Abstract
Objective
To develop a novel diagnostic algorithm for Lyme disease among children with facial palsy by integrating public health surveillance data with traditional clinical predictors.
Design
Retrospective cohort study.
Setting
Children’s Hospital Boston emergency department,1995–2007
Patients
264 children under age 20 years presenting with peripheral facial palsy who were evaluated for Lyme disease
Main outcome measures
Multivariate regression was used to identify independent clinical and epidemiologic predictors of Lyme facial palsy.
Results
65% of children from high-risk counties during Lyme season tested positive, compared to 5% of children without geographic or seasonal risk factors present. Among patients with both seasonal and geographic risk factors, 80% with one clinical risk factor (fever or headache) and 100% with two clinical factors had Lyme. Factors independently associated with Lyme facial palsy were presentation from June-November (odds ratio 25, 95% CI 8.3–113), residence in a county where the most recent three year average Lyme incidence exceeded 4 cases/100,000 (18, 6.5–69), fever (3.9, 1.5–11), and headache (2.7, 1.3–5.8). Clinical experts correctly treated 68/94 (72%) patients with Lyme facial palsy, but a tool incorporating geographical and seasonal risk identified all 94 cases.
Conclusions
Most clinicians intuitively integrate geographic information into Lyme disease management, but we demonstrate quantitatively how formal use of geographically-based incidence in a clinical algorithm improves diagnostic accuracy. These findings demonstrate potential for improved outcomes from investments in health information technology that foster bidirectional communication between public health and clinical settings.
Keywords: Lyme disease, clinical decision support systems, tick-borne diseases, epidemiologic methods, public health informatics
Introduction
Background
When the possible causes of a patient’s condition vary geographically, knowledge about local scale disease incidence could help steer clinicians towards the most likely diagnosis. Children with peripheral facial palsy pose a diagnostic challenge, because optimal management at the point of care requires correctly identifying the etiology for the palsy. Rapid point-of-care testing for Lyme disease is not available, so diagnostic test results, if ordered, often are not known for several days, leaving clinicians to choose a treatment strategy without confirmatory serology. Over-diagnosis of Lyme is associated with excessive antibiotic use, and under-diagnosis with progression to more complications. At one time otitis media accounted for most identifiable cases of facial palsy in children.1 Infections with Borrelia burgdorferi have increased over the past several decades, so Lyme disease, the most frequently reported vector-borne disease in the United States, now accounts for a substantial proportion of cases in endemic areas.2–4 Antivirals and corticosteroids may be helpful in adults with facial palsy.5 For children with Lyme facial palsy, early initiation of appropriate antibiotics is the optimal strategy, and according to the American Academy of Pediatrics Red Book Committee on Infectious Diseases, steroids should not be given.6
Importance
Prediction rules traditionally factor in historical elements, physical exam findings and sometimes seasonality to identify the correct cause of the facial palsy, but to date, none of the rules have incorporated residential location as a predictor.7 Epidemiological context– the recent regional incidence of a disease, often calculable from clinical or public health datasets – may be an important predictor, in the absence of timely diagnostic data.8, 9 Even within endemic areas, Lyme disease incidence varies by location and season, in part because the irregular local and regional distribution of ticks depends on landscape ecology, and micro- and macrometerologic conditions.10, 11 While prior studies have considered ecologic and entomologic risk to generate community-level Lyme prevention recommendations and vaccination strategies,12–15 this analytic approach has not yet extended to manipulating formal management algorithms for symptomatic patients by combining geographic risk with clinical features.
Goals of this Investigation
To optimize management of peripheral facial palsy in children, clinical decision models would incorporate local epidemiological risk to differentiate Lyme disease from other etiologies. Taking a novel approach, integrating epidemiological information about location and season with traditional clinical variables, we sought to create a model to improve diagnostic accuracy and management of children with peripheral facial nerve palsy. We hypothesize that quantitative use of the patient’s geographic risk of Lyme disease would improve the accuracy of diagnosis.
Methods
Design, setting and subjects
Our sample was a retrospective cohort of children under 20 years old presenting to the emergency department (ED) of Children’s Hospital Boston, a large, urban tertiary care hospital, from 1995–2007. The study site ED volume exceeds 50,000 patients annually. We only included children residing in Massachusetts.
Selection of Participants
ED visits of patients with peripheral facial nerve palsy were identified by a computer-assisted key word screening tool and regular expression matching from all ED visits at the study site during the study period.16 We included only those children with facial palsy who were evaluated for Lyme disease (either by obtaining Lyme serology or by the presence of erythema migrans rash). Patients were excluded from the analysis if they had any of the following characteristics causing facial palsy: congenital facial palsy, known central nervous system malignancy, known history of herpes simplex virus, surgery near the facial nerve within one week of presentation, or Todd’s or more generalized paralysis, including hemiparesis.
Case definition
A child was defined to have Lyme disease according to the CDC definition: presence of erythema migrans lesion or serologic evidence of infection with Borrelia burgdorferi via the two-tiered testing strategy.17 Children were only classified positive in our study if the Western blot was positive using the laboratory reference standards. Offsite commercial laboratory personnel (ARUP – Salt Lake City; Immugen – Norwood, MA) performed serologic testing for B burgdorferi. Patients with positive enzyme-linked immunosorbent assay and negative Western blot or no Western blot performed were not considered positive for Lyme disease.18
Predictor variables and data collection
Demographics, onset and duration of symptoms, clinical features, laboratory data and treatment data were collected for each patient via comprehensive chart review by two investigators specializing in pediatric emergency medicine (LEN, ADT). Signs and symptoms included headache, fever, muscle aches, joint pains, rash and potential exposures such as tick bites. Laboratory data were reviewed for Lyme test results. Treatment data included type and duration of treatment with antibiotics or steroids. To assess inter-rater reliability, an independent abstractor specializing in pediatric emergency medicine (AMF) reviewed eight percent of charts chosen at random.19, 20 Candidate predictors with kappa statistics with a lower limit of 95% confidence interval of > 0.4 were considered for the multivariate analyses.21 Visit date and county of residence for each patient were obtained from the chart review. County-level annual Lyme incidence was calculated from available public health surveillance data from the Massachusetts Department of Public Health Office of Integrated Surveillance Informatics Services.2, 22–24 These data were used to calculate the average Lyme incidence over the prior three years in the home county for each patient. For example, for a patient presenting from Essex County in 2004, the incidence in that county was averaged from 2001–2003.
Building the decision models
Three decision models were built with clinical and epidemiological variables: 1) Clinical model – candidate predictors included traditional elements – data on demographics, history and physical exam; 2) Epidemiologic model – candidate predictors included the timing of presentation (month or season) and the incidence variables associated with the county of residence; and 3) Contextualized model – variables not included in the prior two models still qualified for inclusion into this model, which combined clinical and epidemiological predictors.
Univariate and multivariate analysis
Univariate and multivariate analytic techniques were used to identify predictors of Lyme disease among patients with peripheral facial palsy. Significance of association of categorical variables with Lyme disease was tested by Chi square. Continuous variables (i.e. average county incidence of Lyme disease in prior 3 years) were dichotomized at categorical cutoffs (e.g. average incidence > 20 cases/100,000 people). Recursive partitioning was used to identify thresholds for testing univariate and multivariate associations.
In the multivariate analyses, candidate variables were entered into a backward stepwise logistic regression to identify independent predictors of patients with Lyme disease. P value cutoffs for entry and departure for the multivariate regression models were 0.25 and 0.10, respectively. The final models contained variables where p<0.05.
Several seasonal variables were considered independently for entry into the models. A range of cutoffs was considered to define patients who presented in “Lyme season,” (June–October, May–December, June–November), because “Lyme season” varies by geography, climate, suitability for tick populations and annual trends.25 For the spatial variables, the annual county Lyme disease incidence in each of the prior three years and the overall three year average incidence were considered as independent predictors. Recursive partitioning was used to identify a cutoff for the three year average Lyme incidence in the county of residence, and this cutoff was considered as an independent candidate predictor. Final models underwent bootstrap validation. Predictors selected in over 50% of 1000 bootstrap analyses were retained in the final models.26–28
Methods of measurement of model performance
Sensitivity, specificity, positive and negative predictive values, and area under the ROC curve, were used to compare performances of the models. Actual management by pediatric emergency medicine experts was compared to management guided by the decision models. Correct management of Lyme facial palsy was defined as use of a correct antibiotic for a correct duration and omission of corticosteroids and antivirals, as defined by the expert panel in the American Academy of Pediatrics Red Book Committee on Infectious Diseases.6
The Committee on Clinical Investigation of Children’s Hospital Boston approved the study.
Results
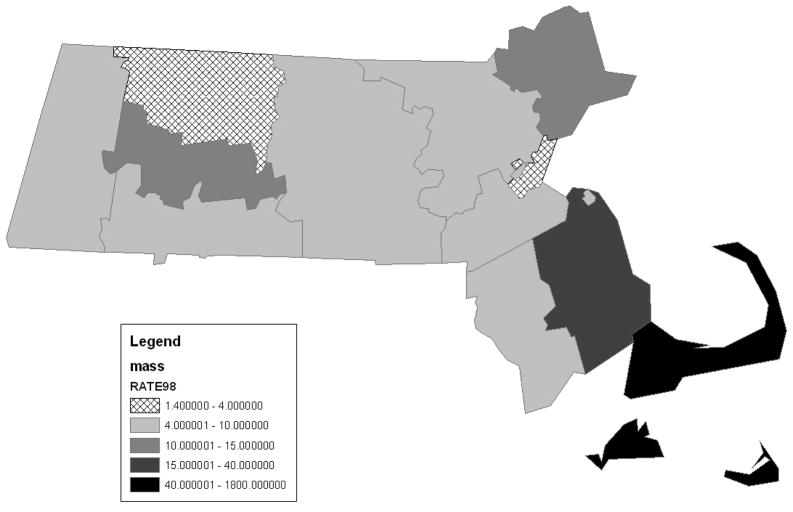
From 1995–2007, there were 609,671 visits to this emergency department for patients under age 20 years. Table 1 displays the characteristics of the 264 patients (0.04% of all ED visits) who presented with peripheral facial palsy, were evaluated for Lyme disease and met study criteria. Patients evaluated for Lyme disease (n=264) were similar to those not evaluated for Lyme disease (n=156) with respect to age, gender and presence of fever and were more likely to have headache (28% vs 12 %, p=0.001) and present during Lyme season (49% vs 31%, p=0.001). The patients came from the nine Eastern-most of the 14 counties in Massachusetts. Figure 1 shows county-level average incidence of Lyme disease for Massachusetts over one three year period of the study.
Table 1.
Characteristics of the 264 Patients with Peripheral Facial Palsy
| Characteristic | Lyme disease absent (n=170) | Lyme disease present (n=94) | P value |
|---|---|---|---|
| N (%) | N (%) | ||
| Male gender | 75 (44%) | 65 (69%) | <0.0001 |
| Mean age (years) (median/IQ range) | 10.9 (12,7–15) | 9.8 (9.5,7–13) | 0.08 |
| Lyme season (present June–November) | 87 (51%) | 91 (97%) | <0.0001 |
| Trauma to face/head | 12 (7.1) | 1 (1.1) | 0.036 |
| Otitis media | 11 (6.5) | 2 (2.1) | 0.15 |
| Fever | 14 (8.2) | 30 (32) | <0.0001 |
| Headache | 28 (16) | 48 (51) | <0.0001 |
| Systemic symptoms/myalgias | 12 (7.1) | 19 (20) | 0.0024 |
| Neck pain | 1 (0.6) | 2 (2.1) | 0.29 |
| Arthritis | 1 (0.6) | 5(5.3) | 0.023 |
| Prior three year mean Lyme incidence (median, IQ range)in county of residence | 11 (3.9, 2.9–17) (per 100,000) | 19 (20, 13–24) (per 100,000) | <0.0001 |
Figure 1.
This map of Massachusetts shows the average incidence (# of cases per 100,000 people) of Lyme disease by county over a three year period. It displays the data used to measure the risk associated with home location for patients who presented with facial palsy to the pediatric emergency department in the year following this three year interval.
Development of clinical decision model
Univariate analysis
Patients with Lyme were more likely to be male, have a history of fever, headache, systemic symptoms like myalgias and arthritis and no history of trauma to the face or head (Table 1). There were no significant differences between those with and without Lyme for age, neck pain, or otitis media. Exposure to tick bite was not captured in the vast majority of charts, and so could not be considered for the analyses.
Multivariate analysis
In the clinical model, headache (OR 4.4, 95% CI 2.2–7.5) was the most significant predictor of Lyme facial palsy, followed by fever (3.3, 1.6–7.1) (Table 2). Presence of either of these two predictors identified children with Lyme with 60% sensitivity, 79% specificity, and area under the ROC curve (AUC) of 0.71. The PPV was 61% and the NPV was 79%.
Table 2. Multivariate Analyses.
High-risk predictors for Lyme disease among patients with facial palsy for the three models.
| Characteristic | Odds Ratio | 95% Confidence Intervals | P value |
|---|---|---|---|
| Clinical model (AUC* = 0.71) | |||
|
| |||
| Headache | 4.4 | 2.2 to 7.5 | <0.0001 |
| Fever | 3.3 | 1.6 to 7.1 | 0.0017 |
|
| |||
| Epidemiological model (AUC=0.84) | |||
|
| |||
| Lyme season** | 25 | 8.6 to 107 | <0.0001 |
| High-risk location*** | 20 | 7.4 to 68 | <0.0001 |
|
| |||
| Contextualized Model (AUC=0.89) | |||
|
| |||
| Fever | 3.9 | 1.5 to 11 | 0.0071 |
| Headache | 2.7 | 1.3 to 5.8 | 0.0095 |
| Lyme season** | 25 | 8.3 to 113 | <0.0001 |
| High-risk location*** | 18 | 6.5 to 69 | <0.0001 |
AUC: Area under Receiver Operator Characteristic Curve
Lyme season = June to November
High risk location: 3 year average Lyme incidence > 4/100,000 in county of residence.
Development of models incorporating epidemiological context: Selection of seasonal variable
Univariate analyses were conducted using a range of cutoffs to define Lyme season. Recursive partitioning identified candidate cutoffs for Lyme season. Patients with Lyme disease were more likely to present during any of the defined Lyme seasons. The Lyme season defined as “June–November” showed a stronger association for Lyme than “June–October” or “May–December” so for further analyses, June–November was used as Lyme season.
Selection of spatial variable
Univariate analysis was used to examine associations between Lyme disease and Lyme incidence rates in the patient’s home county. Recursive partitioning identified cutoffs to classify 3 year county average incidences as high or low risk. The low risk cutoff occurred when the average three year Lyme incidence for a county was less than four cases/100,000 people. Annual incidence and three year average incidence were associated with Lyme disease, but the cutoff incidence of >4 cases/100,000 people was the strongest spatial predictor, and was retained as the spatial predictor for the rest of the analyses.
The best epidemiological model contained two variables—Lyme season (June–November) and high-risk home location (three year average county-specific Lyme incidence > 4 cases/100,000 people). Lyme season (OR 25, 95% CI 8.6–107) and high-risk home location (20, 7.4–68) were both very strong predictors with odds ratios above 20 (Table 2). The AUC for this model was 0.84.
The contextualized model considering all clinical and epidemiologic variables regardless of whether they entered into the previous models contained four variables: fever (OR 3.9, 95% CI 1.5–11), headache (OR 2.7, 1.3–5.8), Lyme season (OR 25, 8.3–113) and high-risk home location (OR 18, 6.5–69) (Table 2). The AUC for this model was 0.89. This model was 100% sensitive and 24% specific with a PPV of 42% and NPV of 100%.
Validation
All predictors from the multivariate analyses were validated by the bootstrap method and retained in the final models. High-risk location was selected in over 99%, Lyme season in over 97%, fever in over 81% and headache in over 77% of 1000 bootstrap analyses.
Measurement of model performance
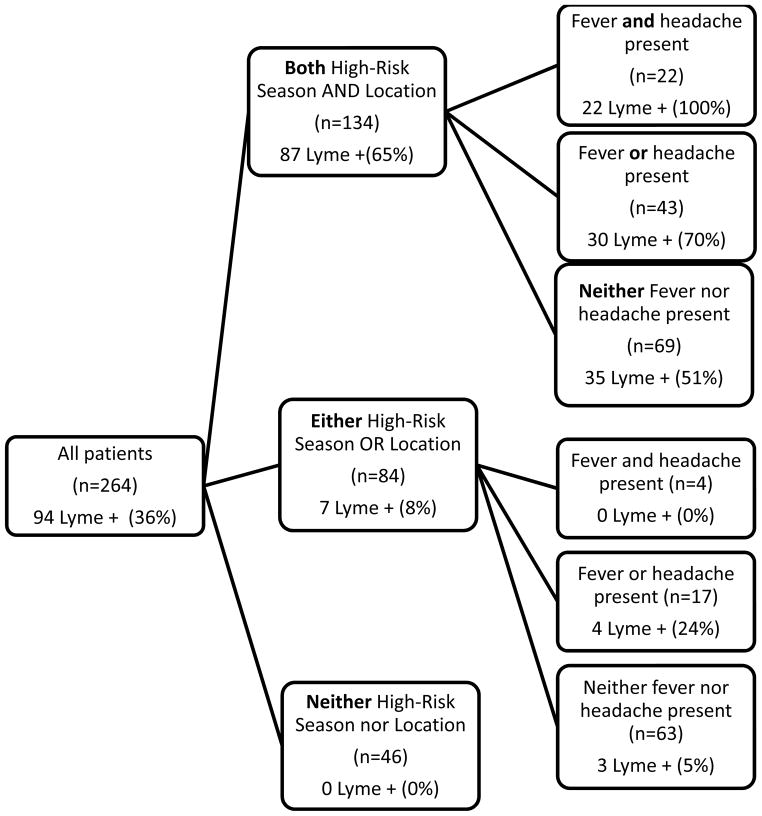
Adding epidemiologic factors (seasonal and spatial variables) to the clinical model improved the AUC from 0.71 to 0.89, whereas adding clinical factors to the epidemiological model improved the AUC more modestly, from 0.84 to 0.89. Figure 2 illustrates the risk of Lyme facial palsy based on the presence of high-risk predictors. Of the 264 patients in the study, 134 presented from high-risk locations during Lyme season, and 87 (65%) had Lyme. In contrast, 7/130 (5%) patients who presented without both high risk location and season were positive for Lyme. A total of 69 patients presented from high-risk locations during Lyme season without either clinical predictor (fever or headache), and 35 (51%) of these patients had Lyme. Of the 65 patients who presented with fever, headache or both, in conjunction with high-risk location and Lyme season, 52 (80%) had Lyme. The combinations that included both season and location identified 87/94 (93%) of Lyme cases. Finally, none of the 42 patients without any of the four identified risk factors had Lyme disease.
Figure 2.
Presence of high-risk predictors among those with and without Lyme facial palsy
Presence of epidemiological and clinical risk factors and rate of Lyme disease among 264 children who presented with peripheral facial palsy. This diagram splits patients by the presence of two, one, or no epidemiological risk factors. Patients presenting from high-risk locations during Lyme season are displayed on the top, further stratified by the presence of clinical risk factors. Patients with only one epidemiological risk factor present are grouped in the middle branch of the tree, and are also further stratified by the presence of clinical risk factors. Patients without either epidemiological risk factor are shown at the bottom of the tree.
Comparing clinician performance with decision models
We compared the proportion of children with facial palsy empirically treated with the appropriate medications by attending physicians in the pediatric emergency department with hypothetical outcomes generated by the three models. These physicians treated 68/94 (72%) Lyme disease patients with the correct type of antibiotics and without steroids or antivirals. The epidemiologic and contextualized models did not miss any cases of Lyme disease.
Discussion
To date, clinical decision rules have relied on clinical factors and to a much lesser extent, seasonality. In the case of Lyme disease, clinicians may informally consider exposure and location when determining the cause of facial palsy, but there are currently no mechanisms that formally facilitate integration of this important contextual information. To the extent that clinicians use contextual epidemiological information to help guide decision making, they tend to use it informally and to rely on personal or pooled collective experiences to reason about diagnosis, testing and treatment.29–31 While most clinicians certainly often intuitively integrate geographic information into their diagnostic workup for Lyme disease, we show that a quantitative, formal integration of geographically based incidence improves diagnosis and treatment.
Within endemic regions of the United States, selected states have higher Lyme rates, and within those states, there is significant variation by county. Our findings support a general approach of estimating clinical risk of disease at the point of care, accounting for recent spatial incidence. This approach emphasizes applying epidemiologic context to the clinical decision making process rather than relying solely on history, physical exam, heuristics and preliminary diagnostic test results.9, 32, 33 Improved collaboration between public health departments and clinicians, the maturation of electronic health records, and advances in disease surveillance and automated reporting now increase the feasibility of delivering readily available and easily computed relevant public health information to clinicians at the point of care.34–36
Previously, we showed that epidemiological information about meningitis from a single hospital provides valuable epidemiological context and enhances a decision model for distinguishing aseptic from bacterial meningitis.8 We have also illustrated how an external public health surveillance source improves a clinical decision model, by incorporating state-wide “epidemiological context.”37 Now, for the first time, we show how spatial incidence data improves the ability of a model to identify cases of an infectious disease. In our analyses, epidemiological context variables like season and home location were stronger than any clinical predictor in identifying patients with Lyme facial palsy, building upon a previous clinical model that did not consider home location.7 Epidemiological context was especially powerful when combined with clinical factors. These findings stress the importance of “situational awareness” in clinical settings. Understanding the epidemiological context in which a patient presents may provide vital information about the etiology of the patient’s problem, but currently, valuable spatiotemporal data are not formally processed, considered, utilized or integrated into the clinical decision-making process.
Clinical and public health datasets offer synergistic information that can be leveraged to generate and refine clinical decision algorithms. Public health data have not typically contributed information to generate decision models because while they contain records about those with confirmed disease, they provide little if any information about those without the disease of interest. This creates unique challenges to the integration of public health data into decision models, which rely on rich information about patients both with and without the disease.38, 39 To capitalize on the use of public health data, we relied on incidence rates to develop and refine a decision model for Lyme disease, a condition with significant morbidity and of increasing public health importance.
Limitations
External validation should be considered prior to integration into a clinical setting, as the performance of predictive indices may deteriorate in subsequent validation studies.40 Our study was confined to Massachusetts, a state endemic for Lyme disease. Specific definitions of thresholds may vary when more geographically diverse data are considered. Second, this study occurred at a single ED. However, this site provides care for 75% of the children who live in and around this large metropolitan area. Third, residential county was taken from ED registration data, which may not accurately reflect home location for patients with multiple home addresses. This residential location also does not reflect exposures during travel, but does represent the best available exposure data. More accurate information about patient home location would probably strengthen the accuracy of a model incorporating residential location. Fourth, due to the retrospective nature of the study, we only were able to include patients in whom a diagnosis of Lyme disease was considered, and not patients with subtle presentations where the clinician did not consider Lyme as an etiology for facial palsy. Lyme incidence data are county-level, and do not account for variation within county, so future investigations using larger data sets, might provide adequate power to obtain finer spatial resolution. For example, availability of zip code level incidence data might provide more refined risk stratification. Future studies could also incorporate surrogate markers for local disease incidence, such as vector surveys and canine serosurveys. Last, compliance with mandatory reporting requirements by laboratories and clinicians is highly variable41, so underreporting of Lyme disease is a limitation.
Conclusions
This study emphasizes the benefit of integrating epidemiologic context into a clinical decision model. We found that, contextual spatial and seasonal epidemiologic factors dominated clinical factors in distinguishing Lyme disease from other causes of pediatric peripheral facial palsy. This study adds to a growing body of evidence that clinical decision support systems can be improved by introducing “epidemiologic context” variables into algorithms. Public health and clinical information simultaneously presented to a decision support application improves diagnostic accuracy. An important goal of national efforts to promote health information technology should be to foster electronic bidirectional communication of data and messaging between public health and clinical sites.
Acknowledgments
This work was supported by the Mentored Public Health Research Scientist Development Award K01HK000055 from the Centers for Disease Control and Prevention (CDC), by Public Health Informatics Center of Excellence Award P01HK000016 and P01HK000088-01 from CDC, and by G08LM009778 and R01 LM007677 from the National Library of Medicine (NIH). The study sponsors had no role in study design, collection, analysis or interpretation of data, writing of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Presented in part at International Society for Disease Surveillance Annual meeting, Dec, 2009, Miami, FL, and at Pediatric Academic Societies Annual meeting, May, 2009, Baltimore, MD.
Conflict of interest: All authors have declared that no competing interests exist.
Ethical approval: The Committee on Clinical Investigation of Children’s Hospital Boston approved the study.
AMF had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Cook SP, Macartney KK, Rose CD, Hunt PG, Eppes SC, Reilly JS. Lyme disease and seventh nerve paralysis in children. American Journal of Otolaryngology. 1997;18(5):320–323. doi: 10.1016/s0196-0709(97)90026-0. [DOI] [PubMed] [Google Scholar]
- 2.Lyme disease--United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2007 Jun 15;56(23):573–576. [PubMed] [Google Scholar]
- 3.Christen H-J, Bartlau N, Hanefeld F, Eiffert H, Thomssen R. Peripheral Facial Palsy in Childhood-Lyme Borreliosis to be Suspected Unless Proven Otherwise. Acta Paediatrica. 1990;79(12):1219–1224. doi: 10.1111/j.1651-2227.1990.tb11413.x. [DOI] [PubMed] [Google Scholar]
- 4.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006 Nov 1;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida JR, Al Khabori M, Guyatt GH, et al. Combined corticosteroid and antiviral treatment for Bell palsy: a systematic review and meta-analysis. Jama. 2009 Sep 2;302(9):985–993. doi: 10.1001/jama.2009.1243. [DOI] [PubMed] [Google Scholar]
- 6.Pickering LK, Baker CJ, Long SS, McMillan JA, editors. American Academy of Pediatrics. Red Book 2006: Report of the Committee on Infectious Diseases. 27. Elk Grove Village: AAP; 2006. [Google Scholar]
- 7.Nigrovic L, Thompson A, Fine A, Kimia A. Clinical Predictors of Lyme Disease Among Children With Peripheral Facial Palsy at an Emergency Department in a Lyme Endemic Area. Pediatrics. 2008 Nov;122(5):e1080–1085. doi: 10.1542/peds.2008-1273. [DOI] [PubMed] [Google Scholar]
- 8.Fine AM, Nigrovic LE, Reis BY, Cook EF, Mandl KD. Linking Surveillance to Action: Incorporation of Real-time Regional Data into a Medical Decision Rule. J Am Med Inform Assoc. 2007 Mar 1;14(2):206–211. doi: 10.1197/jamia.M2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis BY, Pagano M, Mandl KD. Using temporal context to improve biosurveillance. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1961–1965. doi: 10.1073/pnas.0335026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitron U, Kazmierczak JJ. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am J Epidemiol. 1997 Mar 15;145(6):558–566. doi: 10.1093/oxfordjournals.aje.a009145. [DOI] [PubMed] [Google Scholar]
- 11.Waller LA, Goodwin BJ, Wilson ML, Ostfeld RS, Marshall SL, Hayes EB. Spatio-temporal patterns in county-level incidence and reporting of Lyme disease in the northeastern United States, 1990–2000. Environmental and Ecological Statistics. 2007;14(1):83–100. [Google Scholar]
- 12.Frank C, Fix AD, Pena CA, Strickland GT. Mapping Lyme disease incidence for diagnostic and preventive decisions, Maryland. Emerg Infect Dis. 2002 Apr;8(4):427–429. doi: 10.3201/eid0804.000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownstein JS, Holford TR, Fish D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ Health Perspect. 2003 Jul;111(9):1152–1157. doi: 10.1289/ehp.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dister SW, Fish D, Bros SM, Frank DH, Wood BL. Landscape characterization of peridomestic risk for Lyme disease using satellite imagery. Am J Trop Med Hyg. 1997 Dec;57(6):687–692. doi: 10.4269/ajtmh.1997.57.687. [DOI] [PubMed] [Google Scholar]
- 15.Falco RC, McKenna DF, Daniels TJ, et al. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol. 1999 Apr 15;149(8):771–776. doi: 10.1093/oxfordjournals.aje.a009886. [DOI] [PubMed] [Google Scholar]
- 16.Kimia AA, Capraro AJ, Hummel D, Johnston P, Harper MB. Utility of lumbar puncture for first simple febrile seizure among children 6 to 18 months of age. Pediatrics. 2009 Jan;123(1):6–12. doi: 10.1542/peds.2007-3424. [DOI] [PubMed] [Google Scholar]
- 17.Steere AC. Lyme disease. N Engl J Med. 2001 Jul 12;345(2):115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 18.CDC. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. 1995;44(31):590–591. [PubMed] [Google Scholar]
- 19.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996 Mar;27(3):305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 20.Gorelick MH, Yen K. The kappa statistic was representative of empirically observed inter-rater agreement for physical findings. J Clin Epidemiol. 2006 Aug;59(8):859–861. doi: 10.1016/j.jclinepi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 22.CDC. Lyme Disease --- United States, 2003--2005. Morbidity and Mortality Weekly. 2007;56(23):573–576. [PubMed] [Google Scholar]
- 23.Lyme disease--United States, 2001–2002. MMWR Morb Mortal Wkly Rep. 2004 May 7;53(17):365–369. [PubMed] [Google Scholar]
- 24.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992–2006. MMWR Surveill Summ. 2008 Oct 3;57(10):1–9. [PubMed] [Google Scholar]
- 25.Subak S. Effects of climate on variability in Lyme disease incidence in the northeastern United States. Am J Epidemiol. 2003 Mar 15;157(6):531–538. doi: 10.1093/aje/kwg014. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Tu JV. Bootstrap Methods for Developing Predictive Models. American Statistician. 2004;58(2):131–137. [Google Scholar]
- 27.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. The American Statistician. 1983;37:36–48. [Google Scholar]
- 28.Glaser N, Barnett P, McCaslin I, et al. Risk Factors for Cerebral Edema in Children with Diabetic Ketoacidosis. N Engl J Med. 2001 Jan 25;344(4):264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 29.Patel VL, Kaufman DR, Arocha JF. Emerging paradigms of cognition in medical decision-making. J Biomed Inform. 2002 Feb;35(1):52–75. doi: 10.1016/s1532-0464(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 30.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;186:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 31.Wolf FM, Gruppen LD, Billi JE. Differential diagnosis and the competing-hypotheses heuristic. A practical approach to judgment under uncertainty and Bayesian probability. JAMA. 1985 May 17;253(19):2858–2862. [PubMed] [Google Scholar]
- 32.Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003 Aug;78(8):775–780. doi: 10.1097/00001888-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Morse SS. Global infectious disease surveillance and health intelligence. Health Aff (Millwood) 2007 Jul-Aug;26(4):1069–1077. doi: 10.1377/hlthaff.26.4.1069. [DOI] [PubMed] [Google Scholar]
- 34.Daniel JB, Heisey-Grove D, Gadam P, et al. Connecting health departments and providers: syndromic surveillance’s last mile. MMWR Morb Mortal Wkly Rep. 2005 Aug 26;54 (Suppl):147–150. [PubMed] [Google Scholar]
- 35.Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007 Jan-Feb;14(1):29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samore MH, Bateman K, Alder SC, et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. Jama. 2005 Nov 9;294(18):2305–2314. doi: 10.1001/jama.294.18.2305. [DOI] [PubMed] [Google Scholar]
- 37.Fine A, Reis B, Nigrovic L, et al. Use of Population Health Data to Refine Diagnostic Decision Making for Pertussis. J Am Med Inform Assoc. 2010;17(1):85–90. doi: 10.1197/jamia.M3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. Jama. 1997 Feb 12;277(6):488–494. [PubMed] [Google Scholar]
- 39.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985 Sep 26;313(13):793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 40.Charlson ME, Ales KL, Simon R, MacKenzie CR. Why predictive indexes perform less well in validation studies. Is it magic or methods? Arch Intern Med. 1987 Dec;147(12):2155–2161. [PubMed] [Google Scholar]
- 41.Fine AM, Goldmann DA, Forbes PW, Harris SK, Mandl KD. Incorporating Vaccine-Preventable Disease Surveillance Into the National Health Information Network: Leveraging Children’s Hospitals. Pediatrics. 2006 Oct 1;118(4):1431–1438. doi: 10.1542/peds.2006-0462. [DOI] [PubMed] [Google Scholar]