Abstract
It is widely held that the somatic cell population that is responsible for sperm development and output (Sertoli cells) is terminally differentiated and unmodifiable in adults. It is postulated, with little evidence, that Sertoli cells are not terminally differentiated in some phenotypes of infertility and testicular cancer. This study sought to compare markers of Sertoli cell differentiation in normospermic men, oligospermic men (undergoing gonadotropin suppression) and testicular carcinoma in situ (CIS) and seminoma samples. Confocal microscopy was used to assess the expression of markers of proliferation (PCNA and Ki67) and functional differentiation (androgen receptor). As additional markers of differentiation, the organization of Sertoli cell tight junction and associated proteins were assessed in specimens with carcinoma in situ. In normal men, Sertoli cells exhibited a differentiated phenotype (i.e., PCNA and Ki67 negative, androgen 40 receptor positive). However, after long-term gonadotropin suppression, 1.7 ± 0.6% of Sertoli cells exhibited PCNA reactivity associated with a diminished immunoreactivity in androgen receptor, suggesting an undifferentiated phenotype. Ki67-positive Sertoli cells were also observed. PCNA-positive Sertoli cells were never observed in tubules with carcinoma in situ, and only rarely observed adjacent to seminoma. Tight junction protein localization (claudin 11, JAM-A and ZO-1) was altered in CIS, with a reduction in JAM-A reactivity in Sertoli cells from tubules with CIS and the emergence of strong JAM-A reactivity in seminoma. These findings indicate that adult human Sertoli cells exhibit characteristics of an undifferentiated state in oligospermic men and patients with CIS and seminoma in the presence of germ cell neoplasia.
Keywords: Sertoli cells, germ cell, neoplasia, fertility, immunohistology
Introduction
Sertoli cells provide structural and nutritional support to developing germ cells. At puberty in higher mammals, Sertoli cells undergo a complex process of structural and biochemical changes to support spermatogenesis and are thought to become terminally differentiated. This terminal differentiation, occurring during puberty, is defined by the conversion from a proliferative to non-proliferative state, changes in protein expression profile and the formation of Sertoli cell tight junctions, which are a major component of the blood-testis barrier (BTB).1,2
The idea of a terminally differentiated adult Sertoli cell population largely derives from findings that no overt Sertoli cell division or degeneration occurs in normal or hormonally manipulated adult rats.3 In addition, Sertoli cell numbers do not significantly change after chronic gonadotropin suppression in rats4 or in men.5-8 However, some undifferentiated characteristics (immature cellular architecture, presence of an immature protein expression profile, including cytokeratin 18, anti-mullerian hormone, AMH) have been identified in Sertoli cells from adult individuals with testicular germ cell cancer (TGCT) and infertility.9-18 To date, a detailed assessment of Sertoli cell differentiation in men after gonadotropin suppression and in testicular disease has not been made.
There has been ongoing discussion whether the undifferentiated Sertoli cells in men with testicular pathologies and/or infertility are due to a lack of terminal differentiation at puberty or to secondary de-differentiation. Arguments for the lack of differentiation are best summarized in the theory of the testicular dysgenesis syndrome (TDS), which postulates that the main event in the pathogenesis of some forms of cryptorchidism, infertility and TGCT, which disturbed early testicular development that affects the differentiation and function of Leydig, Sertoli and germ cells.19 TGCTs are thought to arise from a common precursor cell known as CIS.20 Similarities in the morphology and protein expression profile between CIS and fetal germ cells suggest that CIS originates from gonocytes that failed to differentiate during fetal development.21 CIS progresses to overt TGCT after puberty, in response to hormonal changes (particularly androgens and gonadotropins), which act via receptors located on the Sertoli cell.22 On the other hand, several pieces of evidence indicate that Sertoli cells may be capable of de-differentiation. In the adult monkey, re-expression of the immature Sertoli cell protein marker cytokeratin-18 occurs after heat treatment.23 Proliferation of adult Sertoli cells is observed in the Djungarian hamster in response to FSH stimulation after gonadotropin suppression.24 These findings are consistent with the idea of adult Sertoli cell de-differentiation in infertility phenotypes. Altogether, these findings challenge the convention that the adult Sertoli cell population is terminally differentiated in phenotypes of infertility and prompted the analysis presented here.
Several factors are known to affect Sertoli cell differentiation,1 such as FSH,25-27 thyroid hormone,28,29 testosterone,30 retinoic acid, activin31 and transforming growth factor β family members,32 however these effects are inducible exclusively in the pre- and pubertal periods. Evidence shows that FSH promotes and thyroid hormone inhibits Sertoli cell proliferation.27-29,33-37 Testosterone, thyroid hormone, retinoic acid and activin play a role in dictating Sertoli cell differentiation prior to puberty through interactions with regulators of the cell cycle,38 but their direct effects are yet to be elucidated.
An important part of normal pubertal Sertoli cell differentiation and, thus, functionality is the formation of a BTB. In the setting of testicular disease, data indicates there is a loss of BTB integrity as shown by dys-localization of junctional proteins in tubules with testicular CIS.16,39,40 The BTB is largely generated by the binding of transmembrane tight junction proteins [occludin, claudins and junction adhesion molecules (JAMs)] on adjacent Sertoli cell membranes, acting to inhibit the exposure of post-meiotic germ cells to factors found in the general circulation.2,41 In the absence of a functional BTB (through knockout of the major tight junction protein claudin-11), Sertoli cells proliferate and undergo apoptosis,42 while germ cells do not progress past early spermatocytes.43
This study sought to clarify whether or not the differentiation state of Sertoli cells is terminal in men. The only human models available were normal healthy men before and after contraceptive-based gonadotropin suppression, and men with testicular cancer (CIS and seminoma). The assessment of the status of Sertoli cells was achieved using two immunofluorescent markers of Sertoli cell proliferation (PCNA and Ki67), as well as expression of a marker of Sertoli cell differentiation (androgen receptor, AR) and a panel of Sertoli cell tight junction and associated junction proteins (claudin 11, JAM-A, ZO-1 and connexin 43).
Results
Sertoli cells show a capacity to proliferate in men after gonadotropin suppression
No PCNA reactivity was detectable in Sertoli cells of sections from untreated normal men (Fig. 1A, green, asterisk) and after treatment of normal men with T and DPMA for 2 wk (Fig. 1B, asterisk). When treatment was extended to 12 wk, PCNA reactivity was detected in 1.7% ± 0.6 of Sertoli cells (Fig. 1C and D, arrows). To verify the proliferating state of Sertoli cells after gonadotropin suppression, an additional marker of proliferation believed to be more specific for the actual growth fraction of a cell population was employed; Ki67.45 No Ki67-positive Sertoli cells were present in untreated normal men (Fig. 1E, red, asterisk); however, positive Sertoli cells were observed after 12 wk of T + DMPA treatment [Fig. 1F and G (enlarged image of IF), green, arrow]. No PCNA was detectable in Sertoli cells of testis sections with CIS (Fig. 1H, asterisk). In the vicinity of seminoma, PCNA-positive Sertoli cells were identified (albeit infrequently) in tubules with a highly abnormal morphology. PCNA reactivity was detectable in germ cells of all assessed groups (Fig. 1A, 1I, triangles). In all sections analyzed, notable non-specific reactivity was observed in the interstitium, making it impossible to assess PCNA and Ki67 reactivity in these regions.
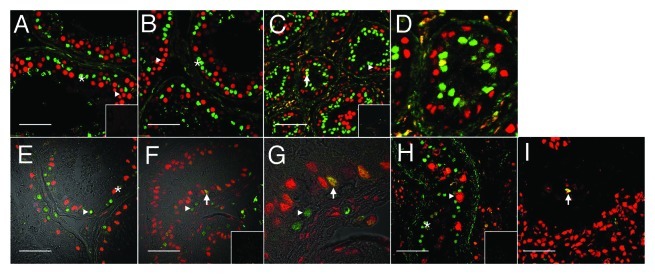
Figure 1. Evidence for Sertoli cell proliferation in men. Confocal immunofluorescence of proliferation markers in testis tissue from normal (A and E) and gonadotropin suppressed men [2 wk, B; 12 wk, C and D (enlarged portion of C) and F and G (enlarged portion of panel F)], in testis with carcinoma in situ (H) and in a tubule adjacent to a seminoma (I). Sections were probed with a combination of either GATA4 (green, in the nuclei of Sertoli cells) within the seminiferous epithelium (asterisks), and PCNA antibodies (red, staining cells with proliferation capacity - triangles) (A–D, H–I), or GATA4 (red) and Ki67 (green, staining proliferating cells) (E–G). Colocalization of GATA4 and PCNA or GATA4 and Ki67 in Sertoli cell nuclei (indicating a proliferative state, arrows) is shown by a yellow color. Figure 1E–G includes a transmitted light channel to illustrate histological detail. Inserts are negative controls. (Bar = 50 µm).
Androgen receptor immunoreactivity appeared lower in PCNA-positive Sertoli cells. AR expression was detected in all somatic cells (Leydig, peritubular myoid and Sertoli cells; as indicated by colocalization with GATA4) of normal men (Fig. 2A, light blue, asterisk), though expression of AR by Sertoli cells was variable, even within a single tubule. This expression pattern persisted after T+DPMA treatment for 2 wk (Fig. 2B) and 12 wk (Fig. 2C–F) though PCNA-positive Sertoli cells consistently appeared to express lower AR reactivity (Fig. 2D–I, yellow, arrow). In Figure 2F–I, a direct comparison of a PCNA-positive, AR low Sertoli cell (arrow) with a PCNA-negative AR-positive Sertoli cell (asterisk) can be seen. Expression of PCNA was detected in germ cells of all assessed groups (Fig. 2A–D, triangles). Samples of tissue from men with testicular cancer could not be reliably assessed with this combination of markers due to their highly variable immunoreactivity.
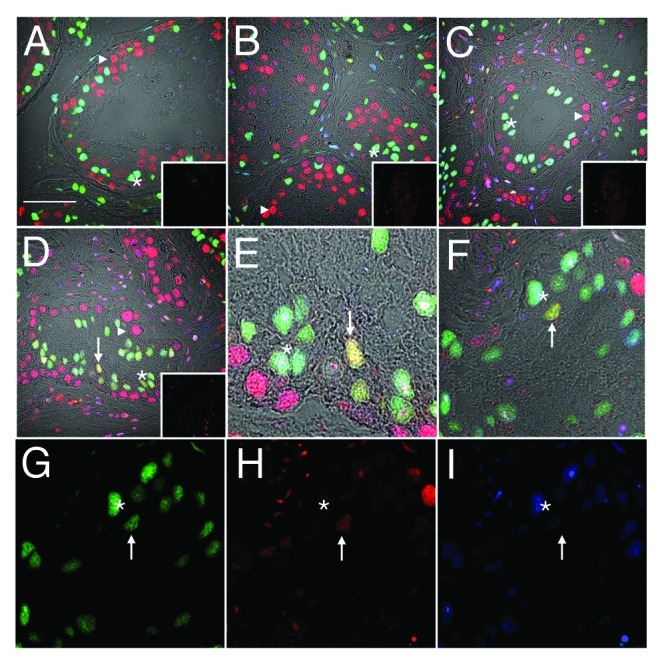
Figure 2. Downregulation of androgen receptor immunoreactivity in PCNA-positive Sertoli cells. Confocal immunofluorescence of human testis sections from normal (A) or gonadotropin-suppressed men (B, 2 wk; C–I, 12 wk). Sections were probed for GATA-4 [green, Sertoli cells within the seminiferous epithelium, asterisks), androgen receptor (blue, Sertoli (asterisks), Leydig and peritubular cells], and PCNA (red, labeling predominantly germ cells, triangles). Colocalization of GATA-4 and PCNA in Sertoli cells indicated by yellow (arrows). G–I are individual channels of the merged image in F, highlighting a GATA4 and PCNA-positive, AR-negative Sertoli cell (arrow) and a GATA4 and AR-positive, PCNA-negative Sertoli cell (asterisk). Inserts are controls where primary antibody was substituted for non-specific antibody of the same isotype. (Bar = 50 µm).
Tight junction proteins are disorganized in testicular cancer
In the normal seminiferous epithelium, claudin-11 reactivity was detected near the basement membrane luminal to pre-meiotic germ cells, in regions associated with inter-Sertoli cell tight junctions (Fig. 3A, red, asterisk). Connexin 43 reactivity was associated with Sertoli cells, spermatogonia and spermatocytes (Fig. 3A, open square) and colocalized with claudin-11 in regions associated with inter-Sertoli cell tight junctions (yellow). Connexin 43 reactivity was also present between Leydig cells of the interstitium (Fig. 3B and C, triangles). Unexpectedly, JAM-A was localized to basally located spermatogonia and spermatocytes (Fig. 4A, asterisk) in the normal testis, as well as being present at inter-Sertoli cell tight junctions. In the same regions, ZO-1 was detected extending from the basement membrane, encircling spermatogonia and spermatocytes (Fig. 5A, asterisk) and also in association with inter-Sertoli cell tight junctions.
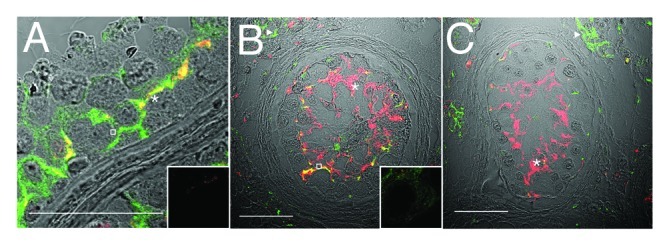
Figure 3. Claudin-11 and Connexin 43 are disorganized in testicular dysgenesis. Confocal immunofluorescence of normal (A) and carcinoma in situ (B and C) human testis sections detecting claudin-11 (red, tight junctions, asterisks) and connexin 43 (green, gap junctions, open squares and triangles). Co-localization appears in yellow. Inserts are negative controls. (Bar = 50 µm).
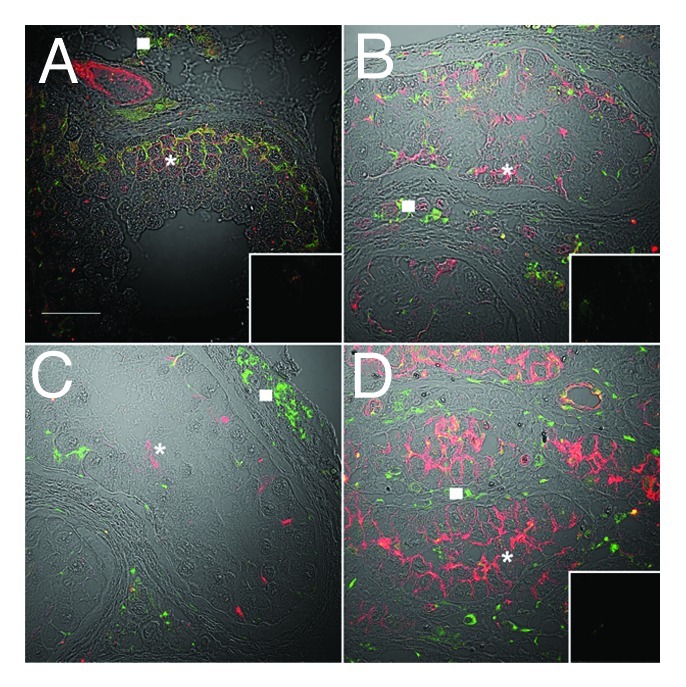
Figure 4. JAM-A localization is disorganized in carcinoma in situ and becomes highly expressed in malignant seminoma. Confocal immunofluorescence of normal human testis (A), carcinoma in situ (B and C) and seminoma sections (D) detecting JAM-A (red, asterisks) and connexin 43 (green, gap junctions, squares). Inserts are negative controls. (Bar = 50 µm).
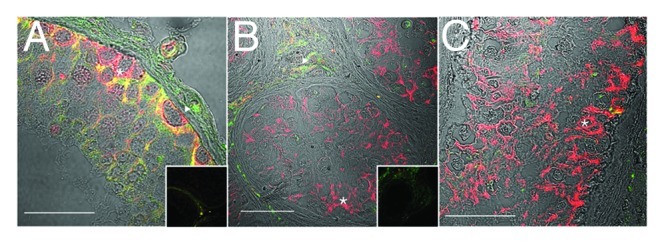
Figure 5. ZO-1 localization is disorganized in carcinoma in situ, in the presence of normal connexin 43 reactivity. Confocal immunofluorescence of normal human testis (A) and carcinoma in situ (B) detecting ZO-1 (tight and associated junctions, red, asterisks) and connexin 43 (gap junctions, green, triangles). Inserts are negative controls. (Bar = 50 µm).
Claudin-11, JAM-A and ZO-1 were expressed in tubules with CIS, but the pattern was highly disorganized compared with that of normospermic men (Figs. 3B and C, 4B and 5B, asterisks) and in some CIS samples a decrease in JAM-A reactivity was seen (Fig. 4C, asterisk). Connexin 43 reactivity was present in samples of CIS (Figs. 3B and C, 4B and C, 5B) an observation indicated elsewhere to be reduced compared with normal spermatogenesis.17
Discussion
This study is the first to identify Sertoli cells with a potential to proliferate in normal men after gonadotropin suppression, and in isolated seminiferous tubules of men with seminoma. These findings are in accord with in vivo data demonstrating adult Sertoli cells exhibiting undifferentiated characteristics in humans with various forms of testicular dysgenesis10,12,15,16,39 and in a rodent model of gonadotropin suppression24 and in vitro data showing adult Sertoli cells from mice and men can regain proliferative ability.46 Taken together, these data provide evidence that adult human Sertoli cells are not a terminally differentiated population,46,47 but rather, have a variable differentiation status and the capacity to de-differentiate,24 at least in the condition of men with suppressed gonadotropins.
While a small fraction of the adult Sertoli cell population in gonadotropin-suppressed men showed signs of proliferative potential (as evidenced by PCNA and Ki67 expression) and, thus, has the potential to expand. In the seasonal breeding Djungarian hamster model, gonadotropin suppression as a result of short day light exposure resulted in changes in the state of Sertoli cell differentiation (using the same markers of proliferation and blood testis barrier as used in this study), similar to those observed in gonadotropin-suppressed men in this study.24 Further to this, the hamster data show that replacement of FSH also promptly (within a week) and completely replenishes the Sertoli cell population48 as a result of increased proliferation and restored blood testis barrier protein localization.24 Notwithstanding differences between hamster and human, the hamster data and that of others46 provide proof that the adult Sertoli cell population is not terminally differentiated and, thus, provide potential for the human Sertoli cell population to also be replenished in infertility syndromes where the Sertoli cell population is subnormal. Sertoli cell numbers were not significantly altered in gonadotropin-suppressed men.7,8 It is possible that Sertoli cells undergo apoptosis as well as proliferation to maintain a relatively stable population, thus small fluctuations in number will fall within the errors of the counting system. Apoptosis of Sertoli cells has been observed in vitro and this effect can be modulated by factors such as FSH.49 In support, Sertoli cells in mice congenitally deficient in gonadotropins undergo apoptosis.50 It is important to note that PCNA has also been shown to be expressed in cells undergoing DNA repair and apoptosis51 and, thus, its expression may be associated with damaged Sertoli cells.
Our data demonstrates that Sertoli cells with proliferative ability have what appears to be diminished AR reactivity, thereby reflecting an altered state of “functional” differentiation. As AR expression varies as part of the normal cycle of the seminiferous epithelium,52 the expression of PCNA may be coincident with those stages that have low AR reactivity. Thus, the normal lowering of AR reactivity may be permissive to PCNA expression rather than a consequence of de-differentiation. As only a small proportion of Sertoli cells displayed features of an undifferentiated state, we speculate that all other Sertoli cells remain differentiated under the influence of residual levels of FSH and testicular testosterone.
In this study, rare proliferating Sertoli cells were also identified in men with TGCT. While the results do not implicate Sertoli cell de-differentiation in the progression from CIS to overt TGCT, the absence of PCNA-positive Sertoli cells in CIS tubules and presence in some tubules adjacent to seminoma, indicate that some secondary de-differentiation may occur, perhaps under the influence of the tumor. In pathological settings of infertility and testicular cancer, Sertoli cells have been reported to exhibit immature characteristics, as assessed either through histology15 or identification of specific markers associated with Sertoli cell differentiation.10,12 These immature cells have also been proposed to be a result of de-differentiation,16,17 although others21 believe that undifferentiated Sertoli cells persist into adulthood after aberrant differentiation in fetal development, resulting from a disruption in hormonal microenvironment, notably androgen signaling.
The organization of trans-membrane tight junction proteins at the BTB in tubules with CIS has not previously been assessed. The results presented here demonstrate claudin-11 and ZO-1 were expressed but the organization of junctions was disrupted in CIS. This disorganization occurred coincident with the described downregulation in connexin 43 expression and loss of BTB functionality in this setting.17,39,40,51,53
Our study demonstrated that JAM-A is present on germ cells in the normal human testis, but only in spermatogonia and spermatocytes. This is supported by evidence in the rat54,55 and hamster.56 JAM-A localization was disorganized in tubules with CIS and, unexpectedly, JAM-A reactivity was relatively high in seminoma. JAM-A is known to be involved in immune cell transmigration across blood vessels via homophilic interactions with JAM-A expressed on endothelial cells.57,58 Thus, the expression of JAM-A by seminoma cells may promote tumor cell migration and infiltration.
In conclusion, there is a mixed phenotype of Sertoli differentiation in men after gonadotropin suppression, a feature not observed in normal men, with a proportion of Sertoli cells expressing Ki67, and PCNA coincident with a possible downregulation of AR. This suggests that Sertoli cells in the men have the capacity to undergo “de-differentiation.” Sertoli cells in testicular carcinoma in situ also exhibited features of an undifferentiated state, such as the disruption of tight junction protein localization but without evidence of proliferation, suggesting an immature phenotype persisting from aberrant differentiation during development or puberty. A novel finding of the aberrant high expression of JAM-A in seminoma warrants further investigation, as the relevance of this to the disease process remains unknown.
Materials and Methods
Study design
Testicular biopsies were obtained from 30 normal men aged 31–46 y who underwent either no treatment or androgen-based male contraceptive treatment for 2 or 12 wk, prior to a previously planned vasectomy and informed consent, and details have been reported.7,8 These studies were approved by the Southern Health Human Research and Ethics Committee, and the Institutional Review Board of the University of Washington.
Briefly, men (n = 5/group) received either a weekly dose of 200 mg of testosterone enanthate (TE: Primotestin depot, Schering AG) alone or in combination with a single dose of 300 mg of depot medroxyprogesterone acetate (DMPA (progestin): Upjohn Pharmaceuticals, Rydalmere, Australia) by intramuscular injections for 2 or 12 wk before surgery.7 These sex steroid treatments resulted in feedback inhibition of gonadotropin release and, thereby, spermatogenic inhibition and rendered oligospermic.7 Five normal men not receiving any treatment served as controls.7 A fragment of the testicular biopsy from one testis of each man was immersion-fixed in Bouin’s solution for 3–5 h and paraffin-embedded for immunohistology.
Samples with TGCT were obtained from pathology departments in Copenhagen. All tumor samples were assessed by experienced pathologists and routinely stained with placental-like alkaline phosphatase (PLAP) to identify CIS cells. The samples included five seminomas, and five samples of the preserved testis tissue adjacent to an overt TGCT (seminoma or nonseminoma), which contained tubules with CIS. Permission for the use of these samples in immunohistochemical studies was obtained from the Regional Committee for Medical Research Ethics.
Immunohistochemistry
Paraffin-embedded sections (5 μm) were cut and adhered to Superfrost-Plus slides (HD Scientific) before drying at 37ºC for 1 h. Sections were de-waxed in Histolene (2 × 8 min) and 100% ethanol (5 min) before air-drying and re-hydration in graded ethanol (90, 75 and 50%) and finally in de-ionized H2O. Antigen retrieval was then performed by immersing sections in 600 ml of 1 mM EDTA-NaOH (pH 8.0)44 and heating in an 800 W microwave at 100% for 5 min, then 20% for 5 min, before cooling for 1 h at room temperature in EDTA buffer. Sections were then washed in de-ionized H2O followed by 0.9% phosphate-buffered saline (PBS). To block non-specific staining, sections were treated with 10% normal serum from the species in which the secondary antibody was raised before application of primary antibodies (outlined in Table 1). Sections were then washed in PBS (2 × 5 min), before addition of secondary antibody diluted 1:400 [goat anti-rabbit Alexa-546/647, goat anti-rat Alexa-546, goat anti-mouse Alexa-488/546, rabbit anti-goat Alexa-488 or donkey anti-goat Alexa-546 (Molecular Probes)] and incubated at room temperature for 30 min. For dual-immunohistological identification of GATA4 and PCNA in Sertoli cells, sections were incubated in 10% normal goat serum (Chemicon International) for 20 min after secondary detection of GATA4 and prior to the secondary detection of PCNA. For the simultaneous detection of GATA4 and Ki67, as well as GATA4, AR and PCNA, all primary antibodies were combined in the same cocktail for incubation as outlined in Table 1. Secondary detection was performed as outlined above. Specificity of primary antibodies was verified by incubating control sections in the equivalent concentration of non-specific antibody of the same isotype. Following secondary detection, all sections were washed in PBS (2 × 5 min), mounted in FluorSave (Calbiochem) and visualized on a confocal microscope (Fluoview FV300, Olympus Australia).
Table 1. Details of primary antibody specificity and incubation conditions.
| Antigen | Marker of: | Supplier | Catalogue # | Host | Concentration (ug/ml) |
Incubation time |
|---|---|---|---|---|---|---|
| GATA-4 | Sertoli, peritubular, interstitial cells | Santa Cruz Biotech | SC-1237 | Goat | 5 | 30 min |
| GATA-4 | Sertoli, peritubular, interstitial cells | eBioScience | 14-9980-82 | Rat | 1.25 | 2 h |
| PCNA | Proliferation capacity | BD Biosciences | 555567 | Mouse | 5 | 2 h |
| Ki67 | Proliferating cells | Affinity Bioreagents | PAI-38032 | Rabbit | 2.5 | 2 h |
| AR | Sertoli, Leydig, peritubular cells | Santa Cruz Biotech | SC-816 | Rabbit | 5 | 2 h |
| Claudin-11 | Sertoli cells | Zymed | 36-4500 | Rabbit | 2.5 | O/N |
| JAM-A | Sertoli cells | Zymed | 36-1700 | Rabbit | 2.5 | 2 h |
| Connexin 43 | Sertoli, peritubular, interstitial cells | Sigma-Aldrich | C-8093 | Mouse ascites |
- | 2 h |
Quantification of PCNA-labeled Sertoli cells
Fields were selected using a systematic uniform approach from a random start and images collected. Between 200–300 Sertoli cells were counted for each man. An unbiased counting frame was superimposed on each image and cells were counted if they fell within the frame or touched the acceptance boundary. GATA4 was used as marker of Sertoli cells within seminiferous tubules. The percent of PCNA-positive Sertoli cells was determined by dividing the number of labeled Sertoli cells by the total number of Sertoli cells (both positive and negative).24 Sertoli cells were observed with varying intensities of PCNA staining and were designated PCNA low, intermediate or high, but only intermediate and high Sertoli cells were included in the quantification of proliferating cells. Samples were run over five experiments.
Acknowledgments
The authors thank Georgia Balourdos for her assistance in preparing tissue sections. PHI manuscript number 08/18. Supported by the National Health and Medical Research Council of Australia (Grants #241000 and #494802, S.J.M., P.G.S., Grants #334011 and #384108, K.L. and #169020, R.I.M.), the Danish Cancer Society (Grant # DP05113, E.R-D.M.), the Australian Research Council (#348239, K.L.) and Monash University (Postgraduate Scholarship, G.T.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/24014
References
- 1.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 2.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec. 1977;187:347–66. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. The effects of recombinant follicle-stimulating hormone on the restoration of spermatogenesis in the gonadotropin-releasing hormone-immunized adult rat. Endocrinology. 1995;136:4035–43. doi: 10.1210/en.136.9.4035. [DOI] [PubMed] [Google Scholar]
- 5.Zhengwei Y, Wreford NG, Schlatt S, Weinbauer GF, Nieschlag E, McLachlan RI. Acute and specific impairment of spermatogonial development by GnRH antagonist-induced gonadotrophin withdrawal in the adult macaque (Macaca fascicularis) J Reprod Fertil. 1998;112:139–47. doi: 10.1530/jrf.0.1120139. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–79. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 7.McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–56. doi: 10.1210/jc.87.2.546. [DOI] [PubMed] [Google Scholar]
- 8.Matthiesson KL, McLachlan RI, O’Donnell L, Frydenberg M, Robertson DM, Stanton PG, et al. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab. 2006;91:3962–9. doi: 10.1210/jc.2006-1145. [DOI] [PubMed] [Google Scholar]
- 9.Stosiek P, Kasper M, Karsten U. Expression of cytokeratins 8 and 18 in human Sertoli cells of immature and atrophic seminiferous tubules. Differentiation. 1990;43:66–70. doi: 10.1111/j.1432-0436.1990.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 10.Steger K, Rey R, Kliesch S, Louis F, Schleicher G, Bergmann M. Immunohistochemical detection of immature Sertoli cell markers in testicular tissue of infertile adult men: a preliminary study. Int J Androl. 1996;19:122–8. doi: 10.1111/j.1365-2605.1996.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 11.Kliesch S, Bergmann M, Hertle L, Nieschlag E, Behre HM. Semen parameters and testicular pathology in men with testicular cancer and contralateral carcinoma in situ or bilateral testicular malignancies. Hum Reprod. 1997;12:2830–5. doi: 10.1093/humrep/12.12.2830. [DOI] [PubMed] [Google Scholar]
- 12.Steger K, Rey R, Louis F, Kliesch S, Behre HM, Nieschlag E, et al. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathological human testis. Hum Reprod. 1999;14:136–43. doi: 10.1093/humrep/14.1.136. [DOI] [PubMed] [Google Scholar]
- 13.Maymon BB, Paz G, Yogev L, Hauser R, Schreiber L, Botchan A, et al. [Immunohistochemical identification of testicular germ cells--preliminary report] Harefuah. 2000;139:179–82, 247. [PubMed] [Google Scholar]
- 14.Maymon BB, Yogev L, Paz G, Kleiman SE, Schreiber L, Botchan A, et al. Sertoli cell maturation in men with azoospermia of different etiologies. Fertil Steril. 2002;77:904–9. doi: 10.1016/S0015-0282(02)03060-1. [DOI] [PubMed] [Google Scholar]
- 15.Hoei-Hansen CE, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol. 2003;200:370–4. doi: 10.1002/path.1372. [DOI] [PubMed] [Google Scholar]
- 16.Brehm R, Rey R, Kliesch S, Steger K, Marks A, Bergmann M. Mitotic activity of Sertoli cells in adult human testis: an immunohistochemical study to characterize Sertoli cells in testicular cords from patients showing testicular dysgenesis syndrome. Anat Embryol (Berl) 2006;211:223–36. doi: 10.1007/s00429-005-0075-8. [DOI] [PubMed] [Google Scholar]
- 17.Brehm R, Rüttinger C, Fischer P, Gashaw I, Winterhager E, Kliesch S, et al. Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia. 2006;8:499–509. doi: 10.1593/neo.05847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nistal M, Gonzalez-Peramato P, Regadera J, Serrano A, Tarin V, De Miguel MP. Primary testicular lesions are associated with testicular germ cell tumors of adult men. Am J Surg Pathol. 2006;30:1260–8. doi: 10.1097/01.pas.0000213361.10756.08. [DOI] [PubMed] [Google Scholar]
- 19.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 20.Skakkebaek NE. Abnormal morphology of germ cells in two infertile men. Acta Pathol Microbiol Scand A. 1972;80:374–8. doi: 10.1111/j.1699-0463.1972.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–23. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 22.Rajpert-De Meyts E, Skakkebaek NE. The possible role of sex hormones in the development of testicular cancer. Eur Urol. 1993;23:54–9, discussion 60-1. doi: 10.1159/000474570. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, et al. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci USA. 2006;103:17718–23. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarulli GA, Stanton PG, Lerchl A, Meachem SJ. Adult sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization. Biol Reprod. 2006;74:798–806. doi: 10.1095/biolreprod.105.050450. [DOI] [PubMed] [Google Scholar]
- 25.Orth JM, Boehm R. Endorphin suppresses FSH-stimulated proliferation of isolated neonatal Sertoli cells by a pertussis toxin-sensitive mechanism. Anat Rec. 1990;226:320–7. doi: 10.1002/ar.1092260308. [DOI] [PubMed] [Google Scholar]
- 26.Zirkin BR, Awoniyi C, Griswold MD, Russell LD, Sharpe R. Is FSH required for adult spermatogenesis? J Androl. 1994;15:273–6. [PubMed] [Google Scholar]
- 27.Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Van Haaster LH, De Jong FH, Docter R, De Rooij DG. The effect of hypothyroidism on Sertoli cell proliferation and differentiation and hormone levels during testicular development in the rat. Endocrinology. 1992;131:1574–6. doi: 10.1210/en.131.3.1574. [DOI] [PubMed] [Google Scholar]
- 29.Simorangkir DR, de Kretser DM, Wreford NG. Increased numbers of Sertoli and germ cells in adult rat testes induced by synergistic action of transient neonatal hypothyroidism and neonatal hemicastration. J Reprod Fertil. 1995;104:207–13. doi: 10.1530/jrf.0.1040207. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology. 2000;141:18–27. doi: 10.1210/en.141.1.18. [DOI] [PubMed] [Google Scholar]
- 31.Buzzard JJ, Farnworth PG, De Kretser DM, O’Connor AE, Wreford NG, Morrison JR. Proliferative phase sertoli cells display a developmentally regulated response to activin in vitro. Endocrinology. 2003;144:474–83. doi: 10.1210/en.2002-220595. [DOI] [PubMed] [Google Scholar]
- 32.Puglisi R, Montanari M, Chiarella P, Stefanini M, Boitani C. Regulatory role of BMP2 and BMP7 in spermatogonia and Sertoli cell proliferation in the immature mouse. Eur J Endocrinol. 2004;151:511–20. doi: 10.1530/eje.0.1510511. [DOI] [PubMed] [Google Scholar]
- 33.Cooke PS, Meisami E. Early hypothyroidism in rats causes increased adult testis and reproductive organ size but does not change testosterone levels. Endocrinology. 1991;129:237–43. doi: 10.1210/endo-129-1-237. [DOI] [PubMed] [Google Scholar]
- 34.Boitani C, Stefanini M, Fragale A, Morena AR. Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology. 1995;136:5438–44. doi: 10.1210/en.136.12.5438. [DOI] [PubMed] [Google Scholar]
- 35.Li LH, Jester WF, Jr., Laslett AL, Orth JM. A single dose of Di-(2-ethylhexyl) phthalate in neonatal rats alters gonocytes, reduces sertoli cell proliferation, and decreases cyclin D2 expression. Toxicol Appl Pharmacol. 2000;166:222–9. doi: 10.1006/taap.2000.8972. [DOI] [PubMed] [Google Scholar]
- 36.Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, et al. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140:5364–73. doi: 10.1210/en.140.11.5364. [DOI] [PubMed] [Google Scholar]
- 37.Buzzard JJ, Wreford NG, Morrison JR. Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction. 2002;124:633–41. doi: 10.1530/rep.0.1240633. [DOI] [PubMed] [Google Scholar]
- 38.Buzzard JJ, Wreford NG, Morrison JR. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat sertoli cells. Endocrinology. 2003;144:3722–31. doi: 10.1210/en.2003-0379. [DOI] [PubMed] [Google Scholar]
- 39.Brehm R, Marks A, Rey R, Kliesch S, Bergmann M, Steger K. Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J Pathol. 2002;197:647–53. doi: 10.1002/path.1140. [DOI] [PubMed] [Google Scholar]
- 40.Fink C, Weigel R, Hembes T, Lauke-Wettwer H, Kliesch S, Bergmann M, et al. Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ. Neoplasia. 2006;8:1019–27. doi: 10.1593/neo.06559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CQ, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol. 2007;178:549–56. doi: 10.1083/jcb.200704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazaud-Guittot S, Meugnier E, Pesenti S, Wu X, Vidal H, Gow A, et al. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod. 2010;82:202–13. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–59. doi: 10.1016/S0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 44.Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, et al. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997;183:116–23. doi: 10.1002/(SICI)1096-9896(199709)183:1<116::AID-PATH1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Oka Y, Takeda K. Retinoic acid combined with GM-CSF induces morphological changes with segmented nuclei in human myeloblastic leukemia ML-1 cells. Anticancer Res. 1997;17(3C):1951–5. [PubMed] [Google Scholar]
- 46.Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, et al. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–91. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- 47.Tarulli GA, Stanton PG, Meachem SJ. Is the adult Sertoli cell terminally differentiated? Biol Reprod. 2012;87:13–, 1-11. doi: 10.1095/biolreprod.111.095091. [DOI] [PubMed] [Google Scholar]
- 48.Meachem SJ, Stanton PG, Schlatt S. Follicle-stimulating hormone regulates both Sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biol Reprod. 2005;72:1187–93. doi: 10.1095/biolreprod.104.039321. [DOI] [PubMed] [Google Scholar]
- 49.Dirami G, Ravindranath N, Kleinman HK, Dym M. Evidence that basement membrane prevents apoptosis of Sertoli cells in vitro in the absence of known regulators of Sertoli cell function. Endocrinology. 1995;136:4439–47. doi: 10.1210/en.136.10.4439. [DOI] [PubMed] [Google Scholar]
- 50.Chausiaux OE, Abel MH, Baxter FO, Khaled WT, Ellis PJ, Charlton HM, et al. Hypogonadal mouse, a model to study the effects of the endogenous lack of gonadotropins on apoptosis. Biol Reprod. 2008;78:77–90. doi: 10.1095/biolreprod.107.060970. [DOI] [PubMed] [Google Scholar]
- 51.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–60. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 52.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–34. doi: 10.1210/en.135.3.1227. [DOI] [PubMed] [Google Scholar]
- 53.Roger C, Mograbi B, Chevallier D, Michiels JF, Tanaka H, Segretain D, et al. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J Pathol. 2004;202:241–6. doi: 10.1002/path.1509. [DOI] [PubMed] [Google Scholar]
- 54.Chalmel F, Rolland AD, Niederhauser-Wiederkehr C, Chung SS, Demougin P, Gattiker A, et al. The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci USA. 2007;104:8346–51. doi: 10.1073/pnas.0701883104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev Biol. 2008;313:246–55. doi: 10.1016/j.ydbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarulli GA, Meachem SJ, Schlatt S, Stanton PG. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction. 2008;135:867–77. doi: 10.1530/REP-07-0572. [DOI] [PubMed] [Google Scholar]
- 57.Ostermann G, Fraemohs L, Baltus T, Schober A, Lietz M, Zernecke A, et al. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–35. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- 58.Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–8. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]


