921. doi: 10.1007/s13361-011-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xia Y, Chrisman PA, Erickson DE, Liu J, Liang X, Londry FA, Yang MJ, McLuckey SA. Implementation of ion/ion reactions in a quadrupole/time-of-flight tandem mass spectrometer. Anal. Chem. 2006;78:4146–4154. doi: 10.1021/ac0606296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang X, Xia Y, McLuckey SA. Alternately pulsed nano-electrospray ionization/atmospheric pressure chemical ionization for ion/ion reactions in an electrodynamic ion trap. Anal.Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia Y, Thomson BA, McLuckey SA. Bi-directional ion transfer between quadrupole arrays: MSn ion/ion reaction experiments on a quadrupole/time-of-flight tandem mass spectrometer. Anal. Chem. 2007;79:8199–8206. doi: 10.1021/ac071448m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.