Abstract
Von Hippel-Lindau (VHL) syndrome is an inherited cancer syndrome in which 8–17 % of germline mutation carriers develop pancreatic neuroendocrine tumors (PNETs). There is limited data on prognostic markers for PNETs other than Ki-67, which is included in the World Health Organization classification system. Recently, specific genes and pathways have been identified by whole exome sequencing which may be involved in the tumorigenesis of PNETs and may be markers of disease aggressiveness. The objective of this study was to identify molecular markers of aggressive disease in VHL-associated PNETs. The protein expression of eight genes (PTEN, CHGA, CHGB, ATRX, DAXX, CC-3, VEGF, and TP53) was analyzed in PNETs by immunohistochemistry and compared to clinical data, VHL genotype, functional imaging results, and pathologic findings. Subcellular distribution of phosphatase and tensin (PTEN), chromogranin A (CHGA), and alpha thalassemia/mental retardation syndrome X-linked (ATRX) were significantly different by WHO classifications (p ≤ 0.05). There was decreased PTEN nuclear to cytoplasmic ratio (p < 0.01) and decreased CHGA nuclear expression (p = 0.03) in malignant samples as compared to benign. Lower cytoplasmic chromogranin B (CHGB) expression (p = 0.03) was associated with malignant tumors and metastasis. Higher nuclear expression of PTEN was associated with VHL mutations in exon 3 (p = 0.04). Higher PTEN and CHGB expression was associated with higher FDG-PET avidity (p < 0.05). Cytoplasmic expression of CC-3 was associated with higher serum chromogranin A levels (ρ = 0.72, p = 0.02). Lastly, greater cytoplasmic expression of p53 was associated with metastasis. Our findings suggest that altered PTEN, ATRX, CHGA, and CHGB expression are associated with aggressive PNET phenotype in VHL and may serve as useful adjunct prognostic markers to Ki-67 in PNETs.
Keywords: Vascular Endothelial Growth Factor, Cytoplasmic Expression, Pancreatic Neuroendocrine Tumor, Positive Immunostaining, Inherit Cancer Syndrome
Introduction
Von Hippel-Lindau (VHL) syndrome is an inherited cancer syndrome due to a germline inactivating mutation in the VHL tumor suppressor gene. Patients with VHL have a high risk of developing multiple neoplasms, including an 8–17 % risk of developing pancreatic neuroendocrine tumors (PNETs). These tumors may be benign or malignant, and can be synchronous or metachronous [1, 2].
Several prognostic classification systems (World Health Organization (WHO), European Neuroendocrine Tumor Society (ENETS), and American Joint Committee on Cancer (AJCC)) for PNETs have been developed [3–5]. While these classification systems have been validated as a risk stratification tool [5–9] there has been little additional prognostic information available for PNETs and currently, only Ki-67 has been used as a tissue marker [10].
A recent study addressed this gap in PNET prognostication and sought to identify the most frequently mutated genes in sporadic PNETs. The investigators used exome sequencing in ten sporadic PNETs, with additional screening for the most common mutations found in an additional 58 samples [10]. Jiao and colleagues identified the presence of somatic mutations in MEN1 (44.1 %), DAXX (25 %), ATRX (17.6 %), and PTEN (7.3 %). Furthermore, the presence of somatic mutations in MEN1 and/or DAXX/ATRX was associated with better survival as compared to patients with PNETs which had wild-type MEN1 and/or DAXX/ATRX [10].
Several candidate markers have also been associated with disease aggressiveness. The expression of CHGA and CHGB varies among different types of neuroendocrine tumors (NETs). In general, greater expression of CHGA is seen in well-differentiated as opposed to poorly differentiated NETs [11]. Increased CHGB expression is associated with a favorable prognosis in patients with sporadic medullary thyroid cancer, but inversely in patients with prostate cancer [11]. Higher expression of the apoptosis-related protein, cleaved caspase-3 (CC-3), has been associated with prognosis in PNETs with greater expression in benign PNETs as compared to malignant sporadic PNETs [12]. The expression of vascular endothelial growth factor (VEGF) has been associated with tumor aggressiveness and patient prognosis in a variety of human malignancies [13]. Lastly, altered p53 expression is frequently seen in many tumors, with tumor suppressor protein-53 (TP53) cited as the most frequently mutated gene associated with malignancy [14]. Yachida et al. recently described abnormal immunolabeling of p53 in nine of 19 poorly differentiated PNETs, but 0 of 11 well-differentiated PNETs [15].
To our knowledge, the protein expression of these genes has not been examined in the context of VHL-associated PNETs and the utility of the WHO, ENET, and AJCC prognostic scoring systems in VHL-associated PNETs is not known. Thus, the aim of this study was to determine if any of the genes were associated with PNET phenotype using immunohistochemistry analysis for phosphatase and tensin (PTEN), alpha thalassemia/mental retardation syndrome X-linked (ATRX), death-associated protein 6 (DAXX), chromogranin A (CHGA), chromogranin B (CHGB), CC-3, VEGF, and p53.
Materials and Methods
Patients and Tissue Samples
Patients with VHL syndrome who had a pancreatic manifestation of their disease were enrolled in a prospective protocol approved by the Office of Human Subject Research at the National Institutes of Health (NIH). All participants gave written informed consent. For all patients who met criteria for an operation, a portion of the surgical specimen was snap-frozen at the time of surgery and stored at −80 °C. A section of each specimen was fixed in formalin and embedded in paraffin. These samples included well-differentiated neuroendocrine tumors with benign characteristics (n = 4), well-differentiated neuroendocrine tumors with uncertain characteristics (n = 7), neuroendocrine carcinoma (n = 4), microcystic serous cystadenoma (n = 4), and normal pancreas (n = 4). Demographic, clinical, radiologic, and pathology data were collected and are summarized in Table 1.
Table 1.
Clinical characteristics of the study cohort
| Clinical feature | Number |
|---|---|
| Number of patients | 21 |
| Sex | |
| Male | 8 |
| Female | 13 |
| Age, years | 48.5 ± 13.4 |
| Range | 19 to 70 |
| Pathology diagnosis | |
| Normal islet cells | 4 |
| Microcystic serous cystadenoma | 4 |
| PNET, well-differentiated, benign | 4 |
| PNET, well-differentiated, uncertain | 7 |
| PNET, neuroendocrine carcinoma | 4 |
| FDG-PET avid | |
| Yes | 10 |
| No | 11 |
| Serum chromogranin A (reference normal <93 ng/ml) | |
| Mean | 212 |
| Range | 98–1,105 |
Immunohistochemistry
Immunohistochemistry was performed using Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, California) according to the manufacturer’s recommendations. Five-micron sections of tissue samples were de-paraffinized for 30 min in xylene then rehydrated first for 30 min in 100 % ethanol, followed by a 5-min rinse each in 95, 70, and 50 % ethanol. Finally, tissue sections were washed for 5 min in de-ionized water. Rehydrated sections underwent a two-step antigen retrieval process, first incubated for 30 min at room temperature in 0.1 % saponin (Calbiochem, Darmstadt, Germany), and then in 1× pH 6.0 citrate buffer (Thermo Scientific, Fremont, California) for 15 min at 100 °C. Tissue sections were incubated for 20 h with respective primary antibodies: PTEN rabbit monoclonal primary antibody against the carboxy-terminal sequence at a 1:100 dilution (#9559, Cell Signaling Technology, Inc., Danvers, Massachusetts); ATRX rabbit polyclonal primary antibody against amino acid sequence 2161–2413 at a 1:100 dilution (#S1800, Epitomics, Inc., Burlingame, California); DAXX rabbit polyclonal primary antibody against amino acid sequence 261–274 at a dilution of 0.9 μg/mL (#NBP1-03157, Novus Biologicals, Littleton, CO); CHGA rabbit polyclonal primary antibody against a site within amino acid sequence 250–457 of P10645 at a 1:2,200 dilution (ab15160, Abcam Inc, Cambridge, Massachusetts); CHGB rabbit polyclonal primary antibody against 15 amino acids in the C-terminal at a 1:2,500 dilution (ab12242, Abcam Inc, Cambridge, Massachusetts); CC-3 rabbit polyclonal primary antibody against a 17/19-kDa fragment resulting from cleavage adjacent to Asp175 at a 1:1,400 dilution (#9661, Cell Signaling Technology, Inc., Danvers, Massachusetts); VEGF rabbit polyclonal primary antibody against isoforms 121, 145, 165, 189, and 206 at a 2.5 μL/mL dilution (ab46154, Abcam Inc, Cambridge, Massachusetts); and p53 mouse monoclonal primary antibody against a site located between the N-terminal amino acids 1–45 at a 1:25 dilution (M7001, Dako North America, Inc., Carpinteria, California). DAB peroxidase substrate kit (Vector Laboratories, Inc.) was used for staining prior to dehydration of the tissue samples. Bronchial epithelial cells were used as a positive control for PTEN staining, gallbladder mucosa was used as a positive control for ATRX, CC-3, and p53 staining; normal pancreas acinar cells were used as a positive control for DAXX and normal pancreatic islet cells were used as a positive control for CHGA, CHGB, and VEGF. Negative control tissue sections from each tumor sample were incubated for 20 h with rabbit serum, rather than primary antibody.
Analyses
Each sample was scored by two independent evaluators for both nuclear and cytoplasmic intensity and percent of cells positive. A semi-quantitative scale was used. Intensity was scored as none (1), faint (2), moderate (3), or strong (4). Percent of positive cells was categorized as <33 % (1), between 33 and 66 % (2) and >66 % (3). Independent scores were evaluated using Spearman’s correlation test prior to averaging for further analysis, with ρ > 0.7 for all categories. The average nuclear intensity and nuclear distribution score for each sample were multiplied to create a total nuclear score [16]. This was similarly performed to calculate a cytoplasmic score. Scores for nuclear and cytoplasmic expression of PTEN immunostaining as well as nuclear-to-cytoplasmic ratio were compared within each pathologic diagnosis. Grubb’s outlier analysis was performed of each group, with significant outliers, if present, discarded prior to determining the average score for each group. Average nuclear scores, cytoplasmic scores, and nuclear-to-cytoplasmic ratios were compared among each histopathologic diagnosis and to patient clinical characteristics.
Results
Phosphatase and Tensin Homolog
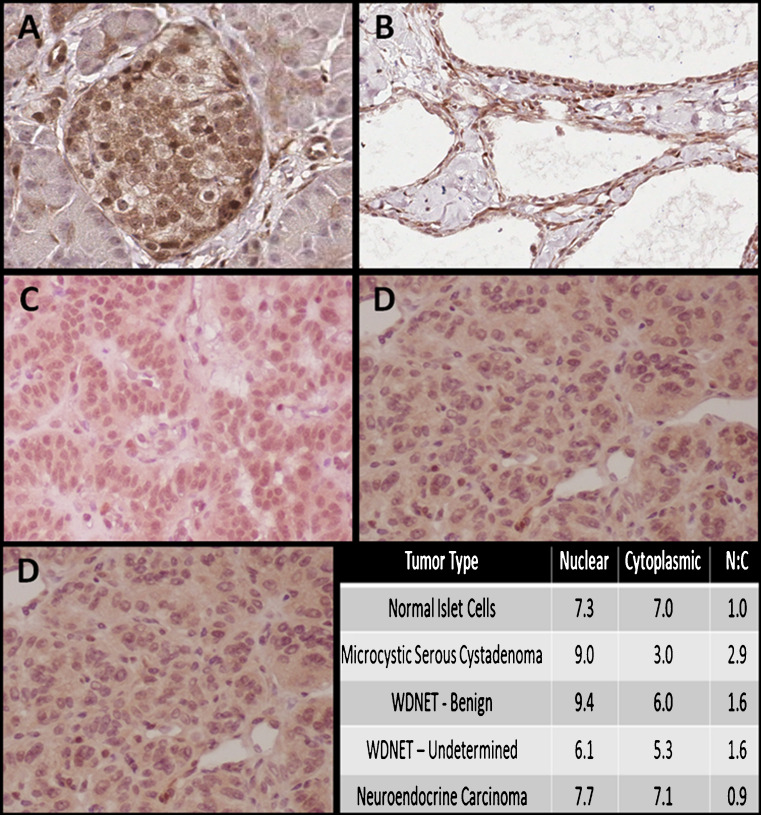
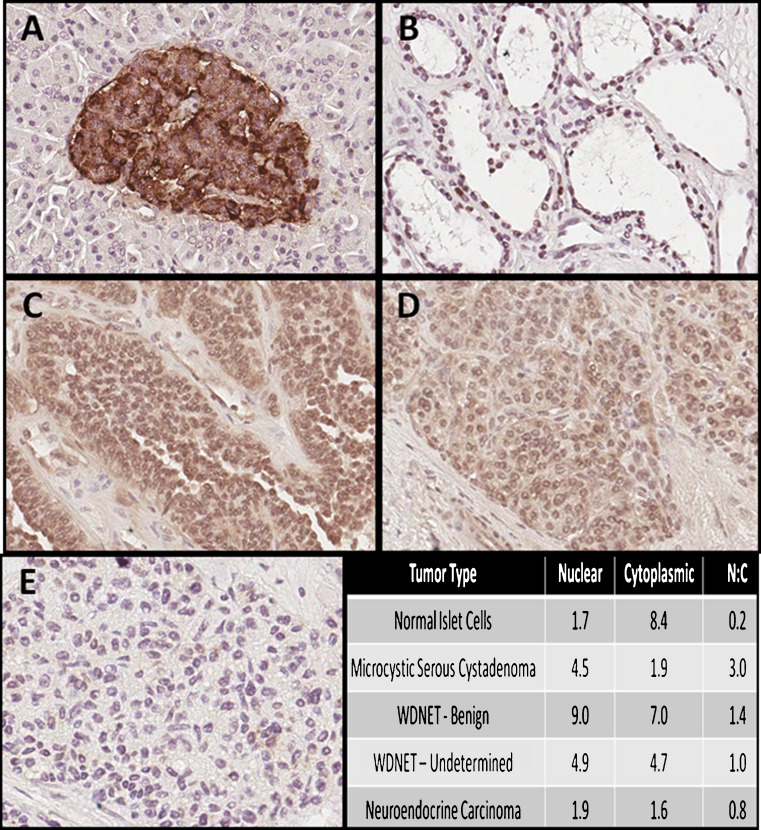
All tissue samples showed positive immunostaining after incubation with anti-PTEN protein antibody. Inter-reader correlation was high (ρ = 0.7060, p < 0.01). Representative images are shown in Fig. 1. Both cytoplasmic expression of PTEN and nuclear-to-cytoplasmic (N/C) ratio of PTEN expression was significantly different by histopathologic diagnostic groups (p < 0.05 and p < 0.02, respectively). The N/C ratio was lowest for carcinomas (p = 0.02).
Fig. 1.
Representative PTEN expression by immunohistochemistry. a Normal islet cells (magnified ×20), b microcystic serous cystadenoma (magnified ×20), c well-differentiated neuroendocrine tumor, benign characteristics (magnified ×20), d well-differentiated neuroendocrine tumor, undetermined characteristics (magnified ×20), e neuroendocrine carcinoma (magnified ×20); bottom right immunohistochemistry scoring averages by diagnosis
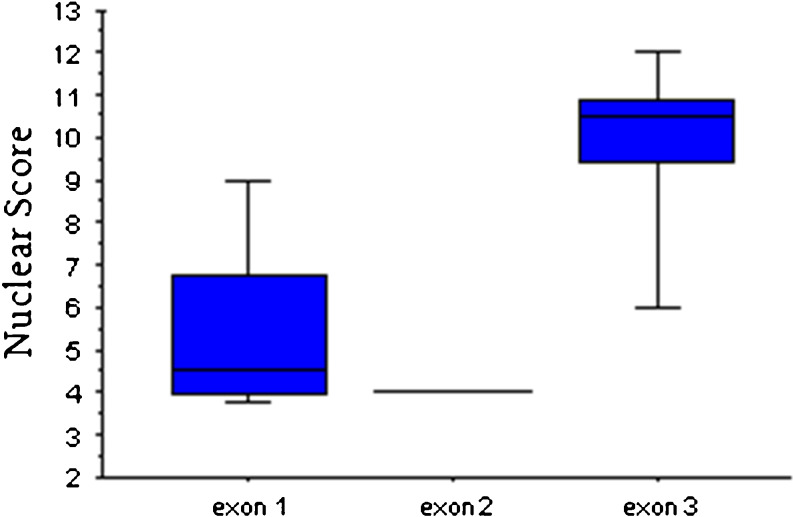
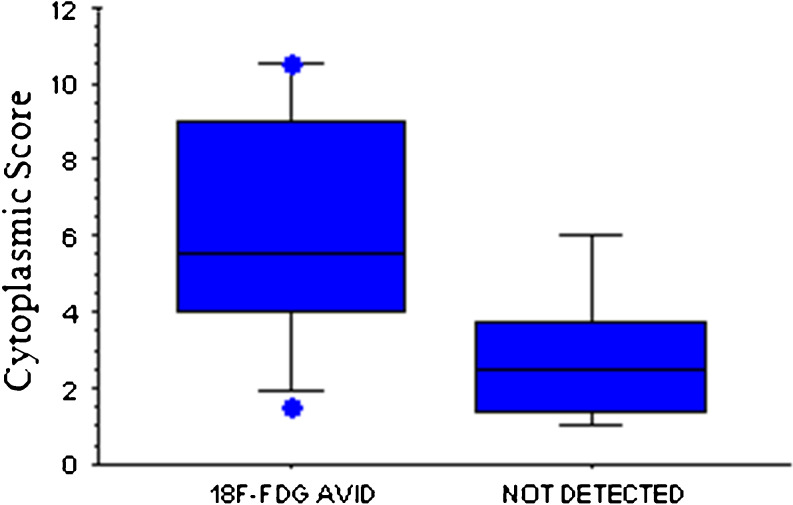
We observed no significant difference in PTEN immunostaining by patient age, gender, Ki-67 index, 6-L-18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET avidity or SUV value. However, patients with a VHL mutation in exon 3 had significantly higher nuclear expression of PTEN compared with patients with mutations in exon 1 or 2 (p = 0.04, Fig. 2). PTEN cytoplasmic expression was higher in patients with F-18-deoxyglucose positron emission tomography (18F-FDG PET) avid tumors (p = 0.04, Fig. 3).
Fig. 2.
Level of PTEN nuclear expression by VHL mutation location. Box plot represents PTEN nuclear score. PTEN expression by immunohistochemistry was significantly different (p = 0.04) with only an 18 % overlap in PTEN expression level by exon mutation status
Fig. 3.
PTEN cytoplasmic expression by 18F-FDG-PET avidity. Box plot represents the PTEN cytoplasmic expression by the presence or absence of an 18F-FDG PET avid tumor on imaging. PTEN expression was significantly different (p = 0.04) by 18F-FDG PET avidity status
Alpha Thalassemia/Mental Retardation Syndrome X-Linked
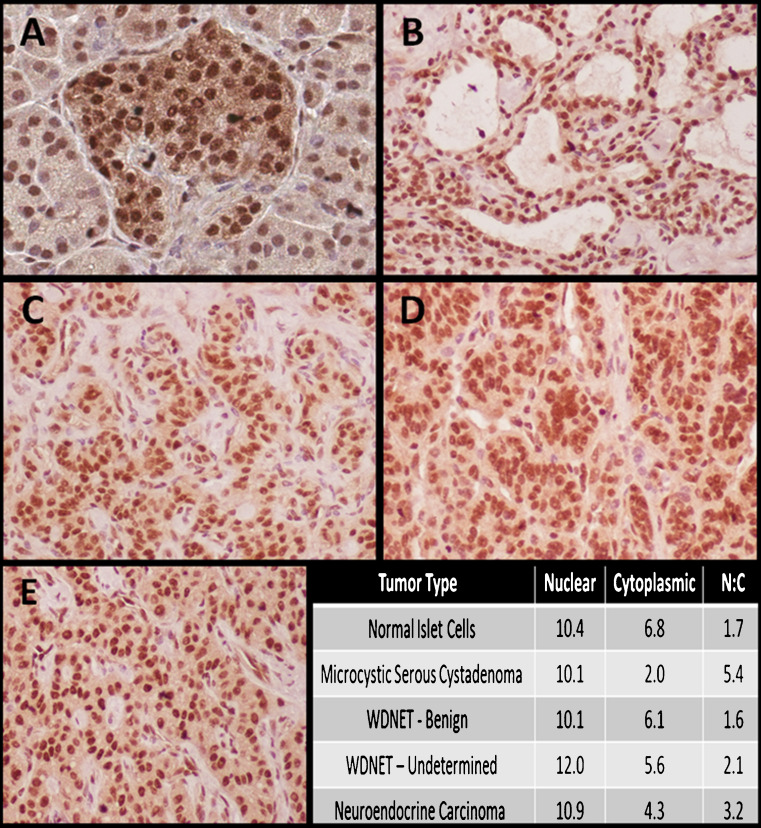
All tissue samples showed positive immunostaining for ATRX. Inter-reader correlation was high (ρ = 0.84, p < 0.01). Representative images are shown in Fig. 4. Within categories of PNETs, the N/C ratio was significantly higher by WHO histologic classification (p = 0.04). No additional statistically significant associations were identified by clinical, radiographic, or genotype data.
Fig. 4.
ATRX expression by immunohistochemistry in representative tissue samples. a Normal islet cells (magnified ×20), b microcystic serous cystadenoma (magnified ×20), c well-differentiated neuroendocrine tumor, benign characteristics (magnified ×20), d well-differentiated neuroendocrine tumor, undetermined characteristics (magnified ×20), e neuroendocrine carcinoma (magnified ×20); bottom right ATRX expression level by diagnosis
Death-Associated Protein 6
All tissue samples showed positive immunostaining for DAXX protein. Inter-reader correlation was high (ρ = 0.86, p < 0.01). We found no statistically significant difference in tumor DAXX protein expression by pathologic diagnosis, clinical data, VHL exon mutation, and functional imaging results.
Chromogranin A
Seventeen of 20 (85 %) tumor tissue samples showed positive immunostaining for CHGA, with one of four MCA samples and two of four malignant samples showing no evidence of staining. Inter-reader correlation was high (ρ = 0.80, p < 0.01). Representative images are shown in Fig. 5. As tumor grade increased, nuclear expression of CHGA decreased (p = 0.03). Additionally, primary tumors from patients with evidence of metastatic disease demonstrated significantly less CHGA protein expression than tumors in patients without metastases (p = 0.03). There was no significant association between serum Chromogranin A values and tumor CHGA immunostaining.
Fig. 5.
CHGA expression by immunohistochemistry in representative tissue samples. a Normal islet cells (magnified ×20), b microcystic serous cystadenoma (magnified ×20), c well-differentiated neuroendocrine tumor, benign characteristics (magnified ×20), d well-differentiated neuroendocrine tumor, undetermined characteristics (magnified ×20), e neuroendocrine carcinoma (magnified ×20); bottom right CHGA expression level by diagnosis
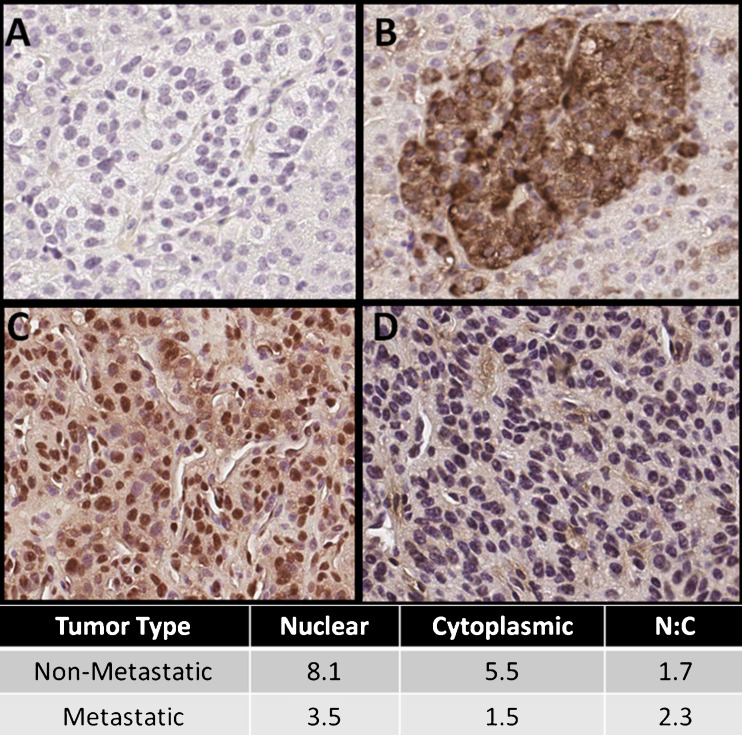
Chromogranin B
All tissue samples, except one malignant tumor, showed positive immunostaining for CHGB. Inter-reader correlation was high (ρ = 0.83, p < 0.01). Representative images are shown in Fig. 6. Primary tumor samples from patients with metastatic disease had less cytoplasmic expression than tissue samples from patients without metastatic disease (p = 0.03). Additionally, if a cytoplasmic outlier sample was excluded (outlier score = 7.5, remaining average score = 1.5), WHO grade III tumors had lower cytoplasmic expression than MCAs and WHO grade I and II tumors.
Fig. 6.
CHGB expression by immunohistochemistry in representative tissue samples. a Negative control (magnified ×20), b normal islet cells, in patient with VHL (magnified ×20), c non-metastatic tumor (magnified ×20), d metastatic tumor (magnified ×20), bottom right CHGB expression level by diagnosis
Cleaved Caspase-3
All tissue samples showed positive immunostaining for CC-3 protein. Inter-reader correlation was high (ρ = 0.73, p < 0.01). There was a significant correlation between cytoplasmic expression of CC-3 and serum values of chromogranin A (ρ = 0.72, p = 0.02).
Vascular Endothelial Growth Factor
All tissue samples showed positive immunostaining for VEGF protein. Inter-reader correlation was high (ρ = 0.74, p < 0.01). No outliers were identified but we found no statistically significant associations with pathologic diagnosis, clinical data, VHL exon mutation, and functional imaging results.
Tumor Suppressor Protein-53
Nineteen of 20 (95 %) tumor tissue samples showed positive immunostaining for p53. Inter-reader correlation was high (ρ = 0.83, p < 0.01). Primary tumor samples from patients with metastatic disease had greater cytoplasmic expression than tissue samples from patients without metastatic disease (p < 0.04). Presence of VHL mutations in exon 3 trended towards higher cytoplasmic expression of PTEN compared with patients with mutations in exon 1 or 2 (p = 0.09).
Discussion
In this study, we performed a comprehensive analysis of the protein expression of eight genes by immunohistochemistry in VHL-associated PNETs to determine if any of these genes may be associated with clinical characteristics and outcome, imaging, and genetic data. We found differences in subcellular compartment expression of PTEN, ATRX, CHGA, and CHGB in VHL-associated PNETs which were associated with more aggressive tumors.
PTEN is a tumor suppressor gene with a well-established role as a susceptibility gene for Cowden’s syndrome which has more recently been implicated in a variety of malignancies, including PNET. Jiao and associates [10] identified a mutation rate of 7 % in the PTEN gene in sporadic PNETs. Perren and colleagues [10, 17] identified loss of heterozygosity in PTEN in one-third of sporadic PNETs. In some, but not all studies, the level of PTEN expression was associated with a worse prognosis and poor response to chemotherapy in patients with PNETs [15, 18–20]. Several investigators have observed that the function of PTEN is dependent on subcellular localization throughout the cell cycle [21–23]. In the nucleus, PTEN maintains chromosome stability [24] and downregulates cyclin D1 with cell arrest, and thereby prevents activation of MAPK [23, 25]. On the other hand, cytoplasmic PTEN inhibits Akt, serving a pro-apoptotic function [23, 26]. Altered trafficking of PTEN has been described in other studies, but none have been in the context of VHL syndrome [17, 27]. In one study, no association was observed based on the subcellular localization of PTEN and clinical outcome [17]. In contrast, Missiaglia and colleagues [27] found that a low cytoplasmic expression of PTEN is associated with a shorter disease-free interval. In our study, we found that patients with VHL-associated PNETs with a higher tumor nuclear-to-cytoplasmic PTEN expression ratio were more likely to have advanced disease by the WHO histologic classification. Interplay of the mTOR/PTEN pathway and VHL has been suggested in a variety of cancers [28–31]. These studies thus support our findings regarding altered PTEN subcellular localization in aggressive PNETs and higher cytoplasmic expression in FDG avid PNETs.
There is growing data of the role of ATRX and DAXX in oncogenesis. For example, ATRX and DAXX have been shown to interact with promyelocytic leukemia nuclear bodies (PML-NBs) and play a role in chromatin remodeling during S-phase [15, 32–37]. Recently, Heaphy and colleagues [38] suggested a mechanism by which a loss of either ATRX or DAXX protein expression may occur as a result of altered telomere length in sporadic PNETs. Other investigators have also shown that ATRX regulates DNA methylation [39–41] and RNA-mediated DNA methylation [42]. In the current study, all tissue samples had strong nuclear staining for ATRX and DAXX. However, altered N/C ATRX staining was observed with increased ratios in more aggressive tumors. To our knowledge, cytoplasmic localization of ATRX has only been described once in the literature, identified by immunofluorescence studies, and isolated to metaphase and anaphase of mitotic cells [43]. Typically, ATRX is described to dissociate with DAXX after chromatin remodeling occurs and then re-locates to the cytoplasm where it interacts with FAS ligand [36, 44]. The presence of cytoplasmic staining in our study may be due to more aggressive tumors having higher number of cells in metaphase and anaphase.
Chromogranins are a family of glycoproteins found in secretory vesicles within neuroendocrine cells. Chromogranin A is commonly used as a biomarker for neuroendocrine tumors: tissue CHGA is helpful for confirming a diagnosis of neuroendocrine cell type, whereas serum CHGA levels have been shown to correlate to patient tumor burden and may be helpful for patient surveillance [11, 45]. Chromogranin B has not been widely studied and does not have a defined clinical application presently. Several immunohistochemistry studies have shown varied results, with high expression related to a better prognosis in sporadic medullary thyroid cancer, worse prognosis in prostate cancer, and mixed results in pheochromocytomas [46–49]. This variety may be accounted for by the unique post-translational processing of the chromogranin family in different neuroendocrine tissues creating a different expression profile of epitopes [50]. Chromogranin’s post-translational processing may even vary within tissue, as demonstrated in different expression patterns of different pancreatic islet cell types within normal pancreatic tissue [51].
Immunostaining within our cohort of patients demonstrated that higher tumor grade was associated with lower nuclear expression of CHGA (p = 0.03), with no significant difference in levels of cytoplasmic expression. Nuclear expression of CHGA has only been reported in one prior publication. The significance of this difference in subcellular localization is unclear [52]. CHGB immunostaining showed less cytoplasmic glycoprotein expression in primary tumors of patients with metastatic disease than in tissue samples from patients without metastatic disease (p = 0.03). This finding is consistent with CHGB’s cytoplasmic role of calcium ion regulation, which affects both cellular proliferation and cell death [53, 54].
The most frequently mutated gene in human tumors is TP53, which encodes the p53 protein [14]. Usually, p53 expression is low in both the nucleus and cytoplasm of cells [55–59]. However, if a cell’s DNA is damaged, p53 expression is increased and functions as a stress-responsive transcription factor [55, 56]. Roe et al. [60] studied the interaction of pVHL and p53, identifying that the α-domain of pVHL directly associates with p53 to strengthen p53’s transcriptional activity in the presence of DNA damage and to activate the p53 apoptotic signaling pathway by nucleating an ATM kinase. In VHL-related renal cell carcinomas, the alteration of the p53 regulatory pathway has been associated with a phenotype of high resistance to radiation and chemotherapy [61, 62]. Point mutations of p53 could also be a potential prognostic variable but this was not analyzed in our study.
In our cohort, cytoplasmic staining was significantly higher (p < 0.05) in tumor samples taken from patients with metastatic disease, suggesting that in these samples p53 is sequestered in the cytoplasm, preventing it from engaging in its DNA-stabilizing function. Similar staining has been described in breast cancer and lung cancer [63–65]. Yachida et al. [15] identified a loss of nuclear staining, not cytoplasmic staining, in poorly differentiated PNETs. This correlated to intragenic mutations in the TP53 gene as identified by sequencing analysis. The immunostaining difference seen in our cohort may be attributed to the interaction between pVHL and p53.
An interesting finding in our analysis was that VHL mutations located in exon 3 were associated with higher nuclear expression of PTEN. Indeed, mutations in exon 3 of the VHL gene have been associated with aggressive PNETs [66]. In our series, microcystic serious cystadenomas and WHO grade I (well-differentiated, benign features) PNETs had the highest nuclear expression, while WHO grade III had the lowest nuclear expression.
Another interesting finding of our study was the association between cytoplasmic expression of CC-3 and serum values of CHGA (ρ = 0.7190, p = 0.02). A similar relationship has been reported in prostate cancer cell lines in which serum CHGA expression created a dose- and time-dependent cell-growth inhibition as measured by the number of apoptotic cells and CC-3 expression [67]. This suggests that serum CHGA may have a naturally intended biologic negative feedback roll on cell proliferation.
There are several limitations in our analysis. One is the number of samples analyzed does not allow us to evaluate the eight genes as possible markers of disease-free survival and overall survival but this represents a relatively large study for VHL-associated PNETs. Although, immunohistochemistry is semiquantitative and may not detect relatively small differences in the expression level of the eight genes studied, we wanted to use a standard technique which could be used in biopsy samples or histologic evaluation of tumors. This could allow the application of the markers, once validated, associated with aggressive PNETs to stratify which patients have aggressive tumor and would warrant resection. However, it will be important to validate our findings in a larger study cohort to make sure the significant statistical associations observed are not due to a limited sample set for this rare tumor.
This study examined the subcellular compartmentalization of eight proteins to identify whether any provided prognostic value. Our results suggest this is true for several markers. A lower PTEN nuclear-to-cytoplasmic ratio, a higher ATRX nuclear to cytoplasmic ratio, a decreased expression of nuclear CHGA, a lower cytoplasmic expression of CHGB, and a greater cytoplasmic expression of TP53 are associated with aggressive VHL-associated PNETs. Furthermore, as each of these protein levels is independent of Ki-67 expression, these markers may act as an adjunctive marker for tumor grading.
Acknowledgments
This research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Kim JJ, Rini BI, Hansel DE. Von Hippel Lindau syndrome. Adv Exp Med Biol. 2010;685:228–249. doi: 10.1007/978-1-4419-6448-9_22. [DOI] [PubMed] [Google Scholar]
- 2.Shehata BM, Stockwell CA, Castellano-Sanchez AA, Setzer S, Schmotzer CL, Robinson H. Von Hippel-Lindau (VHL) disease: an update on the clinico-pathologic and genetic aspects. Adv Anat Pathol. 2008;15(3):165–171. doi: 10.1097/PAP.0b013e31816f852e. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 4.Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1–S16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt AM, Anlauf M, Rousson V, Schmid S, Kofler A, Riniker F, et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol. 2007;31(11):1677–1682. doi: 10.1097/PAS.0b013e31805f675d. [DOI] [PubMed] [Google Scholar]
- 7.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95(5):627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa S, Klersy C, Uccella S, Dainese L, Albarello L, Sonzogni A, et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40(1):30–40. doi: 10.1016/j.humpath.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23(6):824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 10.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. Granins and granin-related peptides in neuroendocrine tumours. Regul Pept. 2010;165(1):12–20. doi: 10.1016/j.regpep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Tomita T. Cleaved caspase-3 immunocytochemical staining for pancreatic islets and pancreatic endocrine tumors: a potential marker for biological malignancy. Islets. 2010;2(2):82–88. doi: 10.4161/isl.2.2.10807. [DOI] [PubMed] [Google Scholar]
- 13.Kajdaniuk D, Marek B, Foltyn W, Kos-Kudla B. Vascular endothelial growth factor (VEGF)—part 2: in endocrinology and oncology. Endokrynol Pol. 2011;62(5):456–464. [PubMed] [Google Scholar]
- 14.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1(3):233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 15.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36(2):173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kebebew E, Peng M, Treseler PA, Clark OH, Duh QY, Ginzinger D, et al. Id1 gene expression is up-regulated in hyperplastic and neoplastic thyroid tissue and regulates growth and differentiation in thyroid cancer cells. J Clin Endocrinol Metab. 2004;89(12):6105–6111. doi: 10.1210/jc.2004-1234. [DOI] [PubMed] [Google Scholar]
- 17.Perren A, Komminoth P, Saremaslani P, Matter C, Feurer S, Lees JA, et al. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am J Pathol. 2000;157(4):1097–1103. doi: 10.1016/S0002-9440(10)64624-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krausch M, Raffel A, Anlauf M, Schott M, Willenberg H, Lehwald N, et al. Loss of PTEN expression in neuroendocrine pancreatic tumors. Horm Metab Res. 2011;43(12):865–871. doi: 10.1055/s-0031-1291333. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Ignat A, Axiotis CA. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10(2):139–146. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 20.O’Toole D, Couvelard A, Rebours V, Zappa M, Hentic O, Hammel P, et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17(4):847–856. doi: 10.1677/ERC-09-0204. [DOI] [PubMed] [Google Scholar]
- 21.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27(41):5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 22.Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 2003;63(2):282–286. [PubMed] [Google Scholar]
- 23.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121(Pt 3):249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 24.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10(6):599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- 26.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65(18):8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 27.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28(2):245–255. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucejova B, Pena-Llopis S, Yamasaki T, Sivanand S, Tran TA, Alexander S, et al. Interplay between pVHL and mTORC1 pathways in clear-cell renal cell carcinoma. Mol Cancer Res. 2011;9(9):1255–1265. doi: 10.1158/1541-7786.MCR-11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Teichman A, Comperat E, Behnke S, Storz M, Moch H, Schraml P. VHL mutations and dysregulation of pVHL- and PTEN-controlled pathways in multilocular cystic renal cell carcinoma. Mod Pathol. 2011;24(4):571–578. doi: 10.1038/modpathol.2010.222. [DOI] [PubMed] [Google Scholar]
- 30.Petrella BL, Brinckerhoff CE. PTEN suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null renal-cell carcinoma. Cancer Biol Ther. 2009;8(14):1389–1401. doi: 10.4161/cbt.8.14.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschlager M. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659(3):284–292. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Ishov AM, Vladimirova OV, Maul GG. Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J Cell Sci. 2004;117(Pt 17):3807–3820. doi: 10.1242/jcs.01230. [DOI] [PubMed] [Google Scholar]
- 33.Lallemand-Breitenbach V, de The H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2(5):a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salomoni P, Betts-Henderson J. The role of PML in the nervous system. Mol Neurobiol. 2011;43(2):114–123. doi: 10.1007/s12035-010-8156-y. [DOI] [PubMed] [Google Scholar]
- 35.Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, et al. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci U S A. 2003;100(19):10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16(2):97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 37.de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol. 2012;9(4):199–208. doi: 10.1038/nrgastro.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kernohan KD, Jiang Y, Tremblay DC, Bonvissuto AC, Eubanks JH, Mann MR, et al. ATRX partners with cohesin and MeCP2 and contributes to developmental silencing of imprinted genes in the brain. Dev Cell. 2010;18(2):191–202. doi: 10.1016/j.devcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Iwase S, Xiang B, Ghosh S, Ren T, Lewis PW, Cochrane JC, et al. ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat Struct Mol Biol. 2011;18(7):769–776. doi: 10.1038/nsmb.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meehan RR, Stancheva I. DNA methylation and control of gene expression in vertebrate development. Essays Biochem. 2001;37:59–70. doi: 10.1042/bse0370059. [DOI] [PubMed] [Google Scholar]
- 42.Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet. 2005;6(1):24–35. doi: 10.1038/nrg1500. [DOI] [PubMed] [Google Scholar]
- 43.Berube NG, Smeenk CA, Picketts DJ. Cell cycle-dependent phosphorylation of the ATRX protein correlates with changes in nuclear matrix and chromatin association. Hum Mol Genet. 2000;9(4):539–547. doi: 10.1093/hmg/9.4.539. [DOI] [PubMed] [Google Scholar]
- 44.Khelifi AF, D’Alcontres MS, Salomoni P. Daxx is required for stress-induced cell death and JNK activation. Cell Death Differ. 2005;12(7):724–733. doi: 10.1038/sj.cdd.4401559. [DOI] [PubMed] [Google Scholar]
- 45.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17(9):2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 46.Scopsi L, Sampietro G, Boracchi P, Collini P. Argyrophilia and chromogranin A and B immunostaining in patients with sporadic medullary thyroid carcinoma. A critical appraisal of their prognostic utility. J Pathol. 1998;184(4):414–419. doi: 10.1002/(SICI)1096-9896(199804)184:4<414::AID-PATH1229>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 47.Pruneri G, Galli S, Rossi RS, Roncalli M, Coggi G, Ferrari A, et al. Chromogranin A and B and secretogranin II in prostatic adenocarcinomas: neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate. 1998;34(2):113–120. doi: 10.1002/(sici)1097-0045(19980201)34:2<113::aid-pros5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 48.Schmid KW, Helpap B, Totsch M, Kirchmair R, Dockhorn-Dworniczak B, Bocker W, et al. Immunohistochemical localization of chromogranins A and B and secretogranin II in normal, hyperplastic and neoplastic prostate. Histopathology. 1994;24(3):233–239. doi: 10.1111/j.1365-2559.1994.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 49.Portela-Gomes GM, Stridsberg M, Grimelius L, Falkmer UG, Falkmer S. Expression of chromogranins A, B, and C (secretogranin II) in human adrenal medulla and in benign and malignant pheochromocytomas. An immunohistochemical study with region-specific antibodies. APMIS. 2004;112(10):663–673. doi: 10.1111/j.1600-0463.2004.t01-1-apm1121003.x. [DOI] [PubMed] [Google Scholar]
- 50.Portel-Gomes GM, Grimelius L, Johansson H, Wilander E, Stridsberg M. Chromogranin A in human neuroendocrine tumors: an immunohistochemical study with region-specific antibodies. Am J Surg Pathol. 2001;25(10):1261–1267. doi: 10.1097/00000478-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Portela-Gomes GM, Stridsberg M. Selective processing of chromogranin A in the different islet cells in human pancreas. J Histochem Cytochem. 2001;49(4):483–490. doi: 10.1177/002215540104900408. [DOI] [PubMed] [Google Scholar]
- 52.Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata SK, Anouar Y. Chromogranins A and B and secretogranin II: evolutionary and functional aspects. Acta Physiol (Oxf) 2008;192(2):309–324. doi: 10.1111/j.1748-1716.2007.01806.x. [DOI] [PubMed] [Google Scholar]
- 53.Thrower EC, Choe CU, So SH, Jeon SH, Ehrlich BE, Yoo SH. A functional interaction between chromogranin B and the inositol 1,4,5-trisphosphate receptor/Ca2+ channel. J Biol Chem. 2003;278(50):49699–49706. doi: 10.1074/jbc.M309307200. [DOI] [PubMed] [Google Scholar]
- 54.Capiod T, Shuba Y, Skryma R, Prevarskaya N. Calcium signalling and cancer cell growth. Subcell Biochem. 2007;45:405–427. doi: 10.1007/978-1-4020-6191-2_15. [DOI] [PubMed] [Google Scholar]
- 55.Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15(2):164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 56.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 57.Shaulsky G, Goldfinger N, Ben-Ze’ev A, Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol. 1990;10(12):6565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rotter V, Abutbul H, Ben-Ze’ev A. P53 transformation-related protein accumulates in the nucleus of transformed fibroblasts in association with the chromatin and is found in the cytoplasm of non-transformed fibroblasts. EMBO J. 1983;2(7):1041–1047. doi: 10.1002/j.1460-2075.1983.tb01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gannon JV, Lane DP. Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature. 1991;349(6312):802–806. doi: 10.1038/349802a0. [DOI] [PubMed] [Google Scholar]
- 60.Roe JS, Youn HD. The positive regulation of p53 by the tumor suppressor VHL. Cell Cycle. 2006;5(18)):2054–2056. doi: 10.4161/cc.5.18.3247. [DOI] [PubMed] [Google Scholar]
- 61.Kawasaki T, Bilim V, Takahashi K, Tomita Y. Infrequent alteration of p53 pathway in metastatic renal cell carcinoma. Oncol Rep. 1999;6(2):329–333. doi: 10.3892/or.6.2.329. [DOI] [PubMed] [Google Scholar]
- 62.Lowe SW. Cancer therapy and p53. Curr Opin Oncol. 1995;7(6):547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Midgley CA, Fisher CJ, Bartek J, Vojtesek B, Lane D, Barnes DM. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli . J Cell Sci. 1992;101(Pt 1):183–189. doi: 10.1242/jcs.101.1.183. [DOI] [PubMed] [Google Scholar]
- 64.Moll UM, Riou G, Levine AJ. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992;89(15):7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iggo R, Gatter K, Bartek J, Lane D, Harris AL. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- 66.Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs) Surgery. 2007;142(6):814–818. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu DS, Hsieh DS, Chang SY. Modulation of prostate carcinoma cell growth and apoptosis by chromogranin A. J Urol. 2003;170(5):2031–2035. doi: 10.1097/01.ju.0000091807.02246.f3. [DOI] [PubMed] [Google Scholar]