Abstract
Background
Communication of lung cancer risk information between providers and African-American patients occurs in a context marked by race-based health disparities.
Purpose
A controlled experiment assessed whether perceived physician race influenced African-American patients’ (n=127) risk perception accuracy following the provision of objective lung cancer risk information.
Methods
Participants interacted with a virtual reality-based, simulated physician who provided personalized cancer risk information.
Results
Participants who interacted with a racially discordant virtual doctor were less accurate in their risk perceptions at post-test than those who interacted with a concordant virtual doctor, F(1,94)=4.02, p=.048. This effect was amplified among current smokers. Effects were not mediated by trust in the provider, engagement with the health care system, or attention during the encounter.
Conclusions
The current study demonstrates that African-American patients’ perceptions of a doctor’s race are sufficient to independently impact their processing of lung cancer risk information.
Keywords: lung cancer, risk perception, race concordance, smoking, virtual reality
Disparities in cancer outcomes between African Americans and Whites are well documented. African Americans are more likely than Whites and several other racial and ethnic groups to be diagnosed with and die from lung cancer (1, 2). Explanations for these disparities are posited to be multi-faceted and complex (3). They include social and environmental causes like socio-economic status and access to care (4), engagement with risk behaviors (e.g., higher rates of menthol cigarette smoking (5)), and, more recently, a growing recognition that genetic variation underpins components of disease risk (6). Another important source of disparities in health outcomes is that African-American patients may have negative encounters in the health care system related to prejudice and discrimination (7), and experience less effective clinical communication with providers (8).
These factors create the context in which objective lung cancer risk information is communicated by providers, and subjectively understood by patients. Indeed, perception of risk is a process whereby a multitude of factors, both internal and external to an individual, influence beliefs about disease risk and reactions to risk information. In the Model of Risk Information Seeking and Processing (RISP), Griffin and colleagues (9) propose three primary factors that influence the processing of risk information: information sufficiency, relevant channel beliefs and perceived information gathering capacity. The first two factors in particular are likely to be influential when patients process objective risk information given by a clinician. Likewise, these factors can affect the accuracy of the risk perceptions that result.
In relation to information sufficiency, communication of medical information is posited to be less effective when patients are of a racial or ethnic minority. This is particularly true in interactions with racially discordant providers (8). For example, providers in racially discordant interactions provide less information (10), answer fewer patient questions (11), and are more dominant and less patient-centered (12). These suboptimal communication processes develop over the course of an interaction between a patient and provider and can lead to poorer transmission of information. Information sufficiency can also be affected by patient-provider racial discordance in that individuals can preferentially attend to and better remember messages provided by a member of their own social group (13). Therefore, patients may be better informed by medical information when it is delivered by a racially-concordant physician.
Patients’ perceptions of and beliefs about racially discordant health care providers (i.e., channel beliefs) may also lead to less successful transmission of risk information. African-American patients often report lower levels of trust in racially discordant physicians (14, 15). In addition, interacting with a physician from the dominant racial group (i.e., White) may activate patients’ beliefs about race-based mistreatment in the health care system (16). Through these mechanisms, African-American patients may be more likely to discount and less likely to act upon health information and recommendations provided by a White physician (17, 18). Therefore, an African-American patient who receives lung cancer risk information from a racially discordant (i.e., White) provider may be less accepting of that information, and may leave the clinical encounter with a less accurate perception of personal risk. Because African-American patients frequently see racially discordant providers (19), the lung cancer risk-related discussions that occur may result in less accurate risk perceptions on a population-wide scale.
The RISP model also posits that characteristics of the individual affect risk processing through several pathways. In the context of lung cancer, patients’ smoking behavior directly exerts great influence on lung cancer risk perceptions (20). Consistent with the model, it is also likely that smoking behavior influences patient beliefs and attitudes toward health care providers and the risk information they supply. For example, being a smoker could lead patients to engage in motivated or self-protective cognition (21). Patients who smoke already feel threatened with respect to lung cancer. They may therefore be more sensitive to cues indicating that risk information might be less trustworthy and can be discounted (22). Smokers may therefore be even more likely than non-smokers to distrust risk information presented by a racially discordant provider.
The current study builds on the RISP to assess processes influencing lung cancer risk perceptions among African-American individuals. Our primary hypotheses were: 1) Participants will have more accurate lung cancer risk perception following an encounter with a racially concordant as opposed to discordant doctor. 2) Smokers will have less accurate risk perception than non-smokers and risk perception will be more inaccurate after receiving information from a racially discordant doctor than non-smokers. 3) The influence of provider race and smoking status on risk perception accuracy will be partially mediated (explained) by trust in the provider, engagement with the health care system, and attention during the risk information transmission session.
We assessed these processes by having participants interact with a virtual reality-based doctor who provided personalized cancer risk information. This allowed us to test whether perceived provider race, independently of any related factor, influenced patient risk perception accuracy. With this study, we aimed to identify a potential intervention point through which to improve the outcome of cancer risk communication between African-American patients and physicians..
Method
Participants
Participants were 127 African-American adults between the ages of 25 and 40, recruited from the Washington, DC area. Participants self-referred to the study which was advertised primarily through flyers and internet postings. Data were collected in 2009–2010. To be eligible for the study, individuals were required to self-identify as African American or Black, have been born in the United States, not have had a diagnosis of the six cancers related to the parent study (lung, stomach, kidney, pancreatic, and colon cancer, and melanoma), and have access to the internet. In addition, due to the use of immersive virtual reality, exclusion criteria included having a seizure or vestibular disorder, being highly prone to motion sickness, and having poor, uncorrected hearing or vision. Participants were compensated for their participation. This study was approved by the appropriate Institutional Review Board.
Design
Data for this analysis were collected as part of a larger project on race concordance and information processing. The study was a controlled experiment in which all participants were randomly assigned to receive cancer risk information from; 1) a racially concordant virtual doctor (i.e., appeared to be Black/African American), or 2) a racially discordant virtual doctor (i.e., appeared to be White/Caucasian).
Procedure
Participants logged in to a website, indicated their initial consent to participate, and completed several items related to their family history, and sociodemographic and lifestyle cancer risk factors. They also completed a measure of numeracy and answered questions related to their beliefs about cancer risk. Once they completed the online questionnaire, participants were scheduled to come into the lab to complete the study.
Before this appointment, we calculated personalized risk estimates for each participant on each of six cancers involved in the parent study. We entered participant-provided information into the Your Disease Risk website (23), which is based on the Harvard Cancer Risk Index (24). This tool provides a qualitative, categorical level of risk (e.g., above average risk). We then converted the qualitative risk information to numerical risk figures using published rates of disease probability by categorical risk status from the National Cancer Institute’s Surveillance Epidemiology and End Results data (25) matched for race, gender, and age. Finally, we rounded to the nearest whole number to arrive at the personalized risk estimates delivered to participants (e.g., 3%).
When they arrived at the lab, participants were greeted by an African-American research assistant. They were told that they would receive objective risk information from a doctor who had recorded a personalized message for them. Participants were blinded to study aims related to race and racial concordance until debriefing.
Participants wore a head-mounted display to interface with the virtual environment. Their head and body movements were tracked using an inertial and optical tracking system to render the appropriate scene in real-time, in stereo, and in three dimensions. The virtual encounter was modeled after a health education scenario. Use of this technology allowed us to manipulate the apparent race of the physician while standardizing every other aspects of his body, voice, and behavior (26, 27). We were also able to situate the encounter in a realistic clinical context as opposed to, for example, using a written vignette (28). Previous research suggests that experiences in virtual environments are psychologically compelling and that findings in the virtual clinical environment are applicable to real social and clinical situations (29–34).
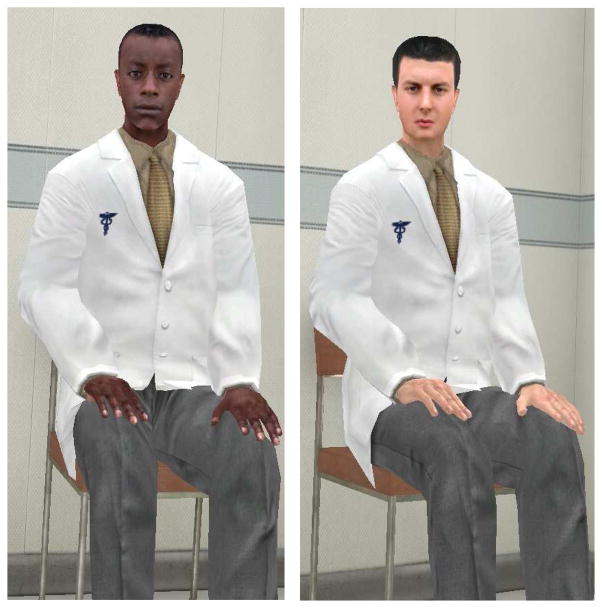
In the encounter, participants sat across the virtual examination room from a male virtual doctor who was either racially concordant or discordant depending upon assigned condition (Figure 1). The virtual doctor gave participants information about each of the six cancers and provided the previously calculated personalized risk estimates. Risk information was provided in terms of a percentage (i.e., “your risk of developing lung cancer in your lifetime is X%”). Although the risk numbers were personalized, all other elements of the encounter were standardized. Participants were able to have portions of the encounter repeated if they desired. During the interaction, the system recorded participants’ gaze direction. At the conclusion of the virtual encounter, participants filled out a post-test questionnaire. Finally, participants were debriefed, informed as to the source of the cancer risk estimates, and given the opportunity to choose cancer information pamphlets to take with them.
Figure 1.
Racially concordant and racially discordant versions of the virtual reality physician
Measures
Pre-Test Assessment
One question assessed participants’ subjective numeric risk perception for lung cancer at pre-test: “on a scale of 0–100%, what do you believe is your chance of getting lung cancer sometime in your lifetime?” Family history, lifestyle and sociodemographic risk factors were assessed using items from the Your Disease Risk tool (23). These items assessed gender, age, weight, smoking status, dietary habits, physical activity, exposure to asbestos and other carcinogens, diagnosis with several chronic diseases (e.g., hypertension), and other factors used for risk calculation. We also assessed educational attainment, and measured participants’ numeracy using an 11-item tool developed by Lipkus and colleagues (35).
Dependent Variable
We assessed participants’ subjective numeric risk perception for lung cancer at post-test using the same question as at pre-test. The absolute value of the difference between participants’ post-test risk perception and the objective number provided by the virtual doctor was calculated to indicate risk perception accuracy.
Manipulation Check
We asked participants to indicate (open-ended) what they believed the race of the virtual doctor to be at post-test. Participants in the racially concordant condition who indicated they believed the doctor was Black, African American, or African, and participants in the racially discordant condition who indicated that they believed the doctor was White, Caucasian, European-American, or European were coded as having ‘passed’ the manipulation check.
Potential Mediators
Trust in the doctor was assessed at post-test using a three-item scale adapted from West and colleagues (36). This measure assessed, on a scale from 1 (not at all) to 9 (very much) the extent to which the doctor was perceived to be trustworthy, dependable, and could be counted on.
Also at post-test, we assessed participants’ personal and race-based discounting of information from the healthcare system (Eccleston, unpublished) using four items adapted from Major and Schmader (37). These items measure whether participants feel that the medical establishment fairly assesses their health and the health of individuals from their racial/ethnic group (e.g., “the medical establishment’s definition of healthy is biased against people from my ethnic or racial group.”) Responses were collected on a scale from 1 (strongly disagree) to 7 (strongly agree).
To assess participants’ attention in the virtual clinic, we unobtrusively measured the extent to which they looked at the virtual doctor and a screen displaying visual aids, versus other elements of the environment. These data were examined to determine the extent to which each element was central in the participant’s view during the portion of the encounter when lung cancer risk was discussed.
Statistical Analysis
Analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago IL). Significance was assessed at p<.05 for all analyses. The comparisons between objective risk information and risk perceptions were performed using a two-tailed paired-samples t-test. Analysis of main effects and interactions were performed using 2-way ANCOVAs where independent variables were racial concordance between the participant and the doctor and participants’ smoking status (current smoker, former smoker, or never smoker). Each ANCOVA contained the following covariates: gender, education, numeracy, whether participants reported having a chronic disease, and whether participants reported exposure to non-tobacco carcinogens related to lung cancer (e.g., asbestos). Covariates were included because they differed between smoking status categories, or had conceptual importance as potential predictors of risk perception (38–40). Because the outcomes on the risk perception accuracy variable were skewed, we used a log transformed version of the variable in analyses. Log transformation normalized the variable, skewness 1.38, kurtosis 1.78. Mediation analyses were performed using Baron and Kenny’s procedure (41).
Results
Ten participants were excluded from analysis due to missing data on post-test risk perception or numeracy measures. An additional 12 were dropped from analysis due to a disqualifying response on the manipulation check.
Demographics
Participant demographics are reported in Table 1. None of the demographic factors varied significantly by condition. As anticipated, chi square analyses revealed that smoking status differed by participant gender and education levels: χ2(2, N=105) = 7.47, p=.024 and χ2(2, N=105) = 7.47, p=.024, respectively. No other demographic factor varied by group.
Table 1.
Demographic factors. Bolded values indicate significant differences at p<.05.
| Total (N=105) | Concordant doctor condition (n=54) | Discordant doctor Condition (n=51) | Current smokers (n=18) | Former smokers (n=15) | Never smokers (n=72) | |
|---|---|---|---|---|---|---|
| Age mean (SD) | 31.6 (4.5) | 31.6 (4.5) | 31.5 (4.5) | 32.1 (4.1) | 29.7 (4.3) | 31.8 (4.6) |
| BMI kg/m2 mean (SD) | 28.3 (6.2) | 27.8 (5.3) | 28.8 (7.0) | 27.4 (5.7) | 29.4 (7.0) | 28.3 (6.2) |
| Numeracy mean (SD) | 6.2 (2.5) | 6.0 (2.6) | 6.5 (2.4) | 6.4 (2.5) | 5.9 (2.8) | 6.3 (2.5) |
| Female gender | 51 (48.6%) | 27 (50.0%) | 24 (47.1%) | 4 (22.2%) | 6 (40%) | 41 (56.9%) |
| Holds college degree | 51 (48.6%) | 27 (50.0%) | 24 (47.1%) | 4 (22.2%) | 6 (40%) | 41 (56.9%) |
| Chronic disease | 7 (6.7%) | 5 (9.3%) | 2 (3.9%) | 2 (11.1%) | 0 (0%) | 5 (6.9%) |
| Other carcinogen exposure | 3 (2.9%) | 1 (1.9%) | 2 (3.9%) | 2 (11.1%) | 0 (0%) | 1 (1.4%) |
| Family history of lung cancer | 23 (21.9%) | 8 (14.8%) | 15 (29.4%) | 4 (22.2%) | 4 (26.7%) | 15 (20.8%) |
| Current smoker | 18 (17.1%) | 10 (18.5%) | 9 (17.6%) | - | - | - |
| Former smoker | 15 (14.3%) | 8 (14.8%) | 7 (13.7%) | - | - | - |
Pre-Test Risk Perception, Objective Risk Information Provided, and Post-Test Risk Perception
At pre-test, participants reported an average perceived risk for lung cancer of 20.5 % (SD=18.54); see Table 2 for all means. Pre-test perception did not differ by provider race condition, but there was a significant difference by smoking status, F(1,64)=12.61, p<.0001. Thirty participants who are otherwise included in analyses did not respond to this item.
Table 2.
Raw means and standard deviations by condition and smoking status
| Concordant Doctor | Discordant Doctor | Total | |
|---|---|---|---|
| Perceived Risk at Pre-Test (% from 0 to 100) | |||
| Current Smoker | 37.0 (21.8) | 36.6 (14.7) | 36.8 (18.2) |
| Former Smoker | 17.0 (19.6) | 34.8 (16.6) | 25.9 (19.5) |
| Never Smoker | 13.2 (15.0) | 14.1 (13.7) | 13.6 (14.3) |
| Total | 19.2 (19.6) | 22.0 (17.5) | 20.5 (18.5) |
| Provided, Objective Risk Information (% from 0 to 100) | |||
| Current Smoker | 7.4 (5.9) | 8.3 (6.5) | 7.8 (6.0) |
| Former Smoker | 3.4 (1.2) | 3.7 (1.1) | 3.5 (1.1) |
| Never Smoker | 2.7 (0.8) | 3.3 (3.5) | 3.0 (2.5) |
| Total | 3.7 (3.1) | 4.1 (4.2) | 3.9 (3.7) |
| Perceived Risk at Post-Test (% from 0 to 100) | |||
| Current Smoker | 11.8 (14.9) | 22.5 (8.3) | 16.6 (19.1) |
| Former Smoker | 2.1 (2.4) | 10.4 (17.5) | 6.0 (12.3) |
| Never Smoker | 2.9 (4.9) | 7.3 (13.6) | 5.1 (10.4) |
| Total | 4.4 (8.2) | 10.1 (16.5) | 7.2 (13.1) |
| Risk Perception Accuracya | |||
| Current Smoker | 5.8 (13.9) | 15.0 (22.7) | 9.9 (18.3) |
| Former Smoker | 2.5 (1.6) | 7.0 (17.2) | 4.6 (11.6) |
| Never Smoker | 1.9 (4.6) | 5.6 (12.7) | 3.8 (9.6) |
| Total | 2.7 (7.0) | 7.2 (15.2) | 4.9 (11.9) |
| Trust in the Doctor (1–9 scale) | |||
| Current Smoker | 7.1 (1.1) | 6.5 (1.9) | 6.8 (1.5) |
| Former Smoker | 7.7 (1.3) | 6.5 (2.0) | 7.1 (1.7) |
| Never Smoker | 6.9 (1.8) | 7.3 (1.6) | 7.1 (1.7) |
| Total | 7.0 (1.6) | 7.1 (1.7) | 7.1 (1.7) |
| Discounting of Information from the Health Care System (1–7 scale) | |||
| Current Smoker | 3.0 (1.4) | 3.8 (1.0) | 3.3 (1.3) |
| Former Smoker | 3.1 (1.3) | 4.1 (0.8) | 3.5 (1.1) |
| Never Smoker | 3.4 (1.0) | 3.2 (0.9) | 3.3 (0.9) |
| Total | 3.3 (1.1) | 3.4 (0.9) | 3.3 (1.0) |
| Visual Attention in the Virtual Encounter - % of time doctor or screen was in view | |||
| Current Smoker | 99.4 (1.9) | 100 (0) | 99.7 (1.4) |
| Former Smoker | 100 (0) | 99.7 (0.7) | 99.9 (0.5) |
| Never Smoker | 98.9 (6.0) | 99.2 (4.2) | 99.1 (5.2) |
| Total | 99.1 (5.0) | 99.4 (3.5) | 99.3 (4.3) |
accuracy defined as the absolute value of the difference between participants’ post-test risk perception and the objective number provided by the virtual doctor
The objective lung cancer risk value provided by the virtual doctor averaged 3.9% (SD=3.7) across all conditions. Values did not differ by provider race condition, however, smokers were given information indicating that their objective risk was higher, F(1,99)=15.51, p<.0001.
At post-test, the average level of lung cancer risk perception reported by participants was 7.2% (SD=13.13). Participants’ perceptions of their risk at post-test were significantly lower than their risk perceptions at pre-test [t(74)=9.59, p<.0001], and significantly higher than the objective values provided by the virtual doctor, t(104)=2.74, p=.007.
Risk Perception Accuracy
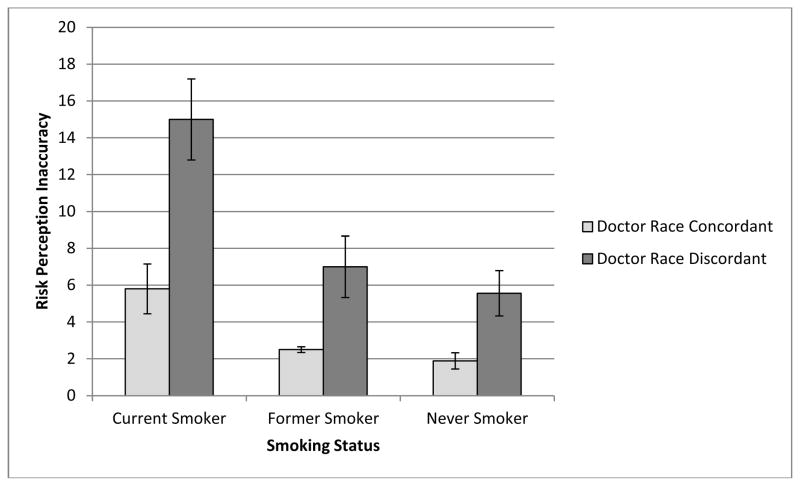
Participants who interacted with the racially discordant doctor were less accurate in their risk perceptions than those who interacted with a racially concordant virtual doctor, F(1,94)=4.02, p=.048 (Table 2). The main effect of participant smoking status on risk perception accuracy was not significant. There was a significant interaction between condition and smoking status, however, F(1,94)=3.58, p=.032. Among current smokers, there was a greater discrepancy in risk perception accuracy between those who saw the racially discordant doctor compared with the concordant doctor (Figure 2).
Figure 2.
Risk perception inaccuracy by condition and smoking status, raw means. Bars represent standard error.
Trust in the Physician
Participants reported trusting the doctor equally whether the doctor was racially concordant or discordant (Table 2). Participants also reported trusting the doctor equally whether they were current smokers, former smokers, or never smokers, and the interaction between condition and smoking status was not significant.
Discounting of Information from the Health Care System
Participants’ discounting of information from the health care system following receipt of their risk information differed significantly by condition. Participants who interacted with the racially discordant doctor reported more discounting than participants who interacted with the racially concordant doctor F(1,94)=4.39, p=.039 (see Table 2). In addition, there was a main effect of smoking status in which former smokers were most likely to discount, F(1,94)=3.21, p=.045. The interaction did not reach significance F(1,94)=2.76, p=.068.
Attention
Participants looked at the virtual doctor and the screen displaying visual aids almost exclusively during the encounter (see Table 2). There were no significant differences by condition or smoking status, and no significant interaction.
Mediation Analyses
There was no significant relationship between provider race condition and trust in the doctor or attention to the encounter. Therefore, there was no evidence of mediation by either variable. Discounting of information from the health care system also did not mediate the relationship between condition and accuracy. Apparent race of the doctor was significantly related to both discounting of information from the health care system and to risk perception accuracy. However, the relationship between discounting and risk perception accuracy was non-significant in the model testing mediation.
Discussion
This study demonstrated that changing a doctor’s apparent race influenced African-American patients’ lung cancer risk perceptions in response to personalized risk information provided by that doctor. Participants’ estimations of their lung cancer risk were closer to the objective figure when the doctor appeared to be Black rather than White. Much of the literature addressing health disparities that stem from clinical encounters centers on the important issue of ineffectiveness in communication processes (8, 42). Importantly, in the current study, we demonstrated that patient perceptions of a physician’s race can lead to less accurate information transmission, independently of any dyadic communication or interaction processes that occur over the course of a clinical visit. Because we simulated the clinical encounter, we were able to vary the physician’s apparent race while holding all other aspects of the interaction constant.
The transmission of objective lung cancer risk created a situation in which participants received information that may have challenged any preexisting assumptions about risk. Indeed, many participants greatly overestimated their lung cancer risk prior to provision of objective risk information. They also continued to overestimate that risk following the encounter. Given extensive public health coverage related to lung cancer, participants may have an inflated perception of the magnitude of “average” and “above-average” risk. It can also be difficult for individuals to accept lower-than-expected risk estimates (43, 44). Indeed, risk perceptions following objective feedback often fall somewhere between preexisting beliefs and objective values (45). Situations where patients face potentially unexpected information about their risk and are required to adjust perceptions, represent a crucial context in which factors like patient-provider racial discordance may influence patient outcomes.
In the current study, we found that the discrepancy in risk perception accuracy between individuals who interacted with a Black versus White doctor was largest among current smokers. Because lung cancer risk information is higher-stakes for current smokers, they may have been more attuned to cues (i.e., provider race) indicating that risk information could be discounted or regarded as less informative (22). Former smokers tended to respond more like non-smokers. This is supported by findings that former smokers often perceive a dramatic reduction in their cancer risk upon quitting smoking (46).
Some literature suggests that smokers under-estimate lung cancer risk (47–49). In line with the current study, other work has shown that individuals tend to overestimate cancer risk from smoking when making numerical estimates (50, 51). Heightened risk perceptions have been considered to be beneficial in their association with decreased smoking behavior. However, having an accurate perception of risk allows individuals to make informed decisions about their health and avoid undue worry. Individuals with an accurate perception of risk may also be less likely to become fatalistic about cancer. Cancer fatalism has been linked to decreases in prevention behavior (52).
Differences in risk perception accuracy predicted by perceived provider race were not mediated by differential levels of trust in the doctor or the health care system. In fact, participants reported trusting the virtual provider equally across conditions. This was a surprising outcome as previous literature reports higher levels of trust in racially similar providers (53, 54). Trust levels in this study were relatively high overall (above 7 on a 9-point scale). It is therefore unlikely that the lack of differential trust between groups is an artifact of unwillingness to endorse trust in a simulated doctor. It may be that other types of trust, such as trust in risk information itself, come into play here. Importantly, trust in health care providers among African-American patients is a multi-faceted and complex construct (14). We may not have adequately measured the most relevant aspects of trust for this type of encounter. In addition, most measures of trust are based on the assumption of an ongoing relationship between patient and provider. There may be different factors influencing trust in a provider with whom a patient is unacquainted. Finally, this sample consisted of individuals who were willing to participate in research within a medical center setting. Therefore, willingness to trust a health care provider may have been higher in this sample than in the population.
The effect of provider race on risk perception accuracy was also not mediated by differing levels of attention paid to the doctor during the encounter. Participants looked almost exclusively at the doctor and the screen where he presented visual aids. Therefore, differences in attention are unlikely to have negatively affected information sufficiency when participants interacted with the White provider. In addition, this argues against the notion that patients might have paid greater attention to the African-American virtual doctor due to his novelty.
Future research should explore whether other aspects of trust and/or other mediating processes explain the relationship between racial concordance and risk perception accuracy. Activation of thoughts about racial group belonging, as can occur when interacting with a racially similar doctor, have been found to affect risk adjustment (55). Interacting with a racially concordant versus discordant doctor could certainly elicit differential affective responses, impacting risk processing. Alternatively, differential levels of cognitive load due to the complexity inherent in intra- versus interracial interactions could impact risk processing or memory for risk information (56). Although there were no differences in visual attention, it may be that participants were more mentally engaged with the Black doctor and the information he provided. This could lead to greater mental elaboration and more complete processing (57). Future work may benefit from assessing information gathering and sufficiency directly.
The current study had several limitations. First, the risk provision encounter between the participant and the doctor was a simple, simulated interaction. Many aspects of interpersonal communication were absent from these encounters. On one hand, these communication processes likely impact the relationships explored here. On the other hand, by simulating the encounter we were able to examine the effect of apparent race in isolation from confounds and extraneous variables. In addition, use of the virtual reality simulation allowed unobtrusive collection of participants’ visual gaze behavior. Such behavioral measurement is difficult to achieve in real clinical encounters.
An additional limitation is that several study participants did not perceive the race of the virtual doctor as we intended. On the open-ended manipulation check, 12 participants indicated that they were unsure of the virtual doctor’s race, or identified it as something other than Black/African-American or White/Caucasian. By excluding these participants from analysis we may have excluded individuals whose concepts of race are different from the majority. It is possible that these individuals may have different reactions to the apparent race of a provider than those who remained in the sample. However, excluded participants did not differ significantly from the rest of the sample on responses to primary study outcomes. In total, 105 participants were included in analysis. The reduced sample size may have somewhat reduced power to detect effects.
There was also the potential for demand characteristics to influence outcomes of the study. We took several steps to reduce this possibility. The study was described as an assessment of telemedicine. All participants were blinded to study aims related to race or racial concordance until debriefing. In addition, the study was conducted by African-American researchers so as to make participants less hesitant to express their attitudes and beliefs.
Finally, the current study focused on numerical risk estimates. Others have suggested that numerical understanding of risk may not be as important a driver for decision making or behavior as more qualitative or categorical understandings (58, 59). These different representations of risk may operate differently and may or may not be impacted by racial concordance between patient and provider. In addition, lay individuals may have more difficulty using numeric risk estimates (60). This may be the reason for an additional limitation, that many participants in this study chose not to answer questions assessing numeric risk perceptions at pre-test. We focused on numeric risk perception because, at present, risk information is frequently presented numerically in the clinic. Therefore, the processes studied here are directly applicable to current clinical practice.
In sum, the current study demonstrates that African-American patients’ perceptions of a doctor’s race are sufficient to independently impact their processing of lung cancer risk information. Several possible mechanisms may underlie these reactions. Many of these mechanisms are posited to stem from previous experiences in the health care system, and other social systems, in which African-American individuals have learned to expect differential treatment from Black versus White individuals (19, 61).Training providers about implicit racial bias and teaching cultural competence skills may improve experiences of African-American patients over time (62). A resulting reduction in negative experiences could change the automatic associations that patients hold with respect to racially discordant doctors, and lead to improved clinical communication for discordant patient-provider dyads (63). Although most research focuses on negative patient reactions to racially discordant doctors, individuals may also have positive reactions to interacting with racially concordant providers. The current results therefore also support the supposition that training and adding additional African-American health care providers to the workforce would be beneficial. Finally, it will be important to uncover the specific mechanisms through which racial discordance leads to less accurate risk information processing, as they are likely to suggest a natural point of intervention.
Acknowledgments
This research was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. The authors thank Collette Eccleston, Paul Han, and Colleen McBride for their insightful comments on an earlier version of this paper, and Isaac Lipkus and Ellen Peters for their valuable advice. The authors also thank Christina Lachance, Jalia Tucker, Wafa Khradouri, and Sabrina Mathenia for assistance with project preparation and data collection.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Contributor Information
Susan Persky, Social and Behavioral Research Branch, National Human Genome Research Institute, Bethesda, MD.
Kimberly A. Kaphingst, Washington University in St. Louis
Vincent C. Allen, Jr., University of California, Los Angeles
Ibrahim Senay, Zirve University, Gaziantep, Turkey.
References
- 1.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 2.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and Racial Differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 3.Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 4.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24(2):325–34. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 5.Giovino G, Sidney S, Gfroerer J, et al. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6:S67–81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- 6.Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nat Clin Pract Oncol. 2007;4:118–29. doi: 10.1038/ncponc0718. [DOI] [PubMed] [Google Scholar]
- 7.Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: The implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67:478–86. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services: Bias, preferences, or poor communication? JGIM. 2003;18:146–52. doi: 10.1046/j.1525-1497.2003.20532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin R, Dunwoody S, Neuwirth K. Proposed model of the relationship of risk information seeking and processing to the development of preventive behaviors. Environ Res Section A. 1999;80:S230–S45. doi: 10.1006/enrs.1998.3940. [DOI] [PubMed] [Google Scholar]
- 10.Gordon HS, Street RL, Jr, Sharf BF, Souchek J. Racial differences in trust and lung cacner patients’ perceptions of physician communication. J Clin Oncol. 2006;24(6):904–9. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 11.Oliver MN, Goodwin MA, Gotler RS, Gregory PM, Strange KC. Time use in clinical encounters: Are African-American patients treated differently? JAMA. 2001;93:380–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R, Roter D, Powe N, Cooper L. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94:2084–90. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGarty C, Haslam SA, Hutchinson KJ, Turner JC. The effects of salience group memberships on persuasion. Small Gr Res. 1994;25(2):267–93. [Google Scholar]
- 14.Jacobs EA, Rolle I, Ferrans CE, Whitaker EE, Warnecke RB. Understanding African Americans’ views of the trustworthiness of physicians. JGIM. 2006;21:642–7. doi: 10.1111/j.1525-1497.2006.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benkert R, Hollie B, Nordstrom CK, Wickson B, Bins-Emerick L. Trust, mistrust, racial identity, and patient satisfaction in urban African American primary care patients of nurse practitioners. J Nurs Scholarsh. 2009;41(2):211–9. doi: 10.1111/j.1547-5069.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 16.Burgess D, Warren J, Phelan S, Dovidio J, van Ryn M. Stereotype threat and health disparities: What medical educators and future physicians need to know. JGIM. 2010;25(S2):S169–77. doi: 10.1007/s11606-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musa D, Schulz R, Harris R, Silverman M, Thomas SB. Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99(7):1293–9. doi: 10.2105/AJPH.2007.123927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Jacobs EA, Moore RD, Beach MC. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS. 2010;24(7):415–20. doi: 10.1089/apc.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159(9):997–1004. doi: 10.1001/archinte.159.9.997. [DOI] [PubMed] [Google Scholar]
- 20.Park ER, Ostroff JS, Rakowski W, et al. Risk perceptions among participants undergoing lung cancer screening: Baseline results from the National Lung Screening Trial. Ann Behav Med. 2009;37(3):268–79. doi: 10.1007/s12160-009-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croyle RT, Sun Y, Hart M. Processing risk factor information: Defensive biases in health-related judgments and memory. In: Petrie K, JaW JA, editors. Perceptions of Health and Illness: Current Research and Applications. Amsterdam: Harwood Academic; 1997. pp. 267–90. [Google Scholar]
- 22.Liberman A, Chaiken S. Defensive processing of personally relevant health messages. Pers Soc Psychol Bull. 1992;18(6):669–79. [Google Scholar]
- 23. [Accessibility verified November 5, 2012];Your Disease Risk: The Source On Prevention. Available at http://www.yourdiseaserisk.wustl.edu/english/
- 24.Colditz GA, Atwood KA, Emmons K, et al. Harvard Report on Cancer Prevention Volume 4: Harvard Cancer Risk Index. Cancer Causes Control. 2000;11:477–88. doi: 10.1023/a:1008984432272. [DOI] [PubMed] [Google Scholar]
- 25.SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 26.Loomis JM, Blascovich JJ, Beall AC. Immersive virtual environments as a basic research tool in psychology. Behav Res Meth Ins C. 1999;31:557–64. doi: 10.3758/bf03200735. [DOI] [PubMed] [Google Scholar]
- 27.Blascovich J, Loomis J, Beall A, Swinth K, Hoyt C, Bailenson J. Immersive virtual environment technology as a research tool for social psychology. Psychol Inq. 2002;13:103–25. [Google Scholar]
- 28.Persky S, McBride CM. Immersive virtual environment technology: A promising tool for future social and behavioral genomics research and practice. J Health Commun. 2009;24(8 ):677–82. doi: 10.1080/10410230903263982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall C, Blascovich J. How, when, and why to use digital experimental virtual environments to study social behavior. Soc Personal Psychol Compass. 2009;3:1–15. [Google Scholar]
- 30.Schmid Mast M, Hall JA, Roter DL. Caring and dominance affect participants’ perceptions and behaviors during a virtual medical visit. JGIM. 2008;23(5):53–527. doi: 10.1007/s11606-008-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid Mast M, Hall JA, Roter DL. Disentangling physician sex and physician communication style: Their effects on patient satisfaction in a virtual medical visit. Patient Educ Couns. 2007;68:16–22. doi: 10.1016/j.pec.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Persky S, Eccleston CP. Medical student bias and care recommendations for an obese versus non-obese virtual patient. Int J Obes. 2011;35:728–35. doi: 10.1038/ijo.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persky S, Eccleston CP. Impact of genetic causal information on medical students’ encounters with an obese virtual patient: Health promotion and social stigma. Ann Behav Med. 2011;41:363–72. doi: 10.1007/s12160-010-9242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossen B, Johnsen K, Deladisma AM, Lind S, Lok B. Virtual humans elicit skin-tone bias consistent with real-world skin-tone biases. Lect Notes Comput Sci. 2008;5208:237–44. [Google Scholar]
- 35.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 36.West DS, Wilkin NE, Bentley JP, Gilbert F, Garner DD. Understanding how patients form beliefs about Pharmacists’ trustworthiness using a model of belief processing. J Am Pharm Assoc. 2002;42:594–601. doi: 10.1331/108658002763029571. [DOI] [PubMed] [Google Scholar]
- 37.Major B, Schmader T. Coping with stigma through psychological disengagement. In: Swim J, Stangor C, editors. Prejudice: The Target’s Perspective. Hillsdale, NJ: Academic Press; 1998. pp. 219–41. [Google Scholar]
- 38.Maurel M, Stoufflet A, Thorel L, et al. Factors associated with cancer distress in the Asbestos Post-Exposure Survey (APEXS) Am J Ind Med. 2009;52(4):288–96. doi: 10.1002/ajim.20672. [DOI] [PubMed] [Google Scholar]
- 39.Kelly KM, Graves KD, Harper FWK, Schmidt JE, Dickenson SL, Andrykowski MA. Assessing perceptions of cancer risk: Does mode of assessment or numeracy matter? Cancer Detect Prev. 2007;31(6):465–73. doi: 10.1016/j.cdp.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Kulik JA, Mahler HI. Health status, perceptions of risk, and prevention interest for health and nonhealth problems. Health Psychol. 1987;6(1):15–27. doi: 10.1037//0278-6133.6.1.15. [DOI] [PubMed] [Google Scholar]
- 41.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 42.Verlinde E, De Laender N, De Maesschalck S, Deveugele M, Willems S. The social gradient in doctor-patient communication. Int J Equity Health. 2012;11(1):article number 12. doi: 10.1186/1475-9276-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dillard AJ, McCaul KD, Kelso PD, Klein WM. Resisting good news: Reactions ot breast cancer risk communication. J Health Commun. 2006;19(2):115–23. doi: 10.1207/s15327027hc1902_3. [DOI] [PubMed] [Google Scholar]
- 44.Lerman C, Lustbader E, Rimer B, et al. Effects of individualized breast cancer risk counseling: A randomized trial. J Natl Cancer Inst. 1995;87(4):286–92. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 45.Senay I, Kaphingst KA. Anchoring-and-adjustment bias in communication of disease risk. Med Decis Making. 2009;29(2):193–201. doi: 10.1177/0272989X08327395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Res Nurs Health. 2006;29:359–70. doi: 10.1002/nur.20132. [DOI] [PubMed] [Google Scholar]
- 47.Weinstein ND, Marcus SE, Moser RP. Smokers’ unrealistic optimism about their risk. Tob Control. 2005;14(1):55–9. doi: 10.1136/tc.2004.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayanian JZ, Cleary PD. Perceived risks of heart disease and cancer among cigarette smokers. JAMA. 1999;281(11):1019–21. doi: 10.1001/jama.281.11.1019. [DOI] [PubMed] [Google Scholar]
- 49.Strecher VJ, Kreuter MW, Kobrin SC. Do cigarette smokers have unrealistic perceptions of their heart attach, cancer, and stroke risks? J Behav Med. 1995;18(11):45–54. doi: 10.1007/BF01857704. [DOI] [PubMed] [Google Scholar]
- 50.Viscusi WK. Do smokers underestimate risks? J Political Economy. 1990;98(6):1253–69. [Google Scholar]
- 51.Windschitl PD. Judging the accuracy of a likelihood judgment: The case of smoking risk. J Behav Decis Making. 2002;15:19–35. [Google Scholar]
- 52.Niederdeppe J, AGL Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16(5):998–1003. doi: 10.1158/1055-9965.EPI-06-0608. [DOI] [PubMed] [Google Scholar]
- 53.Street RL, Jr, O’Malley KJ, Cooper LA, Haidet P. Understanding concordance in patient-physician relationships: Personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6(3):198–205. doi: 10.1370/afm.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha S, Sanders DS, Korthuis PT, et al. The role of cultural distance between patient and provider in explaining racial/ethnic disparities in HIV care. Patient Educ Couns. 2011;85(3):e278–e84. doi: 10.1016/j.pec.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowen D, Powers D, Greenlee H. Effects of breast cancer risk counseling for sexual minority. Health Care Women Int. 2006;27:59–74. doi: 10.1080/07399330500377119. [DOI] [PubMed] [Google Scholar]
- 56.Richeson JA, Shelton JN. Negotiating interracial interactions: Costs, consequences, and possibilities. Curr Dir Psychol Sci. 2007;16(6):316–20. [Google Scholar]
- 57.Mackie D, Worth L, Asuncion A. Processing of persuasive in-group messages. J Pers Soc Psychol. 1990;58(5):812–22. doi: 10.1037//0022-3514.58.5.812. [DOI] [PubMed] [Google Scholar]
- 58.Reyna VF. Theories of medical decision making and health: An evidence-based approach. Med Decis Making. 2008;28(6):829–33. doi: 10.1177/0272989X08327069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Windschitl PD, Wells GL. Measuring psychological uncertainty: Verbal versus numeric methods. J Exp Psychol Appl. 1996;2(4):343–64. [Google Scholar]
- 60.Hay J, Shuk E, Cruz G, Ostroff J. Thinking through cancer risk: Characterizing smokers’ process of risk determination. Qual Health Res. 2005;15(8):1074–85. doi: 10.1177/1049732305276682. [DOI] [PubMed] [Google Scholar]
- 61.Bird ST, Bogart LM. Perceived race-based and socioeconomic status (SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11(3):554–63. [PubMed] [Google Scholar]
- 62.Stone J, Moskowitz GB. Non-conscious bias in medical decision making: What can be done to reduce it? Med Educ. 2011;45:768–76. doi: 10.1111/j.1365-2923.2011.04026.x. [DOI] [PubMed] [Google Scholar]
- 63.Dasgupta N, Greenwald AG. On the malleability of automatic attitudes: Combating automatic prejudice with images of admired and disliked individuals. J Pers Soc Psychol. 2001;81(5):800–14. doi: 10.1037//0022-3514.81.5.800. [DOI] [PubMed] [Google Scholar]