Abstract
Purpose
A major limitation of studies reporting a lower prevalence rate of human papilloma virus (HPV) in African American (AA) oropharyngeal cancer (OPSCC) patients than Caucasian Americans (CA), with corresponding worse outcomes, was adequate representation of HPV positive AA patients. This study examined survival outcomes in HPV positive and HPV negative AA with OPSCC
Experimental Design
The study cohort of 121 primary OPSCC had 42% AA. Variables of interest included age, race, gender, HPV status, stage, marital status, smoking, treatment, and date of diagnosis.
Results
CA are more likely to be HPV positive (OR=3.28, p=0.035), as are younger age (age <50 OR=7.14, p=0.023 compared to age >65) or being married (OR=3.44, p= 0.016). HPV positivity and being unmarried were associated with being late stage (OR=3.10, p=0.047 and OR=3.23, p=0.038, respectively). HPV negative patients had 2.7 times the risk of death as HPV positive patients (p=0.004). Overall, the HPV-race groups differed (log-rank p<0.001), with significantly worse survival for HPV negative AA vs 1) HPV positive AA (HR=3.44, p=0.0012); 2) HPV positive CA (HR=3.11, p=<0.049); and 3) HPV negative CA (HR=2.21, p=0.049).
Conclusions
HPV has a substantial impact on overall survival in AA OPSCC. Among AA OPSCC, HPV positive patients had better survival than HPV negative. HPV negative AA also did worse than both, HPV positive CA and HPV negative CA. This study adds to the mounting evidence of HPV as a racially-linked sexual behavior life style risk factor impacting survival outcomes for both AA and CA OPSCC patients.
Introduction
There is abundant epidemiological evidence that self-identified race/ethnicity is associated with differences in cancer incidence and mortality(2, 3). The high mortality rate for head and neck squamous cell carcinoma (HNSCC) continues to be driven by the disparate unfavorable diagnosis and prognosis outcomes for African Americans (AA)(2–4). AA have been shown to have a worse overall survival compared to whites after controlling for age, disease stage and treatment received(5). The 5-year relative survival is lower in AA than in Caucasian Americans (CA) for every stage of diagnosis for nearly every cancer site(6). There is no consensus on the causes of the differences in the higher incidence of and the mortality from HNSCC for AA when compared to CA, but they can include differences in access to care, stage at diagnosis, insurance status, attitudes of health providers, as well as human papilloma virus (HPV) infection status(3, 5, 7–10).
In AA with oropharyngeal squamous cell cancer (OPSCC), survival disparities were attributed to racial differences in the prevalence of HPV positive tumors. Settle et al.(10) found that a worse survival outcome for AA versus CA in OPSCC was attributable to racial differences in the prevalence of HPV positive tumors. This was also confirmed by Chernock et al. (11) with corresponding worse disease free survival in AA and a trend toward worse overall survival for AA. A major limitation of these studies was the lack of adequate representation of HPV positive AA patients. For this study we compared survival outcomes in HPV positive and HPV negative AA with OPSCC in a retrospective primary OPSCC cohort with 42% AA.
Material and Methods
Patients
The study cohort of 121 primary OPSCC was drawn from a large, clinically well characterized multi-ethnic (42% AA), primary care patient population in the Detroit area(1). Patients were identified through tumor registry and ENT clinic records. Eligibility criteria included age of 21 years or older, a primary HNSCC diagnosis (including OPSCC), and availability of tumor tissue blocks. For patients in this analysis, diagnosis dates ranged from 1990–2004, follow up dates from 1999–2008, and death dates from 1991–2007.
HPV-16 Detection by Real-Time Quantitative PCR (qPCR)
Whole 5 micron tissue sections with 70% or more tumor or microdissected tumor lesions were processed for DNA extraction.(12) Tumor HPV DNA was determined using qPCR as previously described.(13) Briefly, primers and probes to a housekeeping gene (β-globin) are run in parallel to standardize the input DNA. By using serial dilutions, standard curves are developed for the HPV viral copy number using CaSki (American Type Culture Collection, Manassas, VA) cell line genomic DNA, known to have 600 copies/genome equivalent (6.6 pg of DNA/genome). The cut-off value for HPV16 positive status was ≥0.03 (≥3 HPV genome copy/100 cells).(13)
Statistical Analysis
All analyses were done using SAS 9.2. Categorical data are presented as count (percent) and continuous data as mean (standard deviation). Univariate Wilcoxon rank sum, chi-square, and Fisher’s exact tests were used to examine individual associations with HPV status. Multivariable logistic regression was used to examine the effects of all other variables of interest on the outcomes of interest (HPV status and stage). Kaplan-Meier plots and log-rank tests were used to compare the survival times of HPV positive and HPV negative patients and of African Americans and Caucasians with HPV as compared to those without HPV. Cox regression was used to model the risk of death given age, race, gender, HPV status, stage, smoking status, marital status, treatment, and year of diagnosis. Adjustment for multiple comparisons was done using Hochberg’s method(14). Statistical significance was set at p<0.05.
Results
Patient cohort characteristics
Of the 121 patients identified, 118 have HPV data, of which 67 are HPV negative and 51 HPV positive (43%). Patient characteristics (study variables) for the 118 OPSCC cohort are described in Table 1 and included 68 CA, 49 AA, and 1 unknown race. The median length of follow-up was 46.8 months (range 0.1 to 194 months).
Table 1.
All patient characteristics
| Variable | Response | All patients | HPV Negative (N=67) |
HPV Positive (N=51) |
P- Value |
|---|---|---|---|---|---|
| Surgery | No | 67 (56.8%) | 41 (61%) | 26 (51%) | 0.267 |
| Yes | 51 (43.2%) | 26 (39%) | 25 (49%) | ||
| Age | <=50 | 17 (14.4%) | 6 (9%) | 11 (22%) | 0.149 |
| 51–65 | 60 (50.9%) | 37 (55%) | 23 (45%) | ||
| >65 | 41 (34.8%) | 24 (36%) | 17 (33%) | ||
| Race | White | 68 (57.6%) | 32 (48%) | 36 (71%) | 0.024 |
| Black | 49 (41.5%) | 34 (51%) | 15 (29%) | ||
| Unknown | 1 (0.9%) | 1 (1%) | 0 (0%) | ||
| Gender | Male | 92 (78.0%) | 49 (73%) | 43 (84%) | 0.181 |
| Female | 26 (22.0%) | 18 (27%) | 8 (16%) | ||
| Stage | Early | 25 (21.2%) | 18 (27%) | 7 (14%) | 0.206 |
| Late | 90 (76.3%) | 47 (70%) | 43 (84%) | ||
| Other/Missing | 3 (2.5%) | 2 (3%) | 1 (2%) | ||
| Smoking | Current | 63 (53.4%) | 42 (63%) | 21 (41%) | 0.059 |
| Past | 40 (33.9%) | 19 (28%) | 21 (41%) | ||
| Never | 15 (12.7%) | 6 (9%) | 9 (18%) | ||
| Treatment | Surgery alone | 15 (12.7%) | 12 (18%) | 3 (6%) | 0.031 |
| Surgery+Radiation | 18 (15.3%) | 8 (12%) | 10 (20%) | ||
| Surgery+Radiation+Chemo | 18 (15.3%) | 6 (9%) | 12 (24%) | ||
| Radiation alone (no surgery) | 36 (30.5%) | 20 (30%) | 16 (31%) | ||
| Radiation+Chemo (no surgery) | 22 (18.6%) | 13 (19%) | 9 (18%) | ||
| No treatment | 9 (7.6%) | 8 (12%) | 1 (2%) | ||
| Married | Not married | 49 (43.4%) | 35 (56%) | 14 (28%) | 0.004 |
| Married | 64 (56.6%) | 28 (44%) | 36 (72%) | ||
| Status | Alive | 54 (45.8%) | 23 (34%) | 31 (61%) | |
| Dead | 64 (54.2%) | 44 (66%) | 20 (39%) | ||
| Year of Diagnosis | 1998.2 ± 3.8 | 1996.3 ± 4.0 | 1997.7 ± 3.9 | 0.090 |
HPV prevalence and associated outcomes
In univariate tests (Table 1), being HPV positive was associated with Caucasian race (p=0.024), not smoking (p=0.059), and being married (p=0.004). In multiple logistic regression modeling of 88 OPSCC with complete data (Table 2), CA are more likely to be HPV positive (OR=3.28, p=0.035), as are younger age (age <50 OR=7.14, p=0.023 compared to age >65) or being married (OR=3.44, p= 0.016). In a model using stage (early or late) as the outcome (Supplementary Table 1) and controlling for age, race, gender, smoking status, and year of diagnosis, late stage (stage III & IV) was associated with HPV positive status (OR=3.10, 95% CI 1.01, 9.45, p=0.047) and as was being unmarried (OR=3.23, 95% CI 1.06, 9.78, p=0.038).
Table 2.
Multivariable associations with positive HPV status (N=88; positive=43, negative=45)
| Variable | Odds Ratio | 95% Confidence Limits | P-Value | |
|---|---|---|---|---|
| Age (51–65 vs >65) | 1.25 | 0.41 | 3.80 | 0.690 |
| Age (≤50 vs >65) | 7.14 | 1.31 | 39.00 | 0.023 |
| Race (White vs Black) | 3.28 | 1.09 | 9.87 | 0.035 |
| Gender (Male vs Female) | 1.63 | 0.42 | 6.32 | 0.476 |
| Stage (Early vs Late) | 1.74 | 0.12 | 24.29 | 0.681 |
| Smoke (Never vs Current) | 1.03 | 0.22 | 4.83 | 0.970 |
| Smoke (Past vs Current) | 1.48 | 0.48 | 4.57 | 0.501 |
| Married (Yes vs No) | 3.44 | 1.26 | 9.33 | 0.016 |
| Year of diagnosis | 1.10 | 0.96 | 1.26 | 0.189 |
HPV status and survival outcomes
Of the 121 patients with survival data available, 56 lived and 65 died. Patients who are HPV positive have significantly higher survival probability than patients who are HPV negative (univariate log rank p=0.003, Figure 1). The median survival time for HPV negative patients was 2.2 years (95% C.I. 1.3, 5.2), but for HPV positive patients median survival was over 13 years.
Figure 1.
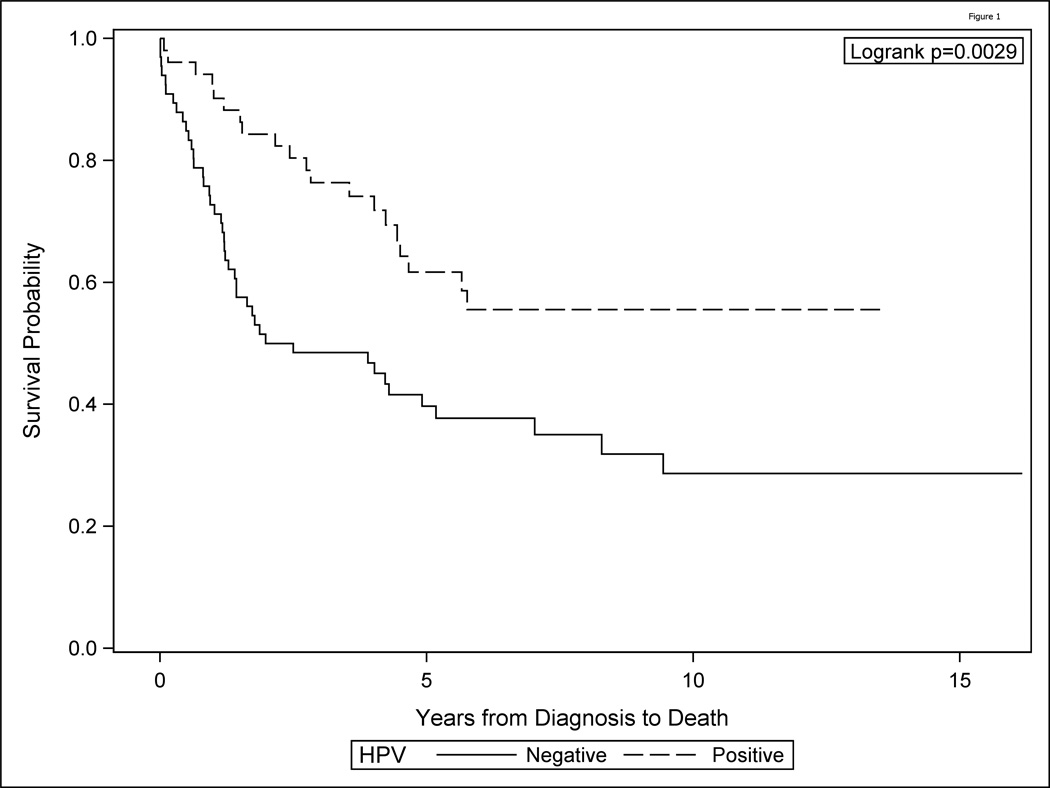
HPV positive OPSCC show improved survival as compared to HPV negative oropharyngeal (OPSCC)
Cox regression given age, race, gender, HPV status, stage, treatment, smoking status, marital status, and year of diagnosis indicated that HPV negative patients had 2.7 times the risk of death compared to HPV positive patients (95% CI 1.37, 5.31, p=0.004) (Table 3), late-stage patients had worse survival than early-stage patients (OR=2.44, 95% CI 1.15, 5.16, p=0.020), and current smokers had 3.4 times the risk of death versus never-smokers (95% CI 1.13, 10.38, p=0.030). Race as AA was not independently associated with worse outcome in the entire cohort in multivariate analysis. No interactions were significant.
Table 3.
Multivariate associations with death (N=108, death=59, censored=49)
| Hazard Ratio | 95% CL | P-Value | ||
|---|---|---|---|---|
| Age (>65) | 2.67 | 1.43 | 4.99 | 0.002 |
| Age (<=50) | 1.49 | 0.63 | 3.54 | 0.364 |
| Race (Black) | 1.15 | 0.64 | 2.07 | 0.645 |
| Gender (Male) | 2.12 | 1.02 | 4.42 | 0.044 |
| HPV (Negative) | 2.69 | 1.37 | 5.31 | 0.004 |
| Stage (Late) | 2.44 | 1.15 | 5.16 | 0.020 |
| Both Radiation & Chemo | 2.89 | 1.47 | 5.72 | 0.002 |
| Neither Radiation & Chemo | 2.53 | 1.18 | 5.43 | 0.017 |
| Smoking (Current) | 3.42 | 1.13 | 10.38 | 0.030 |
| Smoking (Past) | 1.51 | 0.49 | 4.70 | 0.478 |
| Married (Yes) | 1.18 | 0.64 | 2.18 | 0.600 |
| Year of diagnosis | 0.95 | 0.87 | 1.02 | 0.169 |
Survival of the HPV and race groups (combined to form 4 groups), differed significantly overall (log-rank p<0.001, Figure 2, Table 4). HPV negative AA had worse overall survival than HPV positive AA (HR=3.44, raw p=<0.001, Hochberg adjusted p=0.0012), worse survival than HPV positive CA (HR=3.11, raw p=0.012, adjusted p=0.0496), and worse survival than HPV negative CA (HR=2.21, raw p=0.02, adjusted p=0.0496). HPV positive AA patients had no differences in survival from that of HPV positive and HPV negative CA (p=0.84 and p=0.66, respectively). Also HPV positive CA and HPV negative CA OPSCC showed no differences in survival outcomes (p=0.84).
Figure 2.
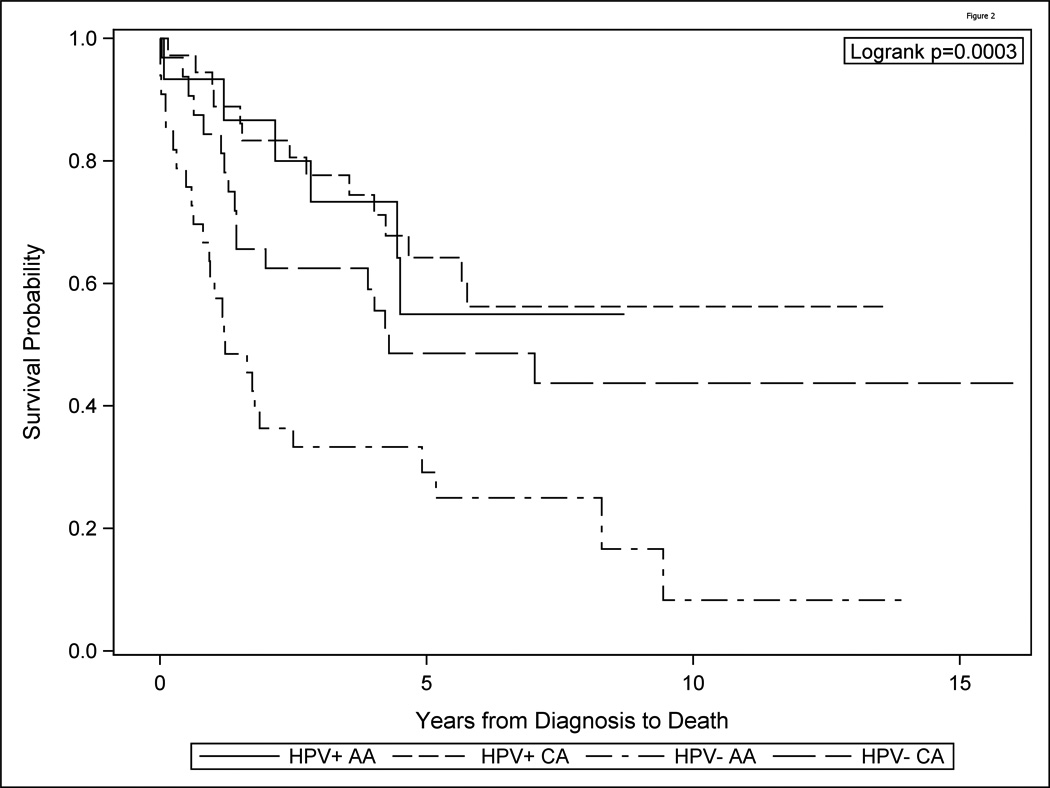
Survival of the HPV and race groups (combined to form 4 groups), differed significantly overall (log-rank p<0.001). HPV negative African American (AA) had worse overall survival than HPV positive AA, worse survival than HPV positive Caucasian American (CA), and worse survival than HPV negative CA. HPV positive AA patients had no differences in survival from that of HPV positive and HPV negative C. Also HPV positive CA and HPV negative CA OPSCC showed no differences in survival outcomes.
Table 4.
Cox proportional survival hazard model for race and HPV with contrasts (N=116)
| Hazard Ratio |
95% CL | Raw P- Value |
Adjusted P-Value |
|
|---|---|---|---|---|
| Model | ||||
| HPV− CA (Ref) | 1.00 | |||
| HPV− AA vs HPV− CA | 2.21 | (1.19,4.08) | 0.012 | 0.0496 |
| HPV+ C vs HPV− CA | 0.71 | (0.28,1.80) | 0.469 | 0.8403 |
| HPV+ AA vs HPV− CA | 0.64 | (0.32,1.30) | 0.220 | 0.6600 |
| Contrasts | ||||
| HPV− AA vs HPV+ CA | 3.11 | (1.28,7.58) | 0.012 | 0.0496 |
| HPV− AA vs HPV+ AA | 3.44 | (1.79,6.60) | <0.001 | 0.0012 |
| HPV+ CA vs HPV+ AA | 1.10 | (0.42,2.88) | 0.840 | 0.8403 |
Discussion
HPV is now regarded, in addition to tobacco and alcohol(15), as a causative agent for oropharyngeal cancer (OPSCC) (16) and an independent risk factor OPSCC(17, 18). The association between HPV and HNSCC (for both incidence and prognosis), is strongest for OPSCC. HPV positive OPSCC has been noted as a distinct variant of HNSCC characterized by high prevalence of HPV infection, better patient outcome, nonkeratinizing histology, and overexpression of p16(19), with worse outcomes in OPSCC for AA compared to CA partially explained by less HPV positive cases in AA (10, 20).
Overall HPV prevalence in this cohort was 43%, with diagnosis years ranging from 1990 through 2004 and younger patients <50 years of age were significantly more likely to be HPV positive. Chatutved et al(18) reported a higher HPV prevalence in OPSCC of approximately 70% during 2000 to 2004. They found that HPV positive patients diagnosed from 1984 to 2004 were significantly younger and more likely to be white. The study attributed increases in the population-level incidence and survival of OPSCC in the United States since 1984 to HPV infection, with an estimation that, by 2020, HPV will cause more oropharyngeal cancers than cervical cancers in the United States(18).
In this study, with 42% AA, prevalence of HPV was lower in AA than CA OPSCC. CA patients were significantly more likely to be HPV positive than AA patients supporting HPV tumor status as a disparate determinant in AA patients with OPSCC(10, 11, 21, 22). There is conclusive evidence that HPV has a strong association between sexual behavior and OPSCC, which has been linked to an increase in the number of oral sex partners (23–26). Studies of racial differences in sexual behaviors(27, 28) suggest that a higher proportion of whites engage in oral sex. These sexual behavior differences are thought to be one piece of the puzzle(29) for the reported racial disparity of HPV prevalence in OPSCC and might explain the higher rate of HPV positive oropharyngeal cancers in whites (versus blacks) (10, 11, 21, 22).
In this study, married patients were more likely to be HPV positive than unmarried OPSCC. The latter is the opposite of what was found in a report that never-married status may be a surrogate for sexual practices associated with HPV transmission based on the dramatically elevated and reciprocal risk of second primary anogenital and oral cavity/pharyngeal cancers risk among never-married men compared with ever-married men (30). Significant changes in marriages and living together dynamics have occurred over the years, resulting in changes in sexual behavior and co-habitation choices emphasizing the need for larger studies that address sexual practices to further define the interaction between marital status and HPV infection in OPSCC.
Our study supports improved survival for HPV positive as compared to HPV negative OPSCC. Tumor HPV status has been shown to be the single strongest predictor, followed by measures of tobacco exposure and tumor stage(31). Overall, in multivariate associations, HPV16-negative patients had a significantly poorer survival probability than HPV positive, a finding that concurs with several reported studies (HR=2.9, p=0.003). Also, late stage patients had better survival than early stage OPSCC (OR=2.21, p=0.034) and support studies of late stage as an important predictor of poor survival with patients dying of uncontrolled loco-regional disease (32).
Current smokers were nearly 4 times more likely to die than never smokers (OR=3.8, p=0.019); no difference in survival for past smokers was noted. Tobacco exposure has been associated with clinical trial outcome(33) and nicotine has been reported to reduce the cytotoxic effects of cisplatin and radiation of HNSCC cell lines(34). A recent finding of an increase of risk of OPSCC progression and death as a direct function of tobacco exposure at diagnosis and during therapy, independent of tumor p16 status and treatment(35) concurs with tumor HPV status as a strong and independent prognostic factor for survival in OPSCC(17). Thus, the most important risk factors for development of head and neck cancer, HPV and smoking, have utility as predictors of response to therapy and patient survival and likely determine the molecular profile of this disease.
Late stage OPSCC were more likely to be HPV positive status (OR=3.10, p=0.47) and unmarried (OR=3.23, p=0.038) as compared to early stage patients. HPV positive HNSCC are more likely to be detected as late-stage cancers, which traditionally indicate poor prognosis(25, 36, 37). Despite this, survival has been shown to be better for patients with HPV-positive when compared with HPV-negative HNSCC(38), underscoring HPV as a reliable biomarker that can be used to not only help diagnose HNSCC, but to also risk stratify patients and help direct treatment plans based on disease behavior and prognosis (38, 39).
A unique contribution of this study is the observation that among AA, those that were HPV positive did better than those that were HPV negative (HR=3.44, raw p=<0.001, Hochberg adjusted p=0.0012), a finding not previously reported presumably due to a paucity of multi-ethnic cohorts and limited number of AA OPSCC patients. This is illustrated in the frequency distribution of HPV by race in TAX 324 by Settle et al.(10), which had only 1 AA HPV positive OPSCC (of 28 AA). Chernock et al. (11) had 3 HPV positive cases (of 26 AA), and Weinberger et al(22) had zero HPV positive AA OPSCC (of 16 AA with HPV data). For HPV and race groups, HPV negative AA had worse overall survival than both HPV positive and HPV negative CA (p=<0.0001 raw, p=0.0005 adjusted and p=0.01 raw, p=0.05 adjusted, respectively). HPV negative AA patients were more likely to be current smokers (79%) than HPV positive AA (31%), HPV negative CA (43%) or HPV positive CA (34%) [p=0.005]. However, even if the comparisons among the four race/HPV groups are made in a multivariable model adjusted for smoking and other factors, the HPV negative AA patients still do worse than the other groups. A larger sample size and assessment of genetic heterogeneity using ancestry informative marker analysis may be needed to further explain this.
The primary health care environment of the Henry Ford Health System (HFHS), which has a high proportions of insured patients (40), may partially explain the lack of survival differences between AA and CA in this OPSCC cohort. Our study, highlighting survival differences based only on HPV-race groups, indicates that HPV has a substantial impact on overall survival in AA with OPSCC. HPV negative AA not only had worse survival than HPV positive AA, but also did worse than both, HPV positive CA and HPV negative CA. Whether these findings reflect cultural differences in sexual lifestyle preferences evident from a significantly lower prevalence for HPV in the AA OPSCC group is not estimable from this study. Drawn from a multi-ethnic access to care population, this study adds to the mounting evidence of HPV as a likely racially-linked sexual behavior life style risk factor impacting survival outcomes for both AA and CA OPSCC patients.
Supplementary Material
Translational Relevance.
In African Americans (AA) with oropharyngeal squamous cell cancer (OPSCC), survival disparities are attributed to racial differences in the prevalence of HPV positive tumors. A major limitation of these studies is the lack of adequate representation of HPV positive AA patients. This study examined survival outcomes for HPV positive and HPV negative AA in a study cohort of 121 primary OPSCC (42% AA), drawn from a large, clinically well characterized multi-ethnic primary care patient population in the Detroit area(1).
A unique contribution of this study is the observation that among AA OPSCC, HPV positive patients had better survival than HPV negative. HPV negative AA also did worse than both, HPV positive CA and HPV negative CA. This study adds to the mounting evidence of HPV as a racially-linked sexual behavior life style risk factor impacting survival outcomes for both AA and CA OPSCC patients.
Acknowledgments
Grant Support
This work was supported by NIH: R01 DE 15990 (to M. J. Worsham)
Footnotes
Disclosure of Potential Conflicts of Interest: None
Authors' Contributions
Conception and design: Maria J. Worsham, Kang Mei Chen, Josena K. Stephen, George Divine
Development of methodology: Maria J. Worsham, Shaleta Havard, Kang Mei Chen, Josena K. Stephen, Meredith Mahan, George Divine, Vanessa Schweitzer
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Maria J. Worsham, Shaleta Havard, Kang Mei Chen, Josena K. Stephen, Vanessa Schweitzer
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Maria J. Worsham, Kang Mei Chen, George Divine, Vanessa Schweitzer
Writing, review, and/or revision of the manuscript: Maria J. Worsham, Shaleta Havard, Kang Mei Chen, Josena K. Stephen, Meredith Mahan, George Divine, Vanessa Schweitzer
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Maria J. Worsham, Kang Mei Chen, George Divine, Vanessa Schweitzer
Study supervision: Maria J. Worsham
References
- 1.Worsham MJ, Stephen JK, Lu M, Chen KM, Havard S, Shah V, et al. Disparate Molecular, Histopathology, and Clinical Factors in Head and Neck Squamous Cell Carcinoma Racial Groups. Otolaryngol Head Neck Surg. 2012 doi: 10.1177/0194599812440681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 3.Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22:25–38. doi: 10.1023/a:1022255800411. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Facts & Figures 2011. American Cancer Society; 2011. [Google Scholar]
- 5.Murdock JM, Gluckman JL. African-American and white head and neck carcinoma patients in a university medical center setting. Are treatments provided and are outcomes similar or disparate? Cancer. 2001;91:279–283. doi: 10.1002/1097-0142(20010101)91:1+<279::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RWE, Brawley O. Jemal A Cancer statistics: 2011:The impact of eliminating socioeconomic and racial disparities on premature cancer deaths CA. Cancer Journal Clinical. 2011 doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 7.Arbes SJ, Jr, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Causes Control. 1999;10:513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin WJ, Thomas GR, Parker DF, Joseph D, Levis S, Franzmann E, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30:358–371. doi: 10.1002/hed.20710. [DOI] [PubMed] [Google Scholar]
- 9.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 10.Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila Pa) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Sawhney R, Khan M, Benninger MS, Hou Z, Sethi S, et al. Methylation of multiple genes as diagnostic and therapeutic markers in primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1131–1138. doi: 10.1001/archotol.133.11.1131. [DOI] [PubMed] [Google Scholar]
- 13.Stephen JKCK, Havard S, Lu M, Shah V, Schweitzer VP, Gardner G, Worsham MJ. HPV outcomes in an access to care laryngeal cancer cohort. Otolaryngology-Head and Neck Surgery. doi: 10.1177/0194599811434482. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini YHY. Controlling the false discovery rate - a practical and powerful approach to multiple testing. 1995 [Google Scholar]
- 15.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 16.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363:1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrank TP, Han Y, Weiss H, Resto VA. Case-matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States--possible role for human papillomavirus in survival disparities. Head Neck. 33:45–53. doi: 10.1002/hed.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberger PM, Merkley MA, Khichi SS, Lee JR, Psyrri A, Jackson LL, et al. Human papillomavirus-active head and neck cancer and ethnic health disparities. Laryngoscope. 2010;120:1531–1537. doi: 10.1002/lary.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 25.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 26.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 27. http://www.cdc.gov/nchs/NSFG.htm. The 2002 National Survey of Family Growth
- 28.Gates GJ, Sonenstein FL. Heterosexual genital sexual activity among adolescent males: 1988 and 1995. Fam Plann Perspect. 2000;32:295–297. 304. [PubMed] [Google Scholar]
- 29.Brawley OW. Oropharyngeal cancer, race, and the human papillomavirus. Cancer Prev Res (Phila) 2009;2:769–772. doi: 10.1158/1940-6207.CAPR-09-0150. [DOI] [PubMed] [Google Scholar]
- 30.Sikora AG, Morris LG, Sturgis EM. Bidirectional association of anogenital and oral cavity/pharyngeal carcinomas in men. Arch Otolaryngol Head Neck Surg. 2009;135:402–405. doi: 10.1001/archoto.2009.19. [DOI] [PubMed] [Google Scholar]
- 31.K Ang QZ, Wheeler RH, Rosenthal DI, Nguyen-Tan F, Kim H, Lu C, Axelrod RS, Silverman CI, Weber RS. A phase III trial (RTOG 0129) of two radiation-cisplatin regimens for head and neck carcinomas (HNC): Impact of radiation and cisplatin intensity on outcome [Google Scholar]
- 32.Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15:1179–1186. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 33.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 34.Onoda N, Nehmi A, Weiner D, Mujumdar S, Christen R, Los G. Nicotine affects the signaling of the death pathway, reducing the response of head and neck cancer cell lines to DNA damaging agents. Head Neck. 2001;23:860–870. doi: 10.1002/hed.1125. [DOI] [PubMed] [Google Scholar]
- 35.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco Smoking and Increased Risk of Death and Progression for Patients With p16-Positive and p16-Negative Oropharyngeal Cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:259–266. doi: 10.1111/j.1749-4486.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 37.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. Apmis. 2010;118:510–519. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 38.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 39.Mydlarz WK, Hennessey PT, Califano JA. Advances and Perspectives in the Molecular Diagnosis of Head and Neck Cancer. Expert Opin Med Diagn. 2010;4:53–65. doi: 10.1517/17530050903338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worsham MJSJ, Lu M, Chen KM, Havard S, Shah V, Schweitzer VG. Disparate molecular, histopathology, and clinical factors in HNSCC racial groups. Otolaryngolog-Head and Neck Surgery. 2012 Mar 12; doi: 10.1177/0194599812440681. [Epub ahead of print] PMID: 22412179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


