Abstract
Consumption of a high-fat diet (HFD) in experimental animal models initiates a series of molecular events and outcomes, including insulin resistance and obesity, that mimic the metabolic syndrome in humans. The relationship among, and order of, the molecular events linking a diet high in fat to pathologies is often unclear. In the present study, we provide several novel insights into the relationship between a HFD and AMP-activated protein kinase (AMPK), a key regulator of cellular metabolism and whole-body energy balance. HFD substantially decreased the activities of both iso-forms of AMPK in white adipose tissue, heart, and liver. These decreases in AMPK activity occurred in the absence of decreased AMPK transcription, systemic inflammation, hyperglycemia, or elevated levels of free fatty acids. The HFD-induced decrease in AMPK activity was associated with systemic insulin resistance and hyperleptinemia. In blood, >98 % of AMPK activity was localized in agranulocytes as the α1 isoform. In contrast to the solid tissues studied, AMPK activities were not altered by HFD in granulocytes or agranulocytes. We conclude that HFD-induced obesity causes a broad, non-tissue, or isoform-specific lowering of AMPK activity. Given the central position AMPK plays in whole-body energy balance, this decreased AMPK activity may play a previously unrecognized role in obesity and its associated pathologies.
Keywords: Obesity, Diabetes, Adipose, Blood
Introduction
The World Health Organization considers obesity to be one of the most prevalent epidemics of this century, with incidents more than doubling since 1980 [54]. Obesity reflects a state of energy imbalance resulting from a combination of excessive energy intake and inadequate energy expenditure, often leading to insulin resistance, type II diabetes, and systemic inflammation [48, 57].
AMP-activated protein kinase (AMPK) has been studied for more than two decades as a master regulator of energy balance. A large array of intracellular processes are regulated in part by AMPK, including the cellular uptake of glucose and free fatty acids (FFAs), cell cycling, mRNA stability, and apoptosis [8, 19–21]. Additionally, recent data indicate that AMPK plays a more global role, regulating multiple aspects of whole-body energy balance including appetite, insulin sensitivity, and the actions of adipokines/cytokines [23, 25, 34, 53].
Due to the central role of AMPK in regulating these facets of energy balance, it is not surprising that alterations in AMPK have been implicated in the onset of obesity and metabolic syndrome [35, 41, 42]. The activation of AMPK is thought to play a role in some of the beneficial effects of common treatments for obesity, including exercise, caloric restriction, and the anti-diabetic compounds such as metformin and thiazolidinediones [10, 31, 55]. Furthermore, AMPK is required for the resveratrol-dependent increases in insulin sensitivity and weight loss in high-fat diet (HFD)-fed mice, as illustrated by a study using the α1AMPK knockout mouse model [51]. Thus, multiple lines of evidence suggest a link between AMPK activity and the pathogenesis of obesity as well as its treatment.
Despite the critical role AMPK plays in cellular and whole-body energy balance, the effect of a HFD, a major cause of obesity and its comorbidities [14, 22, 39, 56], on AMPK has not been comprehensively studied. To date, the effect of a HFD [14, 22, 39, 56] on AMPK has most frequently been studied in a single tissue type, usually in liver and/or skeletal muscle, using immunoblots. These types of studies have yielded inconsistent results, some reporting decreased AMPK activity [3, 5, 12, 18, 27, 28, 35] while others reporting increased or no change in activity [5, 49].
Therefore, the goals of this study were to determine the effect of HFD-induced obesity in rats on the activity of both isoforms (α1 and α2) of the catalytic subunit of AMPK in four tissues important in the regulation of whole-body energy balance: white adipose tissue (WAT), brown adipose tissue (BAT), liver, and heart. Because AMPK has been implicated as a mediator of the inflammatory response [44, 59], AMPK activities were also measured in granulocyte and agranulocytes. Finally, serum markers of nutrient status and inflammatory state were measured to assess their relationships to HFD-induced changes in AMPK activity.
Methods
Animals
Eight-week old, adult, sexually mature, male Sprague–Dawley rats were purchased from Harlan Laboratories (n=16) and housed at a University of Wisconsin Animal Care Facility. The facilities and research protocols were approved by the University of Wisconsin Institutional Animal Care and Use Committee. After acclimatization, half the rats were fed a standard chow diet (3 kcal/g, 14 % calories from fat, 32 % calories from protein, 54 % calories from carbohydrate; 8604 Teklad rodent diet, Harlan) and the other half fed a high-fat diet (5.25 kcal/g, 60 % calories from fat, 20 % calories from protein, 20 % calories from carbohydrate; Open Source Diets D12492) for 18 weeks. Eighteen weeks was chosen based on the different lengths of time used in previous studies [2, 15, 17]. Each rat was singly caged and had unrestricted access to food and water. Rats underwent an 18-h fasting period prior to killing.
Blood glucose was measured using a glucometer (OneTouch UltraMini, Lifescan) and blood from a tail snip of anesthetized rats just prior to killing. Blood was collected via cardiac puncture from rats under deep CO2 anesthetization: one sample with a non-heparinized syringe for serum isolation and a second sample with a heparinized syringe for blood fractionation. Rats were then killed via carbon dioxide asphyxiation. Interscapular brown adipose tissue liver, heart, and epididymal fat pads were rapidly collected. The heart and liver were frozen in liquid nitrogen and stored at −80 °C until analyzed. The white adipose tissue was separated into two sections: one frozen in liquid nitrogen and the second fixed in 4 % paraformaldehyde (PFA) and stored at 4 °C for histological analysis.
Serum isolation and measurement of FFA, cytokine, insulin, and leptin
Non-heparinized blood was allowed to clot at room temperature for 30 min. Samples were centrifuged and the supernatant was collected as serum and frozen at −80 °C until analyzed. Serum leptin, insulin, FFA, IL-6, and TNFα concentrations were measured via ELISA kits (Millipore for leptin and insulin, BioVision for FFA, and Thermo Scientific for IL-6 and TNFα) following the manufacturers’ instructions.
Blood fractionation
Three milliliters of heparinized blood was fractionated using Accuspin System Histopaque 1077 (cat no. A6929, Sigma-Aldrich) following the manufacturer’s protocol. Briefly, blood was applied to the Histopaque column and centrifuged at 1,000×g for 15 min at room temperature. The layer above the frit (the agranulocyte fraction) was removed and 1 ml of red blood cell (RBC) Lysis Buffer (cat no. R7757, Sigma-Aldrich) was added. Cells were agitated for 2 min, centrifuged at 1,000×g for 10 min at 4 °C, resuspended in 500 μl of phosphate-buffered saline (PBS), and analyzed using a hemocytometer (Fisher Scientific) to determine the total cell number and the number of granulocytes and agranulocytes in each sample. The cell pellet from the Histopaque column was collected and resuspended in 50 ml of the RBC Lysis Buffer II (8.3 g/L NH4Cl in 0.01 M Tris–HCl buffer), agitated for 5 min, and centrifuged at 1,000×g for 10 min at 4 °C. The pellet and dense layer were collected along with 3–4 ml supernatant. The RBC Lysis Buffer was added to bring the volume to 15 ml. The mixture was agitated, centrifuged at 1,000×g for 10 min at 4 °C, and repeated. The pellet (granulocyte fraction) was collected, resuspended in 500 μl of PBS, and analyzed using a hemocytometer (Fisher Scientific) to determine the total cell number and the number of granulocytes and agranulocytes in each sample.
AMPK activity assay
AMPK activity was measured as previously described [38] and expressed as picomoles of phosphate incorporated per minute per milligram of homogenate protein. Briefly, tissue and cell pellets were homogenized in the presence of protease inhibitors and phosphatase inhibitors. Of the total protein from the resulting supernatant, 20 μg was immunoprecipitated with protein A/G agarose beads (Santa Cruz Biotech) and antibodies against either the α1 (Santa Cruz Biotech) or α2 (raised against the α2 AMPK peptide C-DDSAMHIPPGLKPHP) catalytic subunits of AMPK overnight at 4 °C. For cellular blood fractions, protein from 100,000 cells was immunoprecipitated. The beads containing the immunoprecipitated AMPK were washed, resuspended in reaction buffer with 0.5 mM SAMS peptide [8] and 0.2 mM [γ-32P] ATP, and incubated for 10 min at 37 °C in a thermomixer in the presence of 200 mM AMP. After incubation, the beads were quickly pelleted and a portion of each supernatant was spotted on a P-81 phosphocellulose paper washed in 1 % phosphoric acid, then washed in acetone and air-dried. The incorporated radioactivity was counted in a TriCarb 3000 beta scintillation counter.
Quantitative real-time PCR
RNA was isolated from the tissues according to the specifications of the Invitrogen PureLink RNA Mini Kit (12183018A). The RNA concentrations were determined using the NanoDrop ND-1000 spectrophotometer. The RNA was converted to cDNA using the AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies, 600559). cDNA was then used with the Brilliant II SYBER Green QPCR Master Mix (Agilent Technologies, 600828) and α1 AMPK (F 5′-GGG ATC CAT CAG CAA CTA TCG, R 5′-GGG AGG TCA CGG ATC AGG), α2 AMPK (F 5′-TCG CAG TGG CTT ATC ATC TC, R 5′-TGT CGT ATG GTT TGC TCT GG), and GAPDH (F 5′-TGC ACC ACC AAC TGC TTA GC, R 5′-GGC ATG GAC TGT GGT CAT GAG) primers and ran on an Agilent Technologies Stratagene Mx3005P. The relative amounts of mRNAs were calculated by the comparative CT method using GAPDH as the reference. The data are expressed as the fold change of the HFD group compared to the control, with p values determined by t test of the ΔCT values [6, 61]. CT values were from eight animals/group using duplicates performed at the cDNA synthesis step for each animal.
Histology
Histological samples were processed at the TRIP Histology Lab (University of Wisconsin—Madison). Fixed tissue samples were embedded in paraffin, sliced into 5-μm-thick sections, stained with hematoxylin and eosin, and the adipocyte diameter measured using Micron (Westover Scientific) software.
Statistics
To assess the effects of a high-fat diet, group comparisons were made using a Student’s t test when possible. A p value <0.05 was considered statistically significant. All data are expressed as the mean ± SE.
Results
Rats were divided into two groups and fed either a control or a high-fat diet. Food intake and body weight were monitored weekly for 18 weeks, as shown in Fig. 1. A steady increase in body weight over the 18 weeks was seen for both control and HFD. At the time of killing, the body weight of the HFD group was approximately 10 % higher than the controls (p<0.05, Fig. 1a). Although the control animals consumed more grams of food (p<0.001, Fig. 1c), the kilocalories consumed were essentially equal between the two groups (p>0.05, Fig. 1b).
Fig. 1.
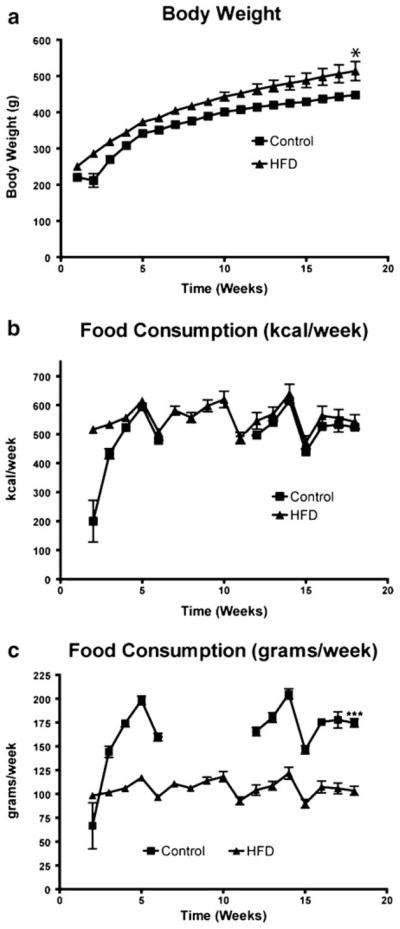
Body weight (a) and food intake expressed as kilocalo-ries per week (b) and grams per week (c) were measured weekly over an 18-week feeding period for control and high-fat diet (HFD) rats. Values are the mean ± SE (n=8/group; *p<0.05, ***p<0.001)
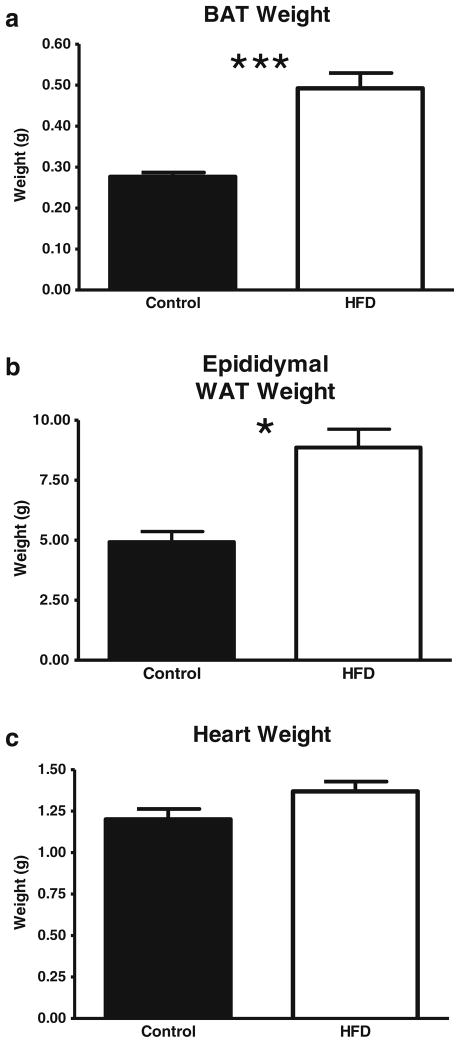
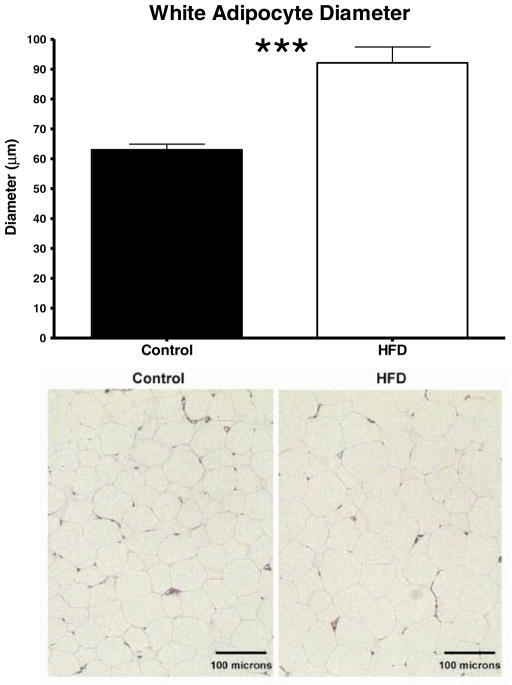
The amount of subscapular BAT and epididymal fat pad WAT was approximately twice as high in HFD rats compared to the controls (p<0.001, Fig. 2a for BAT; p<0.05, Fig. 2b for WAT). The heart weight of HFD animals also tended to be higher (p=0.07; Fig. 2c). As expected in this model, adipocyte diameter was significantly increased by the HFD (p<0.0001, Fig. 3).
Fig. 2.
Effect of a HFD on interscapular brown adipose tissue (BAT), epididymal fat pad white adipose tissue (WAT), and heart weights at the time of killing. Values are the mean ± SE (n=8/group; *p<0.05, ***p<0.001)
Fig. 3.
Effect of a HFD on white adipocyte diameter. Adipocytes were measured in sections of white adipose tissue fixed in 4 % PFA and stained with hematoxylin and eosin. Values are the mean ± SE (n=7–8/group; ***p<0.001)
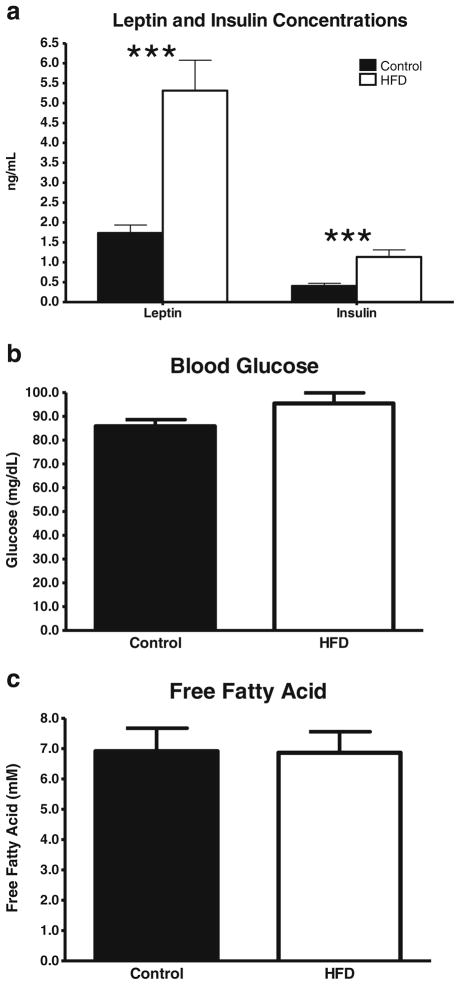
In addition to the effect of HFD on body weight and tissue weights, other hallmarks of a HFD regimen were observed. Leptin levels were over threefold higher in the HFD rats than in the controls (p<0.001, Fig. 4a). While glucose and FFAs were not significantly elevated in the HFD rats (p>0.05, Fig. 4b, c), insulin was increased nearly threefold, suggesting that the HFD rats had developed a moderate degree of insulin resistance (p<0.001, Fig. 4a).
Fig. 4.
Effect of a HFD on serum leptin and insulin (a), blood glucose (b), and FFA (c) concentrations measured at the time of killing. Values are the mean ± SE (n=7–8/group; ***p<0.001)
To determine whether a systemic inflammatory response was present in the HFD group, as often occurs during obesity [47, 48, 57], the serum concentrations of TNF-α and IL-6 were measured via ELISA. In the two groups of rats, the serum concentrations of both cytokines were below the lower limit of detection of the cytokine kits (15 pg/ml; data not shown).
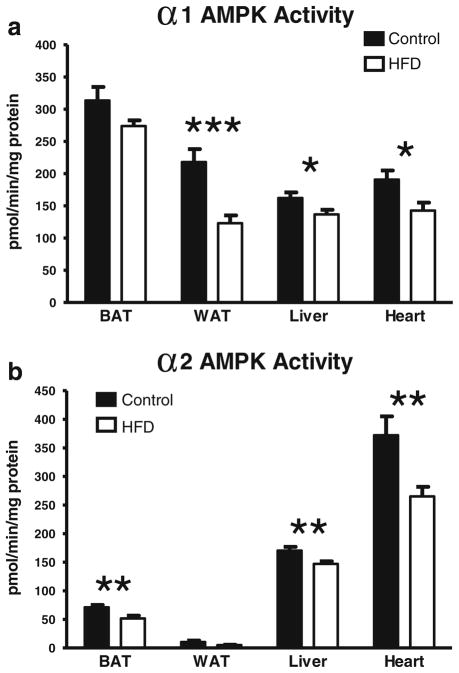
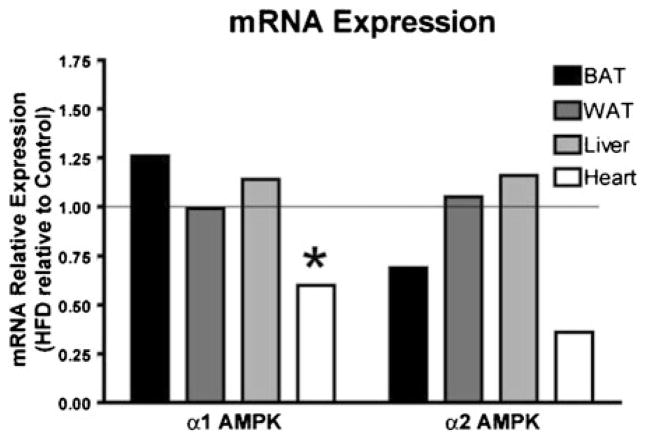
To assess how HFD affects AMPK activity, the enzymatic activities of the α1 and α2 isoforms of AMPK were measured in BAT, WAT, liver, heart, and blood. As shown in Fig. 5a, the activity of α1 AMPK was significantly lower in the HFD group in WAT (p<0.001), liver (p<0.05), and the heart (p< 0.05) than in the controls. In WAT, this represented a nearly 50 % lower α1 AMPK activity in the HFD group. The decreases were approximately 15 and 25 % for the liver and the heart, respectively. For BAT, comparing HFD to the control tissues yielded a p value of 0.11. The activity of α2 AMPK in BAT, the liver, and the heart were significantly lower in the HFD group (27, 13, and 29 %, respectively, p<0.01 for each; Fig. 5b). It should be noted that the α2 AMPK activity in WAT is so low that it is nearly undetectable. These HFD-induced decreases in AMPK activity were unlikely due to the decreased expression of mRNA for WAT, BAT, and the liver since they showed no decreases in mRNA. However, in the heart, HFD decreased the mRNA expression of α1 AMPK (p<0.05, Fig. 6) with a trend toward a decreased expression of α2 AMPK (p=0.08, Fig. 6).
Fig. 5.
Effect of a HFD on α1 (a) and α2 (b) AMPK activities in BAT, WAT, the liver, and the heart. AMPK is expressed as picomoles of phosphate incorporated per minute per milligram of homogenate protein. Values are the mean ± SE (n=8/group; *p<0.05, **p<0.01, ***p<0.001)
Fig. 6.
Effect of a HFD on α1 and α2 AMPK mRNA expressions relative to the control group's expression in BAT, WAT, the liver, and the heart (n=8/group)
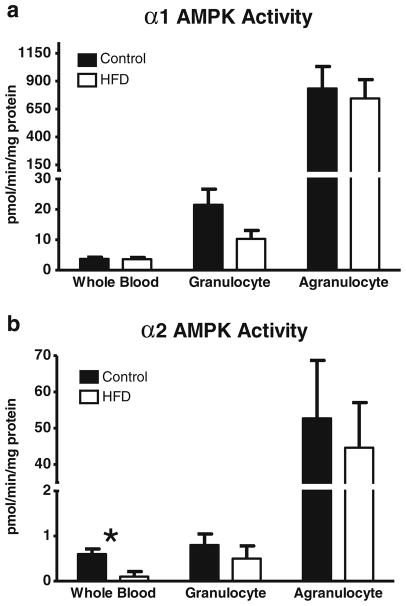
Because AMPK has been linked to inflammatory as well as survival pathways in white blood cells [44, 59], its activity was measured not only in whole blood but also in white blood cell fractions enriched for granulocytes (79–85 % granulocytes) and agranulocytes (>99 % agranulocytes; Fig. 7a, b). In whole blood, AMPK activities were very low, but measurable (Fig. 7a, b). These very low levels of AMPK in whole blood are likely because AMPK is presented as activity per milligram of protein, and the protein-rich red blood cells have little, if any, AMPK activity (data not shown). We did note that in whole blood, α2 AMPK activity, while barely measurable, was significantly lower in the HFD group without there being significantly less AMPK activity in either blood fraction. This can be attributed to the difference in scales on which the whole blood and agranulocytes are plotted in that the whole blood from the HFD group was approximately 0.5 pmol min−1 mg−1 protein less than the controls, whereas in the HFD group the agranulocytes had approximately 20 pmol min−1 mg−1 protein less than the α2 AMPK activity. This was not statistically significant because of the larger variability. The agranulocyte fraction (lymphocytes and monocytes) contained 97–99 % of the total α1 and α2 AMPK activities in each of the groups of rats. Unlike the solid tissues studied, there was no effect of HFD on the AMPK activity in the blood cell fractions. HFD had no significant effect on the total white blood cell count (4.5 vs. 4.2 million cells per milliliter of whole blood, p=0.58).
Fig. 7.
Effect of a HFD on α1 (a) and α2 (b) AMPK activities in whole blood, granulocyte fraction (approximately 85 % granulocytes), and agranulocyte fraction (>99 % agranulocytes) collected at the time of killing. Values are the mean ± SE (n=7–8/group; *p<0.05)
Discussion
In humans as well as in experimental animal models, consuming a diet high in fat initiates a series of molecular events often leading to elements of metabolic syndrome, including insulin resistance and obesity [14, 22, 39, 56]. The relationship among the molecular events linking a diet high in fat to pathologies is often unclear. In the present study, we provide novel insights into the relationship between a HFD and a key regulator of cellular metabolism and whole-body energy balance, AMPK. We found that HFD decreased the activity of both isoforms of AMPK in almost all tissues studied. This widespread reduction of AMPK activity by HFD suggests that AMPK signaling is likely an important aspect of the pathophysiological effects of HFD. While the largest HFD-induced reduction in AMPK activity that we observed was 44 %, evidence is found in several studies that AMPK activity reductions of ≤50 % can be associated with pathologies. For example, a 35 % decrease in WAT AMPK activity was seen in obese, insulin-resistant patients [13], and a 50 % decrease in heart AMPK activity was seen in patients with chronic heart failure [52].
There are several possible mechanisms by which HFD might cause the observed decrease of AMPK activity. The simplest explanation is that while providing tissues with inadequate amounts of metabolic substrates, such as glucose and FFAs, is well known to increase AMPK activity, the converse may be true, with an excess of circulating metabolic substrates decreasing AMPK activity [29, 45]. However, in the model we studied, neither circulating glucose nor the FFA levels were elevated. Thus, elevated circulating levels of metabolic substrates seem unlikely to be the cause of the HFD-induced reduction in AMPK activities. However, since a major regulator of AMPK activity is the ATP/AMP ratio, the possibility exists that the HFD, while not causing elevations in glucose and FFA levels, might inhibit AMPK activities by increasing this ratio above that in control-fed rats. This would demonstrate that under control conditions, AMPK activities are high enough to be inhibited by diet and, thus, challenges the perception that AMPK activity during “control” or “baseline” conditions is near its lowest possible level. This possibility of a HFD lowering AMPK via effects on intracellular energetics is difficult to evaluate since little, if anything, is known regarding how a HFD affects the variables central to intracellular energetics, such as [ATP], [AMP], [AMP], [Pi], and [PCr].
Another possible mechanism for the observed decrease in AMPK activities with HFD is a decrease in the transcription of the catalytic subunits of AMPK, such as has been described in some HFD settings [5, 27, 33]. This possibility was assessed by measuring the transcript levels for the α1 and α2 subunits of AMPK in the WAT, BAT, the liver, and the heart. With the exception of the heart, the transcript levels of the α1 and α2 subunits were not affected by HFD (Fig. 6), indicating that the downregulation of the transcript levels was not the primary mechanism underlying the HFD-induced decrease in AMPK.
AMPK activity has been reported to be modulated by the cytokine IL-6 [5, 26, 43, 60], and HFDs are often associated with elevated levels of circulating inflammatory cytokines [7, 28, 30, 40]. To determined whether the HFD-induced decrease in AMPK activity was associated with altered levels of inflammatory cytokines, we measured the serum levels of two well-established markers of systemic inflammation, TNFα and IL-6 [1, 50]. We found that in our model of HFD, neither cytokine was elevated above the non-detectable levels present in control rats. While the extent to which HFD elevates inflammatory markers is highly model-dependent, our results are consistent with other studies that show that TNFα and IL-6 are not elevated by HFD [4, 36] and allow us to conclude that changes in these inflammatory markers are not obligatory for the HFD-induced decreases in AMPK activities. Furthermore, it suggests that HFD-induced decreases in AMPK do not result in the immediate elevation of these markers of systemic inflammation.
Another possible cause of the HFD-induced decreases in AMPK activities relates to the observation that leptin is a known modulator of AMPK activity [53, 58]. However, leptin is thought to be a stimulator of AMPK activity in peripheral tissues [9, 37], and we found that a high leptin level in the HFD group was associated with a low AMPK activity across the different tissues. This lack of a positive correlation between circulating leptin levels and AMPK activities was also reported by our group in the context of a calorie-restricted diet and fasting, both of which decreased the leptin levels (to as low as 10 % of the control) without altering tissue AMPK activities [16]. In the present study, our finding of a decrease in AMPK activity occurring simultaneously with hyper-leptinemia may be related to tissue leptin resistance, such as occurs in models of HFD [32, 46]. It is possible that HFD-induced tissue leptin resistance results in the impairment of intracellular leptin signaling, leading to a reduced activation of AMPK.
Regardless of the cause(s) of the HFD-induced decreases in AMPK activity, important questions remain about the effect of this lowered AMPK activity. For example, what role, if any, does the decreased AMPK activity play in systemic insulin resistance? Previous reports have indicated that the insulin-sensitizing drugs metformin and TZDs function, in part, via the activation of AMPK [31, 55]. Further establishing a link between insulin sensitivity and AMPK activity are studies demonstrating that the AMPK activity is decreased in tissues of diabetic and non-diabetic animal models of insulin resistance [5, 26, 43, 60]. Few reports have examined whether a reduced AMPK activity is a causative agent in the development of insulin resistance; whole-body α2 AMPK knockout mice showed mild insulin resistance, while whole-body α1 AMPK knockout mice did not [24], and the expression of a muscle-specific, inactive form of α2 AMPK exacerbated HFD-induced insulin resistance in mice [11]. It is tempting to speculate that the HFD-induced reductions of AMPK activities in our study contributed to the observed elevation of plasma insulin. If so, it would appear that AMPK activities were not sufficiently low to lead to hyper-glycemia or hyperlipidemia.
While AMPK has been extensively studied in solid tissues such as the liver and the skeletal muscle, very little is known about AMPK in blood and its cellular components. It has been demonstrated that AMPK is important in white blood cells during inflammation [44, 59]. We found that essentially all (>97 %) AMPK activity in the blood is located in agranulocytes, with the vast majority of this AMPK activity being attributable to the α1 isoform. Since the agranulocyte fraction includes both monocytes and lymphocytes, it is reasonable to infer that AMPK is an important signaling molecule in one, or both, of these cell types (or one of the subsets of lymphocytes).
In contrast to what was observed in WAT, the liver, and the heart, the activity of α1 AMPK was not altered by HFD in agranulocytes. It is important to note that there were no obvious signs of inflammation in our model; plasma FFA was not significantly increased by HFD (Fig. 5), and the plasma levels of the inflammatory cytokines IL-6 and TNFα were not elevated. Thus, while AMPK may be important in the functions of some types of white blood cells in the setting of HFD, activity is not altered, at least in the absence of overt inflammation.
In the present study, we have provided evidence that a HFD caused a marked reduction of AMPK activity (without altered transcript levels) across several tissues. This was associated with an obese and hyperinsulinemic “pre-diabetic” state. While a cause-and-effect relationship between the AMPK activity and the observed pre-diabetic state remains unsolved, the data presented here do shed light on the relationship of AMPK with other aspects seen in the metabolic syndrome. Importantly, our data suggest that hyperglycemia, hyperlipidemia, and systemic inflammation are not prerequisites for the reduction of AMPK activity in HFD-fed rats. Whether this finding holds true for other HFD models, or in humans, remains to be investigated.
Acknowledgments
These studies were supported by the National Institute of Health grants R01DK080345, R01DK080345-02S1, and T32HL007936.
Footnotes
Disclosures: None.
Kurt W. Saupe deceased.
Contributor Information
Christopher R. Lindholm, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
Rebecca L. Ertel, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
Jake D. Bauwens, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
Eric G. Schmuck, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
Jacob D. Mulligan, Email: jdm@medicine.wisc.edu, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
Kurt W. Saupe, Email: kws@medicine.wisc.edu, Department of Medicine, Division of Cardiovascular, Medicine, University of Wisconsin, 1630 Medical Sciences Center, 1300 University Ave., Madison, WI 53706, USA
References
- 1.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: the role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 2.Axelsen LN, Lademann JB, Petersen JS, Holstein-Rathlou NH, Ploug T, Prats C, Pedersen HD, Kjolbye AL. Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocar-dial lipid accumulation. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1560–1570. doi: 10.1152/ajpregu.00392.2009. [DOI] [PubMed] [Google Scholar]
- 3.Barroso E, Rodriguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, Vazquez-Carrera M. The PPARbeta/delta activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1alpha-Lipin 1-PPARalpha pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152(5):1848–1859. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- 4.Bedoui S, Velkoska E, Bozinovski S, Jones JE, Anderson GP, Morris MJ. Unaltered TNF-alpha production by macrophages and monocytes in diet-induced obesity in the rat. J Inflamm (Lond) 2005;2(1):2. doi: 10.1186/1476-9255-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34(1):52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano P, Cardinali DP, Rios-Lugo MJ, Fernandez-Mateos MP, Reyes Toso CF, Esquifino AI. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 2009;17(10):1866–1871. doi: 10.1038/oby.2009.200. [DOI] [PubMed] [Google Scholar]
- 8.Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature's energy sensor. Nat Chem Biol. 2011;7(8):512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- 9.Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574(Pt 1):55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804(3):581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008;57(11):2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298 (4):C961–971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier MS, O'Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, Gokce N, Apovian C, Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404(1):382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giugliano D, Ceriello A, Esposito K. Are there specific treatments for the metabolic syndrome? Am J Clin Nutr. 2008;87(1):8–11. doi: 10.1093/ajcn/87.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Perez Y, Capllonch-Amer G, Gianotti M, Llado I, Proenza AM. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutr Metab (Lond) 2012;9:15. doi: 10.1186/1743-7075-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287(5):E1032–1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 17.Goseki-Sone M, Maruyama R, Sogabe N, Hosoi T. Effects of dietary lactose on long-term high-fat-diet-induced obesity in rats. Obesity (Silver Spring) 2007;15(11):2605–2613. doi: 10.1038/oby.2007.312. [DOI] [PubMed] [Google Scholar]
- 18.Ha SK, Kim J, Chae C. Role of AMP-activated protein kinase and adiponectin during development of hepatic steatosis in high-fat diet-induced obesity in rats. J Comp Pathol. 2011;145(1):88–94. doi: 10.1016/j.jcpa.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93(4):891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546(1):113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 22.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23(2):270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 23.Hegarty BD, Turner N, Cooney GJ, Kraegen EW. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf) 2009;196(1):129–145. doi: 10.1111/j.1748-1716.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5’-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279 (2):1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 25.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320(2):449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Jung JY, Kim HW, Park T. Anti-obesity effects of Juniperus chinensis extract are associated with increased AMP-activated protein kinase expression and phosphorylation in the visceral adipose tissue of rats. Biol Pharm Bull. 2008;31(7):1415–1421. doi: 10.1248/bpb.31.1415. [DOI] [PubMed] [Google Scholar]
- 28.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58(11):2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290(3):E471–479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44(2):87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 32.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6 J mice. Int J Obes Relat Metab Disord. 2000;24 (5):639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339 (2):701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 34.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281(28):18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 36.Meksawan K, Venkatraman JT, Awad AB, Pendergast DR. Effect of dietary fat intake and exercise on inflammatory mediators of the immune system in sedentary men and women. J Am Coll Nutr. 2004;23(4):331–340. doi: 10.1080/07315724.2004.10719376. [DOI] [PubMed] [Google Scholar]
- 37.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 38.Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging elevates basal AMPK activity and eliminates hypoxic activation of AMPK in mouse liver. J Ger-ontol A Biol Sci Med Sci. 2005;60(1):21–27. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Panchal SK, Brown L Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumor-igenesis by enhancing IL-6 and TNF expression. Cell. 2010;140 (2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas J, Arraiz N, Aguirre M, Velasco M, Bermudez V. AMPK as target for intervention in childhood and adolescent obesity. J Obes. 2011;2011:252817. doi: 10.1155/2011/252817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55(Suppl 2):S48–54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 44.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59(10):2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R493–500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15(4):373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg GR. Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle. 2007;6(8):888–894. doi: 10.4161/cc.6.8.4135. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008;295(5):E1076–1083. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- 50.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3(2):185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 53.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45 (4):276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO. Obesity and overweight WHO Fact Sheet N311. 2011 Updated March 2011. [Google Scholar]
- 55.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116 (8):607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133(4):1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 57.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112 (12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574(Pt 1):73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285(25):19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47(11):2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 61.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinforma. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]