Abstract
TB causes 1.4 million deaths annually. HIV-1 infection is the strongest risk factor for TB. The characteristic immunological effect of HIV is on CD4 cell count. However, the risk of TB is elevated in HIV-1 infected individuals even in the first few years after HIV acquisition and also after CD4 cell counts are restored with antiretroviral therapy. In this review, we examine features of the immune response to TB and how this is affected by HIV-1 infection and vice versa. We discuss how the immunology of HIV–TB coinfection impacts on the clinical presentation and diagnosis of TB, and how antiretroviral therapy affects the immune response to TB, including the development of TB immune reconstitution inflammatory syndrome. We highlight important areas of uncertainty and future research needs.
Keywords: antiretroviral therapy, ART, diagnosis, HIV, immunity, immunopathology, TB, TB-associated immune reconstitution inflammatory syndrome, TB-IRIS, tuberculosis
Background
TB causes 1.4 million deaths annually [1]. Despite increasing political and financial commitment to reduce the impact of this disease over recent decades, morbidity due to TB has escalated. In South Africa, a country with one of the highest TB burdens, the incidence of TB quadrupled from a case notification rate of 163 per 100,000 of the population in 1986 to 628 per 100,000 of the population in 2006 [2]. This increase was driven by the emergence of the HIV-1 epidemic. HIV-1 is now recognized as the strongest risk factor for TB. A recent study demonstrated substantially higher TB rates in HIV-infected individuals despite antiretroviral therapy (ART), with 2.70–25.49 TB cases per 100 person-years in HIV-infected individuals on ART compared with 0.62 cases per 100 person-years in HIV-uninfected individuals living in the same community [3]. TB is the commonest cause of death in HIV-infected individuals in Africa and a third of all TB deaths are in HIV-infected patients [1,4].
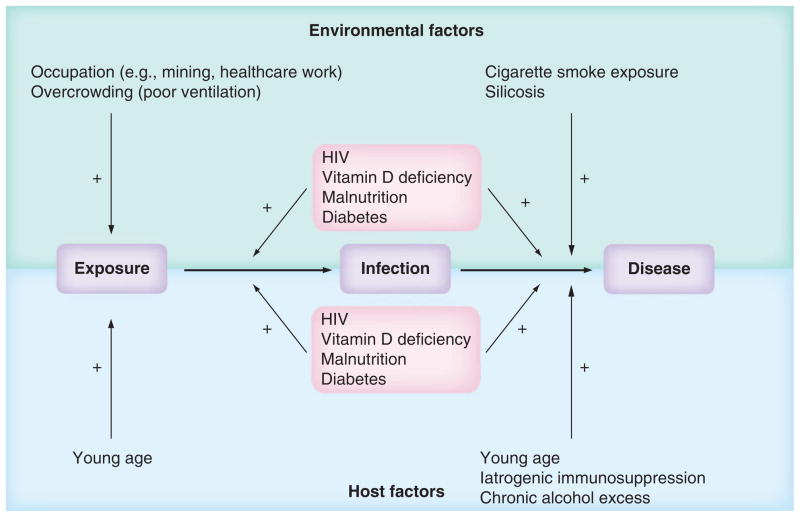
A compromised immune response increases the risk of TB disease. Extremes of age, malnutrition, chronic alcohol excess, diabetes and vitamin D deficiency are all associated with some degree of impaired immune function [5–10]. In many populations affected significantly by TB, HIV coexists with these socioeconomic and environmental factors, affecting TB exposure, risk of infection and progression to disease, and potentially exacerbating the problem (Figure 1) [6,7,11,12].
Figure 1. Major risk factors for TB disease.
Environmental and host factors influence Mycobacterium tuberculosis exposure and the risk of development of active TB once infected. Young age, with increased proximity to infectious adults and a less developed immune response, confers increased risk of exposure and progression to disease. Diabetes, malnutrition, vitamin D deficiency and HIV infection impact on risk following exposure, probably by both increasing the likelihood of exposure leading to chronic infection and progression of infection to active TB. These conditions are affected by a complex interplay of host and environmental factors: for example, vitamin D deficiency occurs as a result of insufficient environmental UVB exposure and also host genetic factors that influence vitamin D metabolism. Host factors (e.g., immunosuppression with anti-TNF-α therapy) also increase risk of progression of infection to TB disease.
The epidemiological background
The epidemiological relationship between TB and HIV is complex. HIV-induced progressive CD4 depletion is associated with increased risk of TB, disseminated TB and death [13]. However, unlike other opportunistic infections, the risk of TB is elevated in HIV-infected individuals throughout the course of HIV infection, even in the first few years after acquisition, when the CD4 cell count remains high [14,15]. ART, while restoring absolute CD4 cell numbers, does not completely reverse the effect of HIV on TB risk [16]. In addition, TB infection is associated with clinical progression of HIV-1 infection [17]. HIV–TB coinfected patients have been found to be at higher risk of new additional opportunistic infections and mortality than HIV-infected patients without TB with the same CD4 count [18–20]. All patients with active TB are now recommended to start ART, irrespective of CD4 cell count [21].
The spectrum of immune responses to TB
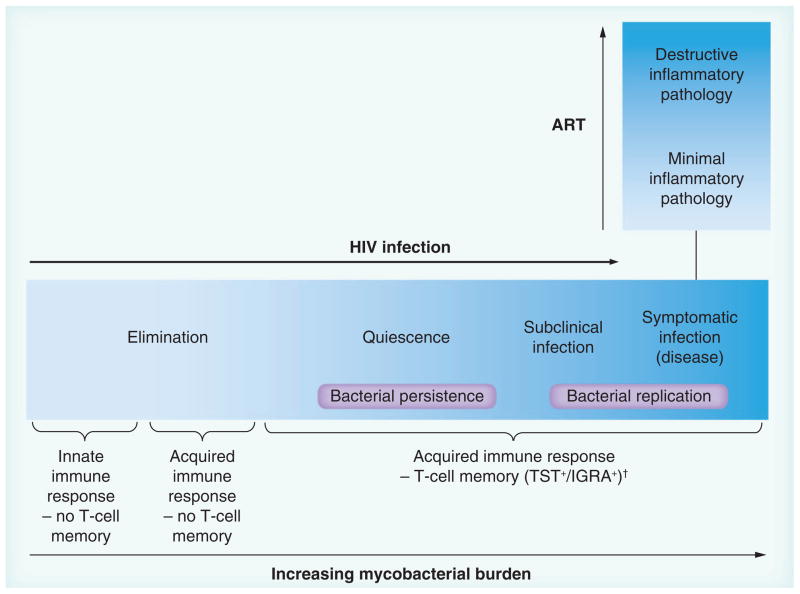
Classically, Mycobacterium tuberculosis (M.tb.) exposure is considered to result in one of three outcomes: uninfected, latently (quiescently) infected or actively infected (diseased). Examining TB in the context of HIV-1 infection has contributed to a new paradigm of TB infection proposing a continuum of immune responses in an infected individual correlating with a spectrum of disease progression risk, rather than binary latently infected or diseased states (Figure 2) [22–24]. At one end of the spectrum, it is proposed that an effective innate immune response can clear the host of M.tb. without T-cell priming and without causing clinical symptoms. At the other end of the spectrum, failure of both innate and acquired immune responses leads to mycobacterial replication and symptomatic disease. ‘Latent’ infection, rather than simply describing the presence of nonreplicating bacteria is considered to more accurately reflect a range of immune responses providing relative control of M.tb. It is now recognized that subclinical TB can occur, particularly in HIV-infected individuals, who may be sputum culture positive for M.tb., without manifesting signs or symptoms of disease [24–26].
Figure 2. The spectrum of immune responses to TB correlates with a spectrum of outcomes of Mycobacterium tuberculosis infection.
HIV infection (and mycobacterial burden) impact on risk of progression towards disease. Despite high mycobacterial burdens, characteristic inflammatory pathology (e.g., dense pulmonary consolidation and cavitation) is rare in HIV when CD4 counts are <200 cells/mm3. This may occur in those with relatively preserved CD4 counts or develop with ART, in TB-associated immune reconstitution inflammatory syndrome.
†In HIV, evidence of T-cell memory may be lost.
ART: Antiretroviral therapy.
In addition, symptomatic active TB presents with a spectrum of pathology (Figure 2). At one end, severe inflammatory TB disease occurs, attributed to exaggerated immune responses, with severe destructive pulmonary pathology and cavitation. This may be paucibacillary and is rarely seen in advanced HIV infection. At the other end of the spectrum disseminated TB infection may occur, with a high M.tb. burden, but only a limited inflammatory response. The latter is more frequently seen in advanced HIV. However, during the TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) when HIV-infected patients start ART, inflammatory TB pathology may rapidly develop despite anti-TB therapy (paradoxical TB-IRIS) or develop acutely in the absence of a previous TB diagnosis (unmasking TB-IRIS) [27].
If a declining absolute CD4 count is not solely responsible for TB risk, what is the defect responsible in HIV infection? What is the mechanism by which HIV-1 and CD4 T-cell decline confer a risk of disseminated and extrapulmonary forms of TB, while localized inflammatory pathology is reduced, and why is TB harder to diagnose in HIV-infected patients, despite higher mycobacterial loads? Why does ART fail to fully reverse TB risk, and in some cases increase immunopathological features of TB disease? In this review we consider these questions, examining firstly, features of the immune response to TB, and how this is affected by HIV-1 infection and vice versa; secondly, how immune responses in HIV–TB coinfection impact on clinical features of active TB and diagnosis; and thirdly, the effect of ART on TB immunity, including immunological aspects of TB-IRIS. We highlight important areas of uncertainty and future research needs.
Immune interactions: TB & HIV-1 infection
What features of the immune response are important in TB infection?
M.tb., the causative organism of TB infection, is spread by respiratory droplets, aerosolized from a pulmonary TB patient when coughing, and inhaled by an exposed person. Not all exposed individuals develop lasting immunological evidence of infection. The reported incidence of ‘latent’ infection following exposure is wide (0–89%) depending on the exposure, environment and population of study [28–30]. The immune response to TB comprises ‘innate’ immune barriers such as the physical components of the airway, cilia and mucus, cells resident in the lung (e.g., alveolar macrophages) and cells recruited rapidly to the site of infection that can mount an immediate response (e.g., neutrophils); and ‘acquired’ cellular responses, that require prior sensitization or priming to effect immunity (e.g., those of CD4 and CD8 T lymphocytes). Th1 cytokines (e.g., TNF-α and IFN-γ) are critical to TB immunity, as demonstrated by mouse knockout models, and increased susceptibility to TB in humans treated with anti-TNF-α agents and with genetic disorders associated with defective IFN-γ function [31–36]. IFN-γ is produced largely by lymphocytes. It promotes macrophage activation and plays a key role in granuloma formation [31,32,37]. B lymphocytes are present within granulomas but their precise role in TB immunity is unclear (as reviewed by Cooper [38]).
The central role of the TB granuloma
The histopathological hallmark of M.tb. infection and the focal point of the immune response to M.tb. is the granuloma, a structure characterized by epithelioid macrophages and Langhans giant cells, around which T lymphocytes cluster. A center of caseous necrotic material distinguishes it from other granulomatous diseases (e.g., sarcoid), although it is not always present. M.tb. granulomas may be non-necrotic or fibrotic. Typically, few if any M.tb. bacilli are detectable at the center of caseous granulomas. However, whether a granuloma provides an ‘effective’ immune response is controversial. Initially, they were believed to facilitate containment of M.tb. bacilli and mycobacterial killing. However, some studies suggest that the granuloma may facilitate microbial persistence by the recruitment of potential uninfected host cells to the disease site, as reviewed by Paige and Bishai, and even favor mycobacterial transmission by promoting host tissue damage (see also later section ‘Tissue destruction’) [39–42]. Parallels have been drawn with the role of the granulomatous inflammation in schistosomiasis transmission, where a correlation has been shown between the magnitude of the inflammatory reaction at the site of the schistosome eggs and the rate of schistosome excretion (and hence transmission) [42].
A limited number of human studies have compared the histopathological features of granulomas found in HIV-uninfected TB patients, with those found in TB patients with advanced HIV. Early post-mortem studies of TB in advanced HIV infection in sub-Saharan Africa demonstrated that the density of Langhans giant cells in TB lesions correlated with pre-mortem blood CD4 counts [43,44]. In patients with relatively preserved CD4 counts, typical granuloma architecture may be seen. In advanced HIV infection, M.tb. lesions are typically found to be multibacillary and highly necrotic, with ill-formed or absent granuloma, and lacking in epithelioid and Langhans giant cells. Similar findings were reported in a post-mortem study from Brazil examining pulmonary lesions in HIV–TB coinfection and histopathological analysis of biopsy specimens from cases of TB lymphadenitis [45–47]. The Brazilian study also reported increased polymorphonuclear cell infiltration in HIV–TB coinfected granulomas and reduced TNF-α staining [45].
More detailed analysis of the influence of HIV-1 infection on the TB granuloma has been impaired by limitations of popular animal models of TB, including the mouse model, where granulomas lack similar structure and caseation to that seen in human M.tb. infection. In a recent review of this literature, Diedrich and Flynn highlight the paucity of studies assessing the effect of HIV–TB coinfection on the granuloma and call for tissue-based studies and representative granuloma models, in order to more closely mimic human tuberculosis lesions and reflect the interactions that may take place between different cellular components [48]. However, despite this, a large number of studies have examined the impact of HIV-1 infection on specific aspects of the immune response that may influence the granulomatous response to M.tb., and in the subsequent sections we review the evidence relating to cells of the innate and acquired immune responses in turn.
TB, HIV & innate immunity
The majority of research into the mechanisms of susceptibility to TB in HIV-infected patients has focused on defects in cell-mediated immunity, particularly relating to CD4 T-cell number and function (examined in subsequent sections). However, the first immune cells to encounter M.tb. are innate immune cells in the lung (e.g., alveolar macrophages and dendritic cells). In recent years, as questions remained unanswered about the susceptibility of HIV-infected patients to TB, greater attention has been paid to defects of the innate immune system and interactions between innate and acquired immune responses.
Interference with macrophage function
Following pulmonary M.tb. infection, alveolar macrophages phagocytose M.tb. bacilli. They are able to present peptide antigen to T cells via MHC class II molecules and secrete cytokines, (e.g., IFN-γ and TNF-α), which influence T-cell differentiation and cytotoxic responses.
An important feature of mycobacterial virulence is resistance to the bactericidal mechanisms of phagocytic cells. M.tb. interferes with phagosomal maturation, is able to resist lysosomal acidification, and potentially inhibits macrophage apoptosis (a process that facilitates mycobacterial killing), therefore, promoting intracellular persistence [49–51].
Evidence suggests that HIV–TB coinfection may potentiate some of these mycobacterial virulence factors. Some in vitro studies suggest that HIV-1 infection of human monocyte-derived macrophages increases M.tb. growth, with reports also suggesting that HIV–TB coinfection reduces macrophage viability and is associated with increased TNF-α and IL-10 production [52,53]. However, other studies have not found a significant effect of HIV-1 infection on M.tb. growth in human monocyte-derived macrophages [54,55]. A study of alveolar macrophages from HIV–TB coinfected patients in Malawi demonstrated intact phagocytosis [56]. However, intracellular M.tb. bacilli were found to reside in vacuoles at a higher pH than that of neighboring M.tb.-uninfected vacuoles, and these vacuoles failed to fuse with lysosomes and acidify. This study was limited by the lack of a TB-infected, HIV-uninfected control group.
There is an increasing body of evidence that suggests that TNF-α dependent macrophage apoptosis is reduced in HIV–TB coinfection. Patel et al. demonstrated reduced M.tb.-induced apoptosis of human alveolar macrophages in a HIV-infected donors compared with HIV-uninfected donors [57]. Their studies suggest that HIV-1 infection inhibits macrophage apoptosis in response to M.tb. infection by inducing IL-10, which reduces TNF-α production and its proapoptotic effects via a BCL-3-mediated pathway. Kumawat et al. have also demonstrated that HIV Nef protein directly reduces M.tb.-induced macrophage apoptosis via the inhibition of TNF-α promoter activation and interference with TNF-α mRNA stability [58].
The process of capture and degradation of cytoplasmic contents by autophagosomes (double-membraned organelles), known as autophagy, is an important mechanism of macrophage resistance to bacterial and viral infections and is likely to have a role in the clearance of mycobacteria [49]. HIV proteins interfere with autophagy in a variety of cells types, including CD4 T cells and macrophages, both promoting the early stages of autophagy (which may facilitate HIV replication in some cells) and arresting maturation of the autophagocytic process [59–61]. The active metabolite of vitamin D, 1α, 25-dihydroxycholecalciferol, has recently been shown to induce autophagy in macrophages coinfected with M.tb. and HIV-1, leading to reduced M.tb. growth and HIV replication [55]. This is interesting in light of epidemiological observations that vitamin D deficiency is associated with an increased risk of TB and progression of HIV [12,62–65].
Neutrophils are innate immune cells that have been potentially underestimated in TB pathogenesis (reviewed by Lowe et al.), despite their ability to phagocytose (although not necessarily kill) mycobacteria and their presence at sites of TB disease [66–68]. Human neutrophil peptides 1–3 have antimycobacterial activity in vitro [69]. Neutrophil activity in TB has been associated both with increased severity of disease and with protection [69,70]. The presence of suppurative necrosis, rather than caseating granulomas found most commonly in human TB lesions in advanced HIV, would suggest a prominent role for the neutrophil in HIV–TB pathology, but this is yet to be defined.
HIV, TB & acquired immunity
CD4 T cells & susceptibility to TB infection: animal studies
The heightened susceptibility of HIV-infected individuals to TB strongly implicates the CD4 T cell in a protective immune response to TB, as the characteristic immunological effect of HIV is on CD4 cell numbers, and CD4 T cells are the main cell type infected by HIV. CD4 T cells are present on the periphery of TB granulomas, and when M.tb. is phagocytosed, antigen presenting cells present mycobacterial peptide to CD4 T cells (via MHC class II), which may then provide cytokine and chemokine signals to activate innate immune cells and recruit cytotoxic lymphocytes and other cells to the disease site, as reviewed by Kwan and Ernst [71].
Mogues et al. attempted to tease out the relative importance of different aspects of cell-mediated immunity in a mouse model of M.tb. infection [72]. Mice devoid of any functional T cells (thymectomy followed by monoclonal antibody infusions to CD4 and CD8) succumbed rapidly to M.tb. infection, by comparison with wild-type mice, who appeared to control the infection within 20 days and survived over 250 days. Mutant mice with defective CD4 T-cell function were unable to control M.tb. infection, whereas those with CD8 T-cell dysfunction (targeted disruption of the β2-microglobulin gene) controlled infection, although notably with a higher bacillary burden than the wild-type mice. Similar results were achieved using blocking antibody to CD4 and CD8 [72].
The mouse model of TB, while regularly used to understand immune mechanisms, fails to mimic human pathology. Mouse M.tb. granulomas lack the organization seen in humans and mice do not develop caseous necrosis or cavitation [73]. Therefore, more recently cynomolgus macaque models have been used to better mimic the spectrum of TB pathology found in humans [74–77]. In macaques challenged with low dose aerosol M.tb. infection, a latently infected state occurs in around 50% animals (defined as tuberculin skin test positive and no clinical symptoms/signs of disease 8–10 months postinfection), which is then maintained for many years [74,77]. Subsequent SIV infection has been used to model the effect of HIV on latent TB [74,78]. After a dip in CD4 and CD8 cell numbers immediately after SIV infection, T-cell numbers recover to pre-SIV levels in all macaques by 8 weeks. All SIV–TB coinfected monkeys developed reactivation of latent TB within 47 weeks, with some reactivating ‘early’ (12–17 weeks post-SIV infection), and some reactivating ‘late’ (26–47 weeks) [74]. There was a significant correlation between the extent of initial peripheral CD4 and CD8 T-cell depletion in primary SIV infection and the time to reactivation of latent TB. However, no differences were found in peripheral CD4 or CD8 T-cell number between monkeys at the time of reactivation and just prior to necropsy, when comparing either early with late reactivating SIV–TB macaques, or coinfected macaques with SIV-uninfected macaques experiencing TB disease.
In these studies, the early-reactivating coinfected monkeys had lower frequencies of CD4 T cells in the airways at 10 weeks post-SIV infection than later-reactivating monkeys (p = 0.05). Coinfected monkeys had significantly fewer CD4 T cells in lung granulomas than M.tb. monoinfected monkeys with active TB and there was a trend towards fewer CD4 T cells in the lung draining lymph nodes of coinfected versus either SIV- or M.tb.-monoinfected monkeys.
Another study using macaques examined the effect of CD4 cell depletion by using huOKT4A antibodies to deplete CD4 T cells in blood and tissues, rather than SIV infection [75]. In CD4-depleted monkeys, an increased incidence of active TB following acute TB infection was seen compared with nondepleted monkeys (80 vs 17%, respectively). In monkeys with established latent M.tb. infection observed over 14 weeks, huOKT4A antibody treatment resulted in reactivation in 50%, compared with 0% control monkeys. Although all huOKT4A monkeys had lower frequencies of CD4 T cells in peripheral blood mononuclear cells (PBMC), bronchoalveolar lavage (BAL) and hilar lymph nodes than non-CD4-depleted monkeys at necropsy, significantly lower frequencies of CD4 T cells were found in hilar lymph nodes of huOKT4A monkeys that reactivated TB compared with huOKT4A monkeys that did not (3.7 vs 16.7%; p < 0.05).
A benefit of animal studies is the ability to control the timing of HIV/SIV and M.tb. infection, allowing observation of early immunological events. Together these studies highlight the importance of CD4 T cells in susceptibility to TB and suggest that consideration of cell dynamics at the site of mycobacterial infection and local lymph nodes, in addition to absolute peripheral blood CD4 T-cell numbers, may be valuable.
CD4 T cells & susceptibility to TB infection: human studies
Human studies have examined immune responses at the site of active TB, as well as in peripheral blood. Pulmonary and pleural TB are typically characterized by a CD4 lymphocyte infiltration at the site of disease in HIV-uninfected populations. In HIV-infected patients with TB, a comparatively reduced number of CD4 T cells are seen in BAL, despite the total number of lymphocytes being increased relative to healthy controls [79,80]. Studies of human disease have also attempted to characterize functional (in addition to quantitative) defects in CD4 T cells in HIV–TB coinfection. This has been demonstrated in two ways: first, by examining production of key cytokines (e.g., IFN-γ) by CD4 lymphocytes in response to stimulation by TB antigens [81–84]; and second, by examining the differences in differentiation phenotype of prevalent T lymphocytes, from antigen-naive cells to antigen-specific memory cells mainly found in lymphoid tissue, and terminally differentiated effector memory cells found in the periphery and at disease sites [81,85,86].
In an example of the former approach, Geldmacher et al. performed a cross-sectional study of PBMC from asymptomatic HIV-infected and -uninfected Tanzanian patients with no signs of TB disease, and a small number of patients with active TB. CD4 T-cell IFN-γ responses were measured by ELISpot and intracellular cytokine staining, performed after overnight stimulation with purified protein derivative (PPD) and the M.tb.-specific antigen ESAT-6 [81]. Chronic HIV-1 infection, in the absence of TB disease, was associated with significantly decreased detectable IFN-γ responses. In active TB, these responses were detectable in the majority of both HIV-infected and -uninfected patients. There was no linear correlation between IFN-γ response and total CD4 cell count in M.tb.-infected or -uninfected HIV-infected patients. In a longitudinal study, they examined five ‘latently’ M.tb.-infected HIV-uninfected subjects who acquired HIV infection. In four out of five of these patients, detectable responses to M.tb. antigens diminished completely within 1 year of seroconversion [81]. None of these four patients developed active TB within 4 years of subsequent follow-up. However, one patient developed dramatically increased responses 1 year after seroconversion. This patient was concurrently diagnosed with active TB [81].
Similarly, Rangaka et al. found reduced IFN-γ responses to PPD in a whole-blood assay, comparing HIV-infected with HIV-uninfected asymptomatic patients in a highly TB-exposed population from Khayelitsha, South Africa [84]. However, in this study, ELISpot responses to ESAT-6 (and additionally CFP10) were not different between HIV-infected and HIV-uninfected individuals. PPD responses significantly correlated with CD4 count, but ESAT-6 and CFP10 responses did not.
These studies suggest that M.tb.-specific CD4 T cells are depleted early in HIV infection. Why should this be the case? CCR5 expression on the surface of these cells may have a role. CCR5, a disease site homing receptor, is the most common coreceptor used by HIV virus to enter cells. M.tb.-specific memory CD4 T cells from latently M.tb.-infected HIV-negative individuals have been shown to frequently coexpress CCR5 (twice the frequency of the total memory CD4 T-cell population), implying that M.tb.-specific CD4 T cells may be particularly susceptible to HIV-1 infection. However, CMV-specific CD4 T cells are depleted less rapidly after acute HIV infection than M.tb.-specific CD4 T cells, despite similar CCR5 expression [81].
Other phenotypic features of these M.tb-specific T cells have been implicated in their susceptibility, such as production of IL-2, which promotes CD4 T-cell proliferation and increases susceptibility to direct HIV infection in cellular models [87]. CMV-specific CD4 T cells produce less IL-2 and more MIP-1β, a CCR5 ligand, which may have a protective role [87,88]. Geldmacher and Koup suggest that in the absence of clinical disease, M.tb.-specific CD4 T cells more commonly express CD27, and lack CD57 (a senescence marker), indicating a transitional or early differentiated phenotype, more commonly associated with IL-2 production, whereas circulating CMV-specific CD4 T cells are more commonly CD27−CD57+, a terminally differentiated phenotype [88]. This difference may correspond to a difference between the two pathogens in the degree to which CD4 T cells are exposed to antigen during latent or subclinical infection, with more persistent low-level antigen exposure after infection with CMV compared with that following M.tb. infection.
In summary, the ideal target cell for HIV infection and depletion appears to be a CD4-and CCR5-expressing T cell, producing abundant IL-2 and little MIP-1β. Increasing evidence suggests that these features are particularly prevalent in M.tb.-specific CD4 T-cell populations and may explain their selective depletion early in the course of HIV infection.
The role of polyfunctional T cells
The role of differentiation phenotype of antigen-specific CD4 T cells, influencing predominant cytokine production and susceptibility to infection, is highlighted by an emerging debate on the role of polyfunctional CD4 T cells in HIV–TB coinfection. Polyfunctional CD4 T cells are antigen-specific CD4 T cells that coexpress IFN-γ, TNF-α and IL-2 and are considered to be an effector memory population, – less well differentiated but with a more durable antigen-specific response – than terminally differentiated effector cells [89]. Mansoor et al. studied infant immune responses following BCG vaccination at birth [83]. In the peripheral blood of HIV-infected infants, they noted a significant reduction in Th1 cytokine production (IFN-γ, TNF-α, IL-2) in response to restimulation with BCG, compared with HIV-uninfected infants, and a significantly reduced number of ‘polyfunctional’ CD4 T cells. Kalsdorf et al. showed that polyfunctional CD4 T cells were reduced in BAL samples from HIV-infected compared with HIV-uninfected healthy but highly TB-exposed patients [82].
In a study of pericardial TB, the phenotypic and functional qualities of CD4 T cells, including polyfunctionality, were examined at the site of TB disease [85]. Measurement of antigen-induced IFN-γ responses in peripheral blood and pericardial fluid, comparing HIV-infected with -uninfected patients, revealed that pericardial fluid responses were elevated compared with those of blood in both HIV-infected and -uninfected patients. However, in HIV infection, pericardial fluid contained more polyfunctional and fewer terminally differentiated CD4 T cells. We hypothesize that terminally differentiated effector cells, which typically express more CCR5, are susceptible to lytic HIV infection, and are rapidly depleted in HIV, leading to the migration of less well-differentiated, polyfunctional T cells with a central or effector memory phenotype, to the site of disease. In addition, central memory T cells are believed to serve as a reservoir of HIV infection, and may harbor virus due to their long half-life [90,91]. It is possible that migration of these cells to sites of M.tb. replication also leads to progression of HIV infection, as TB infection drives HIV replication (see section ‘TB impacts on the immune response to HIV-1 infection’ later).
Polyfunctionality has been associated with TB protection in recent novel TB vaccine studies [92]. However, this is controversial. A correlation with protection from TB disease in humans has not yet been shown [89,92]. In Italian and Gambian patients, higher proportions of polyfunctional CD4 T cells are found in peripheral blood of active pulmonary TB patients following restimulation with M.tb. antigens, compared with latently infected controls, and in the Italian study, these cell populations decreased with TB treatment, suggesting a correlation with viable M.tb. burden [93,94]. However, a similar study in Cape Town, South Africa reported conflicting results [95]. In this study, decreased peripheral blood polyfunctional CD4 T-cell responses to restimulation with M.tb. antigens were found in smear-positive compared with smear-negative pulmonary TB and latently infected patients, with an increase in these cell populations with TB treatment. Interestingly, in this study a much higher proportion of the overall CD4 T-cell response was attributed to polyfunctional T cells for all studied groups, compared with findings in the previous studies. These differences may reflect differences in methodology between studies or variability related to the specific patient populations studied. However, in all cases it is unclear how peripheral blood findings are reflective of cell populations at the site of disease.
Cytotoxic lymphocytes
CD8+ T lymphocytes are important in the human immune response to a number of intracellular pathogens, especially chronic viral infections such as HIV, and are dependent on CD4 T-cell help [96–98]. Although not as critical as CD4 T cells for protection from M.tb., mouse and primate models have demonstrated a role for CD8+ cytotoxic T lymphocytes in TB immunity [38,72,74,99]. CD8-depleted mice develop chronic pulmonary TB with higher mycobacterial burdens but similar survival to wild-type mice [72]. A model of latent TB infection in mice has implicated CD8 T cells as being particularly important in control of chronic rather than acute infection [100].
The mouse model may not be appropriate for fully delineating CD8 T-cell responses, as mice lack comparable expression of group 1 CD1 proteins that may present mycobacterial lipid antigens to cytotoxic T cells, and also lack expression of the cytotoxic molecule granulysin, which is released by cytotoxic T lymphocytes and has direct antimycobacterial activity [101]. Indeed, studies using human cells have shown that perforin and granulysin are important for CD8 T-cell-mediated target cell lysis in TB infection [101–103]. CD8+ effector memory T cells, which express cell surface TNF-α are a major source of granulysin [104]. Anti-TNF-α therapy causes a reduction in this cell population, which may contribute to the increased susceptibility of patients on this treatment to TB disease [104–106]. HIV infection has a number of effects on CD8 T-cell function, including causing persistent cellular activation and increasing proportions of intermediately differentiated CD8 T cells [90,107,108]. Granulysin production by CD8 T cells has not been studied in HIV–TB coinfection. However, HIV-1 infection of CD8 T lymphocytes suppresses the induction of granulysin in response to IL-21 and IL-15 [109] and defective granulysin-mediated cytotoxicity has been shown in HIV-associated cryptococcal infections [110]. In addition, reduced IL-21 levels may potentiate defective cytolytic T-cell responses in HIV infection, which could impact on antimycobacterial immunity. CD4 T-cell-derived IL-21 is essential to sustain CD8 T-cell responses to chronic viral infections in mice and may have a role in the control of human chronic infections, such as hepatitis B and C [111–113]. Low levels of serum IL-21 are found in HIV infection, correlating with CD4 count [114].
Other cytotoxic lymphocytes are affected by both TB and HIV infections and may play a role in HIV-mediated TB susceptibility. Invariant natural killer T (iNKT) cells express an invariant T-cell receptor comprised of Vα24 coupled with Vβ11 in humans, which binds to glycolipid antigen bound to CD1d molecules on APCs [115]. Activation leads to rapid production of both Th1- and Th2-type cytokines without priming and cytotoxicity mediated by perforin and granzymes [116]. This potent response, coupled with lack of requirement for immune memory has lead to classification of iNKT cells as innate lymphocytes, with the potential to bridge the gap between innate and acquired immunity [117]. HIV can productively infect iNKT cells [118]. In human HIV infection, both CD4+ and CD4− iNKT cell subsets are depleted, while institution of ART rapidly reconstitutes iNKT cells populations in blood [119–121]. iNKT cells have the ability to directly restrict M.tb. growth in culture [122]. In mouse models, iNKT cells have been shown to improve immune responses to M.tb. infection, and these responses can be boosted by pharmacological activation of these cells [123,124]. In human studies of TB, iNKT cell populations are consistently found to be reduced in quantity and are functionally impaired [121,125,126]. However, studies examining iNKT cells in HIV–TB coinfection are lacking.
Similarly, NK cells have innate and cytotoxic activity, but their importance in HIV–TB pathogenesis is not well defined. NK cells can lyse M.tb.-infected cells, via the activating receptor NKp46 [127,128]. NK cells isolated from HIV-infected patients have reduced expression of activating receptors, including NKp46, increased expression of inhibitory receptors and are functionally impaired, compared with NK cells from healthy controls [129,130]. Recent studies have implicated NK cell dysfunction in TB-IRIS (see later) suggesting further study in this area is warranted [131,132].
We have discussed immune effects of HIV infection that increase susceptibility to and modulate TB infection. In the next section we examine how TB infection impacts on the immune response to HIV infection.
TB impacts on the immune response to HIV-1 infection
What features of the immune response are important in HIV infection?
HIV predominantly infects CD4 T cells by binding to the CD4 receptor on the cell surface, in addition to a coreceptor, usually the chemokine receptor CCR5 (predominantly expressed by effector T cells, but also present on monocytes and macrophages) or CXCR4 (in later-stage infections, usually expressed by naive T cells). This interaction allows fusion of the viral envelope with the host cellular membrane and permits viral entry. Once inside the cell, HIV viral RNA is transcribed into proviral DNA by HIV reverse transcriptase enzyme. Proviral DNA is integrated into cellular DNA and transcribed by cellular RNA polymerase II [133]. During primary HIV-1 infection, absolute CD4 T-cell numbers decline acutely as viral titers in peripheral blood peak. This is largely due to infection and depletion of transitional effector memory and fully differentiated memory CD4-and CCR5-expressing T lymphocytes [134,135]. However, with the onset of a HIV-specific cell-mediated immune response, in the form of HIV-1-specific cytotoxic CD8 T lymphocytes and with the production of HIV-1 specific antibodies, some degree of control over viral replication occurs. Additionally, cytotoxic CD4 T-cell responses may be important in controlling infection at an early stage [136–138].
During the ‘chronic’ or asymptomatic period of HIV infection, persistent replication of HIV occurs, and the CD4 count gradually declines. During this period, stronger HIV-specific CD8 T-cell responses and preservation of central memory CD4 T-cell subsets are linked to slower progression [96]. A persistent decline in CD4 cell numbers correlates with increasing immunocompromise, susceptibility to opportunistic infections and eventual death in the absence of ART.
Immune activation due to TB drives HIV replication
Proinflammatory cytokine production by innate immune cells in response to M.tb. may facilitate progression of HIV infection (reviewed by Diedrich and Flynn [48]). Monocytes from active pulmonary TB patients infected with HIV-1 support higher levels of HIV replication than monocytes from PPD-positive healthy control donors [139]. Activated monocytes, in addition to CD4 T lymphocytes, contribute significantly to increased plasma HIV viral loads during TB infection [140]. M.tb. infection drives HIV-1 viral replication by transcriptional activation in alveolar and pleural macrophages in respiratory TB, and is dependent on macrophage–lymphocyte contact and M.tb.-induced inflammatory cytokines and chemokines, such as TNF-α and MCP-1 [141–144]. Recent work has shown increased expression of P-TEFb, activation of NF-κβ and loss of an inhibitory C/EBPβ, identifying these as being the transcription factors involved in this process [66,141,145]. Increased viral replication is associated with development of increased viral heterogeneity at the site of TB disease and in blood, and may persist following completion of anti-TB therapy [17,146].
TB infection promotes cellular susceptibility to HIV
Chemokines induced by M.tb. infection of phagocytic cells may also be instrumental in recruiting target cells susceptible to HIV (CCR5-expressing CD4 T cells and monocytes) to the site of HIV-1 replication, further potentiating the increased HIV viral replication at TB infection sites [147]. In addition, ex vivo studies of human CD4 T cells suggest that TB infection may increase the susceptibility of CD4 T cells to new HIV infection by upregulation of Toll-like receptor 2 (TLR2), through which activation of an HIV-long terminal repeat may occur, promoting HIV replication [148,149]. This potentially provides a contributory explanation for the epidemiological associations between TB and HIV disease progression, although further studies are required.
In summary, TB and HIV-1 infection have reciprocal detrimental immune effects. HIV appears to have significant effects on cells of the innate immune system, particularly macrophages, although evidence of this largely comes from in vitro experimental models of HIV–TB coinfection. Cytotoxic lymphocyte function, particularly affecting cells that bridge innate and acquired immune systems (e.g., NK and iNKT cells), appears to be impaired. Further research to define this dysfunction is required. The key role of CD4 T cells has been demonstrated in animal and human studies. Improved animal models, such as the macaque model, and the addition of new cellular models, will be valuable to investigate the interactions between immune cells involved in the granulomatous response to M.tb. and the impact of HIV-1 infection on these interactions.
In the next section we consider in more detail the clinical implications of the immune response to HIV-1 and M.tb. infection, and how it affects patient presentation, diagnosis and management with ART.
Clinical implications of the immune response in HIV-associated TB
HIV infection has exemplified how the state of the immune system impacts not only on the risk of TB disease and HIV progression, but also on the clinical presentation, diagnosis and treatment of TB in HIV-1 infected individuals [150].
The clinical presentation of TB in HIV-infected patients
Pulmonary TB is the most common form of active TB, classically presenting with chronic productive cough, fever, weight loss and night sweats, and may be accompanied by hemoptysis and pleuritic chest pain. Localized pulmonary inflammation is visible on chest radiograph, typically affecting lung apices, and frequently cavitation, the hallmark of pulmonary TB. Although TB may infect any organ and miliary TB (disseminated TB) is sometimes reported, these manifestations are less frequent in immunocompetent patients.
Since the early days of the HIV epidemic it has been clear that active TB in advanced HIV infection does not follow this typical pattern, reviewed recently by Schutz et al. [150]. Pulmonary disease is more likely to be widespread, often causing lower zone infiltration and rarely causing cavitary lung disease in patients with CD4 counts below 200 cells/mm3. Chest radiographic changes may be absent in sputum culture-positive TB. In addition, TB is more likely to present with extrapulmonary forms, particularly lymphadenitis, meningitis, gastrointestinal and disseminated TB. Clinical manifestations of TB in HIV-infected patients with relatively preserved CD4 counts follow a much more typical pattern of predominantly classical pulmonary disease, as described above.
Mechanisms of divergent immunopathology in HIV-associated TB
Although, in the absence of HIV infection, the risk of developing active TB following infection is lower, when it does occur M.tb. bacilli tend to be locally contained (mainly at pulmonary sites). The ensuing immune response may be highly inflammatory, damaging local tissue, with devastating physiological effects and promotion of TB transmission through the formation of cavities. In advanced HIV, the balance appears to be tipped in favor of M.tb. growth and dissemination, while resulting in less inflammatory pathology and therefore reduced cavitation and transmissibility.
The prominent spectrum of TB pathology in HIV-infected patients and the relationship of these manifestations to CD4 count suggests that divergent immune responses are responsible. Studies in immunocompromised animals have failed to adequately model clinical and pathological features of human HIV–TB coinfection. Disruption of T-cell function in murine models of TB produces some similar features, with fulminant disseminated infection, rather than the controlled contained disease seen in wild-type mice [72]. However, it is hard to interpret these findings, as pulmonary disease in immunocompetent mice does not accurately model human pulmonary TB, particularly due to lack of cavitation in most strains [151].
CD4 depletion in the macaque model of acute TB infection causes more widespread dissemination of primary infection than in wild-type monkeys, with greater bacterial burden and increased pathology [75]. Similarly to advanced HIV infection in humans, no chest radiographic abnormalities were found, despite elevated erythrocyte sedimentation rates, higher mycobacterial burdens and macroscopically greater pathology at necropsy, although cellular architecture of the TB granulomas in CD4-depleted and control monkeys was similar.
CD8-depleted macaques also exhibit more extensive TB lesions compared with non-CD8-depleted macaques following high-dose M.tb. infection. In the CD8-depleted monkeys, histopathology was similar to advanced HIV–TB in humans: granulomas were less well-organized and more necrotic, with reduced lymphocytic infiltration [43,152].
In the SIV–TB model of TB reactivation described above, gross pathology scores, bacterial number scores and percentages of positive samples on mycobacterial culture were similar when comparing active infection in SIV–TB coinfected macaques and TB-monoinfected macaques. However, it is worth noting that these monkeys had similar peripheral CD4 counts to TB mono-infected monkeys and therefore were not representative of advanced HIV infection. A wide range of granuloma types were present in these coinfected monkeys, from necrotic to fibrotic, with strikingly more fibrotic granulomas in late-reactivating SIV-infected monkeys compared with early-reactivating coinfected monkeys or monoinfected monkeys [74].
Recent investigation of matrix metalloproteinase (MMP) activity in TB has elucidated the mechanisms of cavitation in TB and shed some light on clinical differences in TB immunopathology in HIV-uninfected and -infected patients. MMPs are a family of zinc-dependent proteases, including collagenases, gelatinases and elastases. They are produced by a range of innate immune cells, epithelial cells and fibroblasts and are upregulated in response to TB infection, without compensatory upregulation of the endogenous tissue inhibitors of MMPs (TIMPs) [153,154]. Together the MMPs are capable of degrading components of the extracellular matrix and are strongly implicated by animal and human studies in M.tb. virulence, correlating with severity of disease in CNS TB, severity of pulmonary inflammatory pathology and the presence of cavitation in pulmonary TB [155–158].
HIV infection is known to modulate MMP production in cellular models and in some HIV-associated neurological conditions [159–161]. Recently we demonstrated that concentrations of MMP-1 (interstitial collagenase) and MMP-2 (gelatinase A) in induced sputum samples from TB patients correlated with the severity of tissue destruction, mycobacterial load in sputum and the presence of cavitation in a mixed HIV-infected and uninfected population [158]. Reduced levels of MMP-1, -2, -8 and -9 were found in induced sputum of TB patients with advanced HIV compared with HIV-uninfected TB patients, suggesting that modulation of TB-driven MMP activity by HIV infection may explain the reduced focal inflammatory pathology and cavitary lesions seen in advanced HIV. MMPs are important in a diverse range of normal immune functions and are immunomodulatory [162,163]. They activate and are activated by a range of inflammatory cytokines and chemokines, including IL-1β and TNF-α and can affect leukocyte migration [164–167]. In a zebrafish model of TB employing Mycobacterium marinum, ESAT-6-dependent production of MMP-9 was shown to promote macrophage recruitment to granulomas, bacterial dissemination and virulence [40]. MMP dysregulation may provide one mechanism for divergent pathology in HIV–TB coinfection. Other immune regulatory factors (e.g., TGF-β) are modulated by HIV–TB coinfection (see also Table 1) [168]. However, the implications of these findings on pathogenesis is not well understood. Further work exploring the impact of TB and HIV on these pathways may elucidate immunomodulatory therapeutic interventions.
Table 1.
Effects of HIV and antiretroviral therapy on key immune factors in TB.
| Immune cell | Response in TB | Effect of HIV | Effect of ART |
|---|---|---|---|
| Macrophage | Phagocytose mycobacteria Produce IFN-γ and TNF-α Secrete MMPs Key role in granuloma formation: epithelioid cell/Langhans giant cell formation Failure of apoptosis linked to intracellular mycobacterial survival |
Increased cellular activation Disruption of autophagy In HIV–TB coinfection: Reduced IL-10-dependent macrophage apoptosis and reduced TNF-α production Increased M.tb. replication (not evident in all studies) Increased HIV-1 replication |
Intracellular persistence of HIV-1 in macrophages despite ART Activation markers reduced by ART but not to normal Effect of ART on role of macrophages in M.tb. immunity is not well defined |
| Neutrophil | Phagocytose mycobacteria Possible role in M.tb. killing via neutrophil peptides (HNP 1–3, cathelicidin LL-37 and lipocalin 2) |
Impaired phagocytic ability and chemotaxis Increased apoptosis |
Improved chemotaxis, but impairment of phagocytosis persists despite ART Protease inhibitors reduce neutrophil apoptosis |
| NK cell | Lysis of M.tb. infected cells in vitro | Reduced functional NK cell populations Reduced activation, more inhibitory receptor expression |
Reconstitution with functional deficits Increased activation and degranulation associated with TB-IRIS |
| CD4 T cell | Recruited to disease site in response to cytokine and chemokine signals from infected antigen-presenting cells; recognize peptide antigens bound to MHC class II molecules: Role in activation of phagocytic and cytotoxic cells: produce Th1 cytokines (e.g., IFN-γ), which are critical to immune response but may also lead to immunopathology |
Infected and depleted: transiently by acute HIV infection; progressively and irreversibly in chronic HIV infection CCR5-expressing, IL-2-producing M.tb.-specific CD4 T cells are particularly susceptible to HIV infection and depletion In HIV–TB coinfection: Reduced at disease sites in HIV–TB coinfection Functionally impaired: reduced IFN-γ response to mycobacterial antigens shown in some studies Reduced IL-21 production may influence CD8 T-cell function |
Early reconstitution of memory T cells (possibly from lymphoid sites); later restoration of naive cells Persistent deficit in CD4 T-cell subsets despite long-term ART Improvement in frequency and function of M.tb.-specific CD4 T cells, although reduced compared with HIV-uninfected populations |
| CD8 T cell | Lyse M.tb.-infected cells via release of granular contents (e.g., granulysin) and restrict mycobacterial growth | Increased cellular activation Defective (granulysin mediated and IL-21 dependent) cytotoxic responses |
Reduced cellular activation in some studies Other studies suggest persistent cellular activation and disruption of cellular maturation pathway |
| Tregs | Expanded populations at TB disease sites; produce IL-10 | Reduced in total number by HIV but increased in proportion In HIV–TB coinfection: Compartmentalization evident in pleural fluid in HIV-associated pleural TB |
Normalization of Treg numbers and proportions with CD4 T-cell reconstitution Reduced IL-10 production in one study of Mycobacterium avium IRIS, which may indicate a defect in regulatory responses to mycobacteria. Other studies suggest no role in TB-IRIS |
| NKT cell | Can restrict TB growth in vitro Reduced frequency in active TB infection with functional impairment |
Activated, productively infected and depleted | Reconstituted rapidly |
ART: Antiretroviral therapy; HNP: Human neutrophil peptide; IRIS: Immune reconstitution inflammatory syndrome; MMP: Matrix metalloproteinase;
M.tb.: Mycobacterium tuberculosis; NKT: Natural killer T cell; TB-IRIS: TB-associated immune reconstitution inflammatory syndrome.
Immunity & TB diagnosis
Despite increased mycobacterial burdens and more widespread disease in HIV-infected TB patients, confirmation of diagnosis is more elusive [150]. Considering that the mainstay of TB diagnosis for many decades has been smear microscopy for acid-fast bacilli in sputum, this is not so surprising. Cavitation correlates well with smear positivity, as M.tb. bacilli enter the airways through pulmonary parenchymal destruction, and such destructive pulmonary TB lesions are rare in advanced HIV. New PCR-based diagnostic tests (GeneXpert®) have improved sensitivity over sputum smear microscopy for pulmonary TB in HIV-infected patients [169]. However, many patients with clinical symptoms of TB fail to have a diagnosis confirmed by smear (and in some instances even culture) and clinicians often treat empirically [170].
Assessment of the immune response to mycobacterial antigens has been historically utilized in diagnosis of latent TB. The tuberculin skin test measures a delayed-type hypersensitivity reaction to mycobacterial PPD injected subcutaneously. A positive response indicates immune sensitization and correlates with M.tb. exposure. However, HIV-infected patients with latent and active TB may have an absent response. Therefore, reliability among HIV-infected individuals is reduced [171,172].
Improved understanding of the immune response to TB has heralded the development of new immunodiagnostics, relying on detection of IFN-γ produced by PBMC or whole blood stimulated with M.tb. antigens, by IFN-γ release assay (e.g., ELISpot, QuantiFERON®-Gold). There was some initial optimism that these may be more reliable than skin tests in HIV-infected individuals for diagnosing ‘latent’ or ‘active’ infection [173]. Studies have not fully substantiated this (reviewed by Oni and Wilkinson) [174]. Little additive value is evident over standard TB diagnostics, in high HIV incidence areas [26]. Meta-analyses of test performance as a predictor of future active TB have demonstrated a weak-to-moderate association, with a relative risk of 2–3 in a mixed HIV-infected and -uninfected population and conclude that a lack of sufficient data exists in HIV-infected patients [175,176]. A recent longitudinal study of South African adolescents demonstrated an eightfold increase in risk of TB disease within 2 years in QuantiFERON TB Gold in-tube converters [177]. However, this study was in a largely HIV-uninfected population.
In a recent longitudinal study of chronically HIV-infected individuals in Durban, South Africa, RD-1 antigen responses were monitored using ELISpot assays every 3 months for up to 2 years. There was a high degree of individual variability in T-cell antigen response over time, without a correlation with stage of HIV disease, ARV status or development of active TB [178]. Only just over half of the ELISpot responses were considered to be consistently positive or negative, and individually in these patients there was a large degree of variability in the number of spot-forming cells, despite not meeting criteria for a change in result from positive to negative. This study demonstrates the complexity of antigen-specific IFN-γ responses to M.tb. in HIV infection and cautions against overinterpretation of IFN-γ responses in cross-sectional studies.
It is likely that a fine balance of pro-and anti-inflammatory factors within TB granulomas and in the immune response to M.tb. is required for optimum containment of TB, without the exaggerated inflammatory response that is associated with the local tissue destruction that characterizes TB in HIV-uninfected individuals. It has been shown that the magnitude of the immune response can correlate with smear positivity, and this relates to difficulty in diagnosis of TB infection in HIV-infected individuals. Currently available immunodiagnostics do not appear to be reliable in HIV-infected patients. Improved understanding of immunity in HIV–TB coinfection is required to aid development of TB diagnostics suitable for HIV-infected patients and therapeutics to target TB tissue destruction.
In the next section we address the effects of ART on the immune response to TB. As ART is now recommended for all HIV-infected patients with TB, and as ART coverage improves globally, this becomes an increasingly important area for consideration.
The effect of ART on the immune response to TB
ART suppresses HIV-1 viral replication, increases CD4 T-cell numbers and in some studies has been shown to reduce CD8 T-cell activation [179–181]. The past decade has seen great advances in ART coverage amongst HIV-infected individuals worldwide. Clear mortality and morbidity benefits have been demonstrated by starting ART following a TB diagnosis. In patients with CD4 counts less than 50 cells/mm3, there is a reduction in mortality and progression to AIDS if ART is started at 2 weeks of TB treatment compared with later time points [182,183]. A new diagnosis of TB is now considered an indication for ART initiation [182–184]. However, in TB-endemic settings, despite ART-mediated CD4 T-cell reconstitution and HIV viral suppression, an increased risk of active TB remains [3]. How does ART affect the immune response to TB and what is lacking?
Several studies have suggested that early reconstitution of CD4 T-cell numbers is comprised largely of memory cells rather than naive cells, suggesting redistribution from lymphoid sites [86,179,181]. This is followed by reconstitution of the naive T-cell population. A study of T-cell subsets in ART-experienced patients in Denmark demonstrated persistent deficits in absolute CD4 T-cell numbers, and subsets (particularly naive CD4 T cells) compared with healthy blood donors, despite up to 14 years of ART with HIV viral suppression [90]. The same study demonstrated differences in the CD8 T-cell maturation pathway, with increased frequencies and proportions of intermediately differentiated (CD45R A−, CD27+, CD28−, CCR7−) CD8 T cells and increased frequencies and proportions of activated CD8 T cells (CD38+, HLA-DR+) in ART-treated patients compared to HIV-negative controls [90].
ART improves antigen-specific CD4 T-cell responses to a number of antigens, including PPD, compared with pre-ART responses [180,185]. However, despite total CD4 T-cell reconstitution and suppression of HIV-1 viral replication, ART-treated patients have significantly depleted M.tb.-stimulated IFN-γ responses and frequencies of PPD-specific IFN-γ-secreting CD4 T cells, compared with HIV-uninfected individuals [186,187]. Our group studied CD4 T-cell reconstitution in detail over the first 48 weeks of ART [86]. In this study, central memory CD4 T cells expanded significantly by 12 weeks. By 36 weeks of ART, naive T cells had also expanded. However, the frequency of terminally differentiated CD4 T cells, including PPD-responsive cells, was less dynamic, and by 12 weeks represented a significantly reduced proportion of total CD4 T cells that was sustained for 48 weeks (although absolute numbers did increase). At week 48, the proportion of CD4+ T cells producing IFN-γ was lower than in healthy HIV-uninfected controls [86].
A study of ART-treated patients with a previous history of treated TB found profoundly reduced ESAT-6 and Ag85B responses in these patients but strong nonspecific mycobacterial (PPD) CD4 T-cell IFN-γ and proliferative responses, when compared with HIV-uninfected patients with previous TB [188]. It suggests that repeat exposure to environmental, nonpathogenic mycobacteria boosts the nonspecific mycobacterial immune response, without causing infection. However, in the context of repeat exposure to the more virulent M.tb., HIV-infected patients succumb to TB disease rather than developing immunity.
The effects of ART on cells of the innate immune system, cytotoxic T cells and regulatory cells are less well defined (Table 1) [60,108,189–191]. Despite successful ART, tissue macrophages contain a reservoir of HIV virus that is considered important in failure of viral eradication by ART and in the pathogenesis of HIV-related neurological conditions that may manifest despite ART [190,192]. The impact of this reservoir on TB immunity in ART-treated patients is not clear.
TB-associated immune reconstitution inflammatory syndrome
In settings where TB is endemic, TB-IRIS is a frequent early complication of ART. TB-IRIS reflects an immunopathological reaction to mycobacterial antigens driven by the recovering immune system. Paradoxical TB-IRIS is better characterized: it occurs in HIV-infected patients on TB treatment when they start ART, who experience recurrence or worsening of TB symptoms and clinical or radiological features during the first weeks on ART. The other form, unmasking TB-IRIS, is less well defined and describes patients with unrecognized TB at ART initiation, who present with an exaggerated inflammatory presentation of TB during early ART [193].
Paradoxical TB-IRIS occurs in approximately 15.9% of patients who start ART while on treatment for TB (95% CI: 9.7–24.5) [194]. The onset of symptoms is typically 1–4 weeks after ART is initiated [27]. Common features are recurrence of TB symptoms, fevers, worsening radiographic pulmonary infiltrates, enlarged lymph nodes, serous effusion enlargement and the formation of tuberculous abscesses [27,195]. Potentially life-threatening manifestations include: enlarging intracerebral tuberculomas, meningitis, renal involvement with renal failure, splenic rupture and cardiac tamponade due to rapid enlargement of pericardial effusions [196–200]. Death due to paradoxical TB-IRIS is infrequent in reports in the literature [194], but patients commonly require hospital admission and diagnostic and therapeutic procedures consuming health care resources [201]. There is one randomized controlled trial of treatment: patients who received prednisone for 4 weeks (2 weeks at 1.5 mg/kg/day, followed by 2 weeks at 0.75 mg/kg/day) had significant reduction in duration of hospitalization and outpatient therapeutic procedures, and more rapid symptom improvement compared to those who received placebo [202]. It is recommended that ART is not interrupted other than in cases where TB-IRIS is imminently life-threatening, such as cases where there is CNS involvement with depressed level of consciousness [203]. Risk factors most consistently associated with paradoxical TB-IRIS are low CD4 count, disseminated TB and the short interval between starting TB treatment and ART [27,204].
During initial ART there is a rapid increase in CD4 T-lymphocyte count in most patients. In the first month of ART, the increase is approximately 75–100 cells/μl [205]. The majority of this initial increase is due to the redistribution of memory T cells from sites of immune activation into peripheral blood [181]. Initial investigations of the pathogenesis of paradoxical TB-IRIS thus focused on T cells, and particularly mycobacterial-specific T cells. Bourgarit et al. demonstrated an association between TB-IRIS and prominent expansions of PPD-specific IFN-γ-producing T cells. Similar expansions were not seen for CMV-specific or ESAT-6-specific T cells in TB-IRIS patients [206]. Similar findings were reproduced in other studies [207]. Subsequently, Bourgarit et al. have reported that these PPD-specific T cells are polyfunctional, coexpressing IFN-γ+ TNF-α+ and IL-2+, are of the CD4+ effector memory phenotype and are highly activated [208].
A tight causal relationship between T-cell expansions and paradoxical TB-IRIS has been questioned on the basis of other findings. In a study published by our group in cross-sectional analyses, patients with TB-IRIS sampled after 2 weeks on ART were found to have higher median ELISpot responses to a broad range of mycobacterial antigens including ESAT-6 (in contrast to Bourgarit et al.’s findings) when compared with control groups [209]. However, the responses were heterogeneous in both cases and controls: some cases showed no expansions and some controls (who did not develop TB-IRIS on ART) showed high levels of IFN-γ-producing T cells in response to mycobacterial antigens. Thus, even though mycobacterial-specific T cells likely play a role in the development of TB-IRIS, the findings suggested they were neither a sufficient nor necessary factor in the development of the condition. This conclusion was reinforced by the findings of a longitudinal study where, in the initial 8 weeks of ART, in both TB-IRIS cases and non-IRIS controls, dynamic expansions and contractions of mycobacterial-specific IFN-γ-producing T cells were evident, with few significant differences between the groups [209]. A Thai study also found no significant differences in concentrations of Th1 cytokines (IL-2, IL-12 and IFN-γ) following PPD and RD1 antigen stimulation between patients who developed TB-IRIS and controls prior to ART and at time of IRIS [210]. Elliott et al. demonstrated similar increases in PPD-specific IFN-γ T-cell responses in both IRIS cases and controls with HIV-associated TB patients who started ART but did not develop IRIS until week 12 [211]. Only at 24 weeks were the responses greater in IRIS cases than controls, long after the onset of the clinical features of TB-IRIS (median: 10 days), suggesting that other immune mechanisms, such as innate immune cells and Tregs, are important in pathogenesis.
Several investigators have explored the role of innate immune cells in TB-IRIS. Pean et al. demonstrated that those HIV-associated TB patients who developed TB-IRIS had higher expression of the degranulation surface marker CD107a on NK cells prior to starting ART than controls who did not develop IRIS [131]. They hypothesize that this reflects higher innate immune responses prior to ART in TB-IRIS cases, and that this may play a role in pathogenesis through increased lysis of M.tb.-infected cells by NK cells and thus higher mycobacterial antigen load. High levels of NK cell activation have also been described to be associated with unmasking TB-IRIS [132]. In a case report of fatal unmasking TB-IRIS, the patient’s dense pulmonary cellular infiltrate was almost entirely composed of CD68+ myeloid cells [212].
A study of five cases of TB-IRIS and nine matched non-IRIS controls suggested a role for TLR2-induced proinflammatory cytokines produced by monocytes and dendritic cells in TB-IRIS pathogenesis. At 24 weeks on ART, TLR2 expression on monocytes and lipomannan-induced TNF-α production was significantly higher in TB-IRIS cases. Lipomannan is a TLR2 ligand. Lipomannan-induced TNF-α and IL-12p40 responses paralleled TB-IRIS in certain patients with high TLR2 expression on monocytes and myeloid dendritic cells, without a parallel increase in IL-10 production [213]. In a study of TB meningitis IRIS, cerebrospinal fluid neutrophil counts were significantly higher at baseline in patients with TB meningitis who developed IRIS, and were more tightly related to clinical improvement prior to ART and deterioration with IRIS than cerebrospinal fluid lymphocyte counts [200]. The suppurative lymphadenitis and abscess formation that is often seen in TB-IRIS also suggests a role for neutrophils.
Ruhwald and Ravn have suggested that paradoxical TB-IRIS is precipitated by a ‘cytokine storm’ [214]. TB-IRIS was accompanied by elevated Th1 and inflammatory cytokines and chemokines, but not Th2 cytokines in one study [206]. In a study conducted by our group, cytokine concentrations were studied in patients who developed TB-IRIS and compared with controls (patients with HIV-associated TB who did not develop TB-IRIS on ART) at 2 weeks on ART [215]. A wide range of cytokines and chemokines were elevated after in vitro restimulation, but most consistently, and additionally significantly elevated in serum, were TNF-α, IFN-γ and IL-6. Many of the cytokines increased in TB-IRIS are of myeloid origin. We have also demonstrated that these cytokines are decreased by corticosteroid treatment [216]. Oliver et al. demonstrated that TB-IRIS was associated with elevated CXCL10 and IL-18 concentrations and lower levels of CCL2 in unstimulated samples, and therefore suggested that pertubations of the innate immune responses may contribute to pathogenesis [217]. A more recent study by the same group demonstrated higher levels of CXCL10 in whole blood cultures stimulated with RD1 antigens at baseline and at week 4, further suggesting a role for this chemokine in pathogenesis [218].
Findings thus suggest a role for innate immune cells and cytokines and chemokines associated with these cells in TB-IRIS pathogenesis. This concurs with findings from an M. avium mouse model of IRIS. T-cell-deficient mice infected with M. avium developed an IRIS-like deterioration after CD4 injections, characterized by weight loss, impaired lung function and rapid death. This syndrome correlated with marked alterations in blood and tissue CD11b+ myeloid cells [219]. On the basis of their findings these investigators hypothesized that IRIS results from hyper-responsiveness of the innate immune system to T-cell help, in the context of recovery of the immune system from a state of prior immunosuppression that permitted the accumulation of large amounts of microbial antigen [220].
An alternative hypothesis has proposed that a delay in recovery of Treg numbers and function, compared with proinflammatory responses is responsible for paradoxical TB-IRIS [214]. However, studies have demonstrated no deficiency of Tregs in TB-IRIS patients and IRIS patients in general [207,209,221]. Seddiki et al., in fact, demonstrated higher levels of CD127loFoxp3+CD25+ Tregs, in addition to an increased ratio of Treg to effector/memory subsets, in patients with IRIS related to nontuberculous mycobacterial infection compared with controls [222]. In this same study, impaired function of and IL-10 secretion from suppressor cells in IRIS patients was demonstrated by in vitro suppression assays, suggesting that despite quantitative increases in Treg numbers, functional ability is impaired. In support of this, a study of two patients with mycobacterial IRIS demonstrated impaired IL-10 and increased IFN-γ production [223]. The authors suggested that an imbalance of regulatory and effector cytokine responses may be the cause of IRIS [223]. Low pre-ART levels of inhibitory NK receptors (CD94/NKG2, CD158a/h and CD158b) on mycobacterial-specific Vδ2 TCRγδ T cells have been shown to predict paradoxical TB-IRIS [224]. Thus, while no study has clearly shown a deficiency in Treg numbers in mycobacterial IRIS, certain studies suggest there may be functional impairment of regulatory components of the immune system that contributes to dysregulated inflammation.
In summary, ART leads to reconstitution of CD4 T-cell populations, initially memory T cells early after initiation and later naive CD4 T cells. Persistent impairments of M.tb.-specific CD4 T-cell populations may be evident despite long-term ART. ART initiation is complicated in a substantial proportion of cases by TB-IRIS, where TB inflammatory pathology may increase or occur where it was previously not clinically evident. TB-IRIS poses specific diagnostic and treatment challenges. Immunopathological mechanisms are incompletely defined, but there is increasing evidence that innate immune dysfunction plays a role. Further research is needed on this condition.
Conclusion
The immunological advances in understanding of TB and HIV infection individually need to be combined in work that describes the complex interactions between these two deadly pathogens, and the effects of ART. This should occur in appropriate animal models (ideally nonhuman primates) and on human samples, allowing advances to rapidly translate into clinical solutions. Specific diagnostics and treatment for TB-IRIS are critically lacking, as are treatments that limit TB immunopathology. Improved understanding of protective immunity will enhance vaccination strategies. Understanding the complex interactions between the individual components of innate and acquired immune responses to TB and HIV infection is likely to be the next step forward.
Future perspective
Mortality occurs at both ends of the immunological spectrum of TB: at one end an HIV-uninfected patient dies from asphyxiation from acute massive hemoptysis due to cavitary TB; at the other end, and far more frequently, a HIV-infected patient with disseminated TB dies from overwhelming infection with less evidence of focal pathology. There is no clear sign that the HIV–TB epidemic is slowing, especially considering the emergence of increasingly drug-resistant strains of M.tb.
A major challenge for the future is to discover immune correlates of TB protection and TB disease risk. Failure to define this conclusively has hindered TB prevention strategies, including the design of new TB vaccines to replace BCG, which provides only short-lived efficacy, preventing severe forms of extrapulmonary disease, and which is contraindicated in HIV-infected patients [225,226]. New candidate vaccines are being developed and some are currently being assessed in clinical trials, including HIV-infected patients, recently reviewed by Rowland and McShane [225]. Hopefully, these studies will help define protective immune responses and demonstrate whether polyfunctional immune responses correlate with protection from TB disease. New immunodiagnostics appropriate for HIV-infected populations would have the potential to improve disease outcomes through early diagnosis and, if able to differentiate exposure, infection and disease, could improve delivery of preventative measures such as isoniazid preventative therapy, to those in greatest need.
Our limited understanding of the immune response to TB has also hampered the development of adjunctive immunomodulatory therapies to prevent the severe tissue destruction frequently seen in pulmonary TB, CNS TB and particularly affecting the HIV-infected TB population, TB-IRIS. As the ideal immune response associated with the clearance of infection is not known, immunomodulatory therapy may be detrimental. However, benefit with corticosteroid therapy in pericardial TB, CNS TB and TB-IRIS has been demonstrated, and anecdotal evidence of response to other immunosuppressants, such as thalidomide, exists [227–230]. With immunological advances in this field, development of targeted immunomodulatory therapies may improve clinical outcomes.
Executive summary.
Epidemiology of HIV-associated TB
HIV infection increases the risk of TB disease, even in individuals with relatively preserved CD4 counts, and those on antiretroviral therapy (ART).
A spectrum of immune responses to TB infection correlates with a spectrum of clinical outcomes following infection, from elimination of infection through to subclinical infection and symptomatic disease.
Immune interactions: TB & HIV-1 infection
HIV-1 infection impacts on important components of the immune response to TB.
-
TB infection impacts on the immune response to HIV-1 infection, including:
Driving transcriptional activation via production of inflammatory cytokines and chemokines;
Increasing cellular susceptibility to HIV-1 infection via increased CCR5 expression and production of cytokines (e.g., IL-2 and Toll-like receptor 2 upregulation).
Clinical implications of the immune response in HIV-associated TB
A greater spectrum of clinical TB disease exists in HIV-1-infected patients, including more disseminated but less inflammatory disease in advanced HIV-1 infection. The etiology of this is unclear, but likely involves a combination of CD4 and CD8 T-cell dysfunction and dysregulation of innate immune factors, such as matrix metalloproteinases.
Diagnosis of TB infection is more challenging in HIV-1-infected patients.
New immunodiagnostics have not been proven to be reliable in HIV-infected populations.
The effect of ART on the immune response to TB
-
ART leads to selective reconstitution of CD4 T-cell populations:
Early memory T-cell reconstitution;
Later naive T-cell reconstitution;
Persistent impairment of Mycobacterium tuberculosis-specific CD4 T-cell populations despite many years of therapy.
-
ART initiation is associated with TB-associated immune reconstitution inflammatory syndrome, reflecting an immunopathological reaction to mycobacterial antigens driven by the recovering immune system:
Precise immunopathological mechanisms remain unclear;
Increasing evidence points to a role for innate immune dysfunction in pathogenesis;
Specific diagnostic tests are lacking;
Immunomodulatory therapy with corticosteroids reduces morbidity but more specific treatments are lacking.
Future perspective
Improved understanding of the immunopathology of HIV-associated TB has the potential to improve preventative measures (e.g., assist vaccine design and diagnostics, and to facilitate early treatment) and elucidate new immunomodulatory therapies. Further research using appropriate animal models and studying human disease is greatly needed.
Acknowledgments
The authors are grateful to CR Diedrich for helpful comments on the manuscript.
Footnotes
Financial & competing interests disclosure
NF Walker, G Meintjes and RJ Wilkinson are supported by the Wellcome Trust (084323, 087537, 088316, 094000, 098316, 081667). RJ Wilkinson received additional support from the MRC (UK; U.1175.02.002.00014.01) and EDCTP (IP.07.32080.002). G Meintjes and RJ Wilkinson also hold support from the European Union via a Marie-Curie award (PIRSES-GA-2011-295214). G Meintjes received additional funding from SATBAT research training that was Fogarty International Center and NIH funded (NIH/FIC 1U2RTW007373-01A1 and U2RTW007370). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.WHO. Global Tuberculosis Control. WHO Press; Switzerland: 2011. [Google Scholar]
- 2.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaisson RE, Martinson NA. Tuberculosis in Africa – combating an HIV-driven crisis. N Engl J Med. 2008;358(11):1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 5.Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25(9):1597–1606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 6.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burki T. Tackling tuberculosis in London’s homeless population. Lancet. 2010;376(9758):2055–2056. doi: 10.1016/S0140-6736(10)62282-9. [DOI] [PubMed] [Google Scholar]
- 8.Dunne FJ. Alcohol and the immune system. BMJ. 1989;298(6673):543–544. doi: 10.1136/bmj.298.6673.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(Suppl 3):S73–S76. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- 10.Deveale B, Brummel T, Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3(4):195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 11.Atun R, Raviglione M, Marais B, Zumla A. Tuberculosis control is crucial to achieve the MDGs. Lancet. 2010;376(9745):940–941. doi: 10.1016/S0140-6736(10)61428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martineau AR, Nhamoyebonde S, Oni T, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci USA. 2011;108(47):19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7(3):268–275. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191(2):150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kizza HM, Rodriguez B, Quinones-Mateu M, et al. Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin Diagn Lab Immunol. 2005;12(11):1298–1304. doi: 10.1128/CDLI.12.11.1298-1304.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151(1):129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 19.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14(9):1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5(3):225–232. [PubMed] [Google Scholar]
- 21.WHO. Rapid Advice: Antiretroviral Therapy for HIV Infection in Adults and Adolescents. WHO Press; Switzerland: 2009. [Google Scholar]
- 22▪▪.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. Important review calling into question the dichotomy between latent and active TB, proposing a new paradigm where infection with Mycobacterium tuberculosis (M.tb.) results in a spectrum of clinical and immunological outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(5):183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS. 2011;25(17):2190–2191. doi: 10.1097/QAD.0b013e32834cda4e. [DOI] [PubMed] [Google Scholar]
- 25.Oni T, Burke R, Tsekela R, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011;66(8):669–673. doi: 10.1136/thx.2011.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangaka MX, Gideon HP, Wilkinson KA, et al. Interferon release does not add discriminatory value to smear-negative HIV–tuberculosis algorithms. Eur Respir J. 2012;39(1):163–171. doi: 10.1183/09031936.00058911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30(4):797–810. x. doi: 10.1016/j.ccm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis. 2009;13(1):39–46. [PubMed] [Google Scholar]
- 29.Pienaar E, Fluitt AM, Whitney SE, Freifeld AG, Viljoen HJ. A model of tuberculosis transmission and intervention strategies in an urban residential area. Comput Biol Chem. 2010;34(2):86–96. doi: 10.1016/j.compbiolchem.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caley M, Fowler T, Welch S, Wood A. Risk of developing tuberculosis from a school contact: retrospective cohort study, United Kingdom, 2009. Euro Surveill. 2010;15(11):19510. [PubMed] [Google Scholar]
- 31.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19(11):491–494. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 34.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 35.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335(26):1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 36.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 37.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21(4):370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 38.Cooper AM. Cell-mediated immune responses in tuberculosis. Ann Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doenhoff MJ. Granulomatous inflammation and the transmission of infection: schistosomiasis – and TB too? Immunol Today. 1998;19(10):462–467. doi: 10.1016/s0167-5699(98)01310-3. [DOI] [PubMed] [Google Scholar]
- 40.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327(5964):466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe’s point of view. Cell Microbiol. 2010;12(3):301–309. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 42.Doenhoff MJ. A role for granulomatous inflammation in the transmission of infectious disease: schistosomiasis and tuberculosis. Parasitology. 1997;115(Suppl):S113–S125. doi: 10.1017/s0031182097001972. [DOI] [PubMed] [Google Scholar]
- 43.Lucas S, Nelson AM. Pathogenesis of tuberculosis in human immunodeficiency virus-infected people. In: Bloom BR, editor. Tuberculosis Pathogenesis, Protection and Control. American Society for Microbiology; Washington, DC, USA: 1994. pp. 503–513. [Google Scholar]
- 44.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7(12):1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 45.De Noronha AL, Bafica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathol Res Pract. 2008;204(3):155–161. doi: 10.1016/j.prp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Shen JY, Barnes PF, Rea TH, Meyer PR. Immunohistology of tuberculous adenitis in symptomatic HIV infection. Clin Exp Immunol. 1988;72(2):186–189. [PMC free article] [PubMed] [Google Scholar]
- 47.Nambuya A, Sewankambo N, Mugerwa J, Goodgame R, Lucas S. Tuberculous lymphadenitis associated with human immunodeficiency virus (HIV) in Uganda. J Clin Pathol. 1988;41(1):93–96. doi: 10.1136/jcp.41.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diedrich CR, Flynn JL. HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79(4):1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Ann Rev Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 50.Toossi Z, Wu M, Rojas R, et al. Induction of serine protease inhibitor 9 by Mycobacterium tuberculosis inhibits apoptosis and promotes survival of infected macrophages. J Infect Dis. 2012;205(1):144–151. doi: 10.1093/infdis/jir697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263(5147):678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 52.Pathak S, Wentzel-Larsen T, Asjo B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect Immun. 2010;78(9):4022–4032. doi: 10.1128/IAI.00106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imperiali FG, Zaninoni A, La Maestra L, Tarsia P, Blasi F, Barcellini W. Increased Mycobacterium tuberculosis growth in HIV-1-infected human macrophages: role of tumour necrosis factor-alpha. Clin Exp Immunol. 2001;123(3):435–442. doi: 10.1046/j.1365-2249.2001.01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meylan PR, Munis JR, Richman DD, Kornbluth RS. Concurrent human immunodeficiency virus and mycobacterial infection of macrophages in vitro does not reveal any reciprocal effect. J Infect Dis. 1992;165(1):80–86. doi: 10.1093/infdis/165.1.80. [DOI] [PubMed] [Google Scholar]
- 55.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8(5):e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mwandumba HC, Russell DG, Nyirenda MH, et al. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol. 2004;172(7):4592–4598. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 57.Patel NR, Swan K, Li X, Tachado SD, Koziel H. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J Leukoc Biol. 2009;86(1):53–60. doi: 10.1189/JLB.0908574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumawat K, Pathak SK, Spetz AL, Kundu M, Basu J. Exogenous Nef is an inhibitor of Mycobacterium tuberculosis-induced tumor necrosis factor-alpha production and macrophage apoptosis. J Biol Chem. 2010;285(17):12629–12637. doi: 10.1074/jbc.M109.073320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Killian MS. Dual role of autophagy in HIV-1 replication and pathogenesis. AIDS Res Ther. 2012;9(1):16. doi: 10.1186/1742-6405-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src–Akt and STAT3. PLoS One. 2010;5(7):e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borel S, Espert L, Biard-Piechaczyk M. Macroautophagy regulation during HIV-1 infection of CD4+ T cells and macrophages. Front Immunol. 2012;3:97. doi: 10.3389/fimmu.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5(1):e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case–control study. Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 64.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 65.Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200(7):1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoshino Y, Hoshino S, Gold JA, et al. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis. 2007;195(9):1303–1310. doi: 10.1086/513438. [DOI] [PubMed] [Google Scholar]
- 67.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33(1):14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137(1):122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berry MPR, Graham CM, Mcnab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mogues T, Goodrich ME, Ryan L, Lacourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193(3):271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dartois V. Immunopathology of tuberculosis disease across species. In: Leong FJ, Dartois V, Dick T, editors. A Colour Atlas of Comparative Pathology of Pulmonary Tuberculosis. Taylor and Francis Group, LLC; UK: 2011. [Google Scholar]
- 74▪▪.Diedrich CR, Mattila JT, Klein E, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5(3):e9611. doi: 10.1371/journal.pone.0009611. Study investigating the immunological and pathological effects of acute SIV infection on latent TB in the macaque SIV–TB model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin PL, Rutledge T, Green AM, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0028. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin PL, Pawar S, Myers A, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74(7):3790–3803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77(10):4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol. 2011;186(6):3527–3537. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Law KF, Jagirdar J, Weiden MD, Bodkin M, Rom WN. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 80.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142(4):1114–1119. [PubMed] [Google Scholar]
- 81▪.Geldmacher C, Schuetz A, Ngwenyama N, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198(11):1590–1598. doi: 10.1086/593017. Cross-sectional and longitudinal analysis demonstrating functional impairment of M.tb.-specific CD4 T-cell responses in human HIV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalsdorf B, Scriba TJ, Wood K, et al. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med. 2009;180(12):1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mansoor N, Scriba TJ, De Kock M, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette–Guerin vaccine. J Infect Dis. 2009;199(7):982–990. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rangaka MX, Diwakar L, Seldon R, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis. 2007;44(12):1639–1646. doi: 10.1086/518234. [DOI] [PubMed] [Google Scholar]
- 85▪.Matthews K, Ntsekhe M, Syed F, et al. HIV-1 infection alters CD4+ memory T-cell phenotype at the site of disease in extrapulmonary tuberculosis. Eur J Immunol. 2012;42(1):147–157. doi: 10.1002/eji.201141927. Detailed immunological analysis of pericardial TB infection, comparing peripheral blood and pericardial fluid compartments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪.Wilkinson KA, Seldon R, Meintjes G, et al. Dissection of regenerating T-cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2009;180(7):674–683. doi: 10.1164/rccm.200904-0568OC. Comprehensive analysis of the effect of antiretroviral therapy on M.tb.-specific CD4 T-cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪.Geldmacher C, Ngwenyama N, Schuetz A, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207(13):2869–2881. doi: 10.1084/jem.20100090. Study examining the susceptibility of pathogen-specific CD4 T cells to depletion in HIV infection, comparing differential characteristics of CMV and M.tb. -specific cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geldmacher C, Koup RA. Pathogen-specific T cell depletion and reactivation of opportunistic pathogens in HIV infection. Trends Immunol. 2012;33(5):207–214. doi: 10.1016/j.it.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 2010;40(8):2139–2142. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 90.Ronsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. T cell subset distribution in HIV-1 infected patients after 12 years of treatment induced viraemic suppression. J Acquir Immune Defic Syndr. 2012;61(3):270–278. doi: 10.1097/QAI.0b013e31825e7ac1. [DOI] [PubMed] [Google Scholar]
- 91.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scriba TJ, Tameris M, Smit E, et al. A Phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185(7):769–778. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40(8):2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 94.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39(3):723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 95.Day CL, Abrahams DA, Lerumo L, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187(5):2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Descours B, Avettand-Fenoel V, Blanc C, et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis. 2012;54(10):1495–1503. doi: 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 97.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300(5617):339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279(2):459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 99.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30(12):3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 101.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 102.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181(12):8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4+ and CD8+ T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167(5):2734–2742. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 104.Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119(5):1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50(2):372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 106.Miller EA, Ernst JD. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J Clin Invest. 2009;119(5):1079–1082. doi: 10.1172/JCI39143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410(6824):106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 108.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 109.Hogg AE, Bowick GC, Herzog NK, Cloyd MW, Endsley JJ. Induction of granulysin in CD8+ T cells by IL-21 and IL-15 is suppressed by human immunodeficiency virus-1. J Leukoc Biol. 2009;86(5):1191–1203. doi: 10.1189/jlb.0409222. [DOI] [PubMed] [Google Scholar]
- 110.Zheng CF, Ma LL, Jones GJ, et al. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109(5):2049–2057. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 111.Franks I. Viral hepatitis: interleukin 21 has a key role in age-dependent response to HBV. Nat Rev Gastroenterol Hepatol. 2011;8(5):243. doi: 10.1038/nrgastro.2011.36. [DOI] [PubMed] [Google Scholar]
- 112.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iannello A, Tremblay C, Routy JP, Boulassel MR, Toma E, Ahmad A. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 2008;21(3):385–388. doi: 10.1089/vim.2008.0025. [DOI] [PubMed] [Google Scholar]
- 115.Borg NA, Wun KS, Kjer-Nielsen L, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 116.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24+V beta 11+ NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100(1):11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 117.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol. 2008;181(4):2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 118.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195(7):869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Der Vliet HJ, Van Vonderen MG, Molling JW, et al. Cutting edge: rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J Immunol. 2006;177(9):5775–5778. doi: 10.4049/jimmunol.177.9.5775. [DOI] [PubMed] [Google Scholar]
- 120.Van Der Vliet HJJ, Von Blomberg BME, Hazenberg MD, et al. Selective decrease in circulating Vα24+Vβ11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168(3):1490–1495. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 121.Montoya CJ, Catano JC, Ramirez Z, Rugeles MT, Wilson SB, Landay AL. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin Immunol. 2008;127(1):1–6. doi: 10.1016/j.clim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 122.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4(12):e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sada-Ovalle I, Skold M, Tian T, Besra GS, Behar SM. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2010;182(6):841–847. doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venkataswamy MM, Baena A, Goldberg MF, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette–Guerin. J Immunol. 2009;183(3):1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kee SJ, Kwon YS, Park YW, et al. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012;80(6):2100–2108. doi: 10.1128/IAI.06018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sutherland JS, Jeffries DJ, Donkor S, et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis. 2009;89(6):398–404. doi: 10.1016/j.tube.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Garg A, Barnes PF, Porgador A, et al. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol. 2006;177(9):6192–6198. doi: 10.4049/jimmunol.177.9.6192. [DOI] [PubMed] [Google Scholar]
- 128.Vankayalapati R, Wizel B, Weis SE, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168(7):3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 129.Parasa VR, Sikhamani R, Raja A. Effect of recombinant cytokines on the expression of natural killer cell receptors from patients with TB or/and HIV infection. PLoS One. 2012;7(6):e37448. doi: 10.1371/journal.pone.0037448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Azzoni L, Papasavvas E, Chehimi J, et al. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168(11):5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 131.Pean P, Nerrienet E, Madec Y, et al. Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood. 2012;119(14):3315–3320. doi: 10.1182/blood-2011-09-377523. [DOI] [PubMed] [Google Scholar]
- 132.Conradie F, Foulkes AS, Ive P, et al. Natural killer cell activation distinguishes Mycobacterium tuberculosis-mediated immune reconstitution syndrome from chronic HIV and HIV/MTB coinfection. J Acquir Immune Defic Syndr. 2011;58(3):309–318. doi: 10.1097/QAI.0b013e31822e0d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sierra S, Kupfer B, Kaiser R. Basics of the virology of HIV-1 and its replication. J Clin Virol. 2005;34(4):233–244. doi: 10.1016/j.jcv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 136.Soghoian DZ, Jessen H, Flanders M, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4(123):123ra125. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klatt NR, Silvestri G. CD4+ T cells and HIV: a paradoxical Pas de Deux. Sci Transl Med. 2012;4(123):123ps124. doi: 10.1126/scitranslmed.3003862. [DOI] [PubMed] [Google Scholar]
- 138.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5(5):518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 139.Toossi Z, Sierra-Madero JG, Blinkhorn RA, Mettler MA, Rich EA. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177(5):1511–1516. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toossi Z, Mayanja-Kizza H, Lawn SD, Hirsch CS, Lupo LD, Butera ST. Dynamic variation in the cellular origin of HIV type 1 during treatment of tuberculosis in dually infected subjects. AIDS Res Hum Retroviruses. 2007;23(1):93–100. doi: 10.1089/aid.2006.0050. [DOI] [PubMed] [Google Scholar]
- 141.Hoshino Y, Nakata K, Hoshino S, et al. Maximal HIV-1 Replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195(4):495–505. doi: 10.1084/jem.20011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawn SD, Pisell TL, Hirsch CS, Wu M, Butera ST, Toossi Z. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J Infect Dis. 2001;184(9):1127–1133. doi: 10.1086/323649. [DOI] [PubMed] [Google Scholar]
- 143.Canaday DH, Wu M, Lu S, et al. Induction of HIV type 1 expression correlates with T cell responsiveness to mycobacteria in patients coinfected with HIV type 1 and Mycobacterium tuberculosis. AIDS Res Hum Retroviruses. 2009;25(2):213–216. doi: 10.1089/aid.2008.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mayanja-Kizza H, Wu M, Aung H, et al. The interaction of monocyte chemoattractant protein-1 and tumour necrosis factor-alpha in Mycobacterium tuberculosis-induced HIV-1 replication at sites of active tuberculosis. Scand J Immunol. 2009;69(6):516–520. doi: 10.1111/j.1365-3083.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 145.Toossi Z, Wu M, Hirsch CS, et al. Activation of P-TEFb at sites of dual HIV/TB infection, and inhibition of MTB-induced HIV transcriptional activation by the inhibitor of CDK9, indirubin-3′-monoxime. AIDS Res Hum Retroviruses. 2012;28(2):182–187. doi: 10.1089/aid.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Collins KR, Quinones-Mateu ME, Wu M, et al. Human immunodeficiency virus type 1 (HIV-1) quasispecies at the sites of Mycobacterium tuberculosis infection contribute to systemic HIV-1 heterogeneity. J Virol. 2002;76(4):1697–1706. doi: 10.1128/JVI.76.4.1697-1706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toossi Z, Mayanja-Kizza H, Baseke J, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005;142(2):327–332. doi: 10.1111/j.1365-2249.2005.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thayil SM, Ho YC, Bollinger RC, et al. Mycobacterium tuberculosis complex enhances susceptibility of CD4 T cells to HIV through a TLR2-mediated pathway. PLoS One. 2012;7(7):e41093. doi: 10.1371/journal.pone.0041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Equils O, Schito ML, Karahashi H, et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol. 2003;170(10):5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 150.Schutz C, Meintjes G, Almajid F, Wilkinson RJ, Pozniak A. Clinical management of tuberculosis and HIV-1 co-infection. Eur Respir J. 2010;36(6):1460–1481. doi: 10.1183/09031936.00110210. [DOI] [PubMed] [Google Scholar]
- 151.Helke KL, Mankowski JL, Manabe YC. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis (Edinb) 2006;86(5):337–348. doi: 10.1016/j.tube.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 152.Chen CY, Huang D, Wang RC, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5(4):e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Price NM, Farrar J, Tran TT, Nguyen TH, Tran TH, Friedland JS. Identification of a matrix-degrading phenotype in human tuberculosis in vitro and in vivo. J Immunol. 2001;166(6):4223–4230. doi: 10.4049/jimmunol.166.6.4223. [DOI] [PubMed] [Google Scholar]
- 154.Elkington PT, Nuttall RK, Boyle JJ, et al. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172(12):1596–1604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 155.Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121(5):1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elkington PT, D’Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med. 2011;3(71):71ps76. doi: 10.1126/scitranslmed.3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hrabec E, Strek M, Zieba M, Kwiatkowska S, Hrabec Z. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int J Tuberc Lung Dis. 2002;6(8):713–719. [PubMed] [Google Scholar]
- 158.Walker NF, Clark SO, Oni T, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185(9):989–997. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liuzzi GM, Mastroianni CM, Santacroce MP, et al. Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J Neurovirol. 2000;6(2):156–163. doi: 10.3109/13550280009013159. [DOI] [PubMed] [Google Scholar]
- 160.Dhawan S, Toro LA, Jones BE, Meltzer MS. Interactions between HIV-infected monocytes and the extracellular matrix: HIV-infected monocytes secrete neutral metalloproteases that degrade basement membrane protein matrices. J Leukoc Biol. 1992;52(2):244–248. doi: 10.1002/jlb.52.2.244. [DOI] [PubMed] [Google Scholar]
- 161.Mastroianni CM, Liuzzi GM. Matrix metalloproteinase dysregulation in HIV infection: implications for therapeutic strategies. Trends Mol Med. 2007;13(11):449–459. doi: 10.1016/j.molmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 162.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61(3):259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elkington PT, O’Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005;142(1):12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 165.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289(5482):1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 166.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100(4):1160–1167. [PubMed] [Google Scholar]
- 167.Harris JE, Fernandez-Vilaseca M, Elkington PT, Horncastle DE, Graeber MB, Friedland JS. IFNgamma synergizes with IL-1beta to up-regulate MMP-9 secretion in a cellular model of central nervous system tuberculosis. FASEB J. 2007;21(2):356–365. doi: 10.1096/fj.06-6925com. [DOI] [PubMed] [Google Scholar]
- 168.Toossi Z, Hirsch CS, Wu M, Mayanja-Kizza H, Baseke J, Thiel B. Distinct cytokine and regulatory T cell profile at pleural sites of dual HIV/tuberculosis infection compared to that in the systemic circulation. Clin Exp Immunol. 2011;163(3):333–338. doi: 10.1111/j.1365-2249.2010.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cox JA, Lukande RL, Nelson AM, et al. An autopsy study describing causes of death and comparing clinico–pathological findings among hospitalized patients in Kampala, Uganda. PLoS One. 2012;7(3):e33685. doi: 10.1371/journal.pone.0033685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Graham NM, Nelson KE, Solomon L, et al. Prevalence of tuberculin positivity and skin test anergy in HIV-1-seropositive and -seronegative intravenous drug users. JAMA. 1992;267(3):369–373. [PubMed] [Google Scholar]
- 172.Selwyn PA, Sckell BM, Alcabes P, Friedland GH, Klein RS, Schoenbaum EE. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA. 1992;268(4):504–509. [PubMed] [Google Scholar]
- 173.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131(6):1898–1906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 174.Oni T, Wilkinson RJ. Immunodiagnosis of tuberculosis in HIV-infected adults in settings of high HIV/tuberculosis burden. Eur Respir Dis. 2012;8(2):148–153. [Google Scholar]
- 175.Santin M, Munoz L, Rigau D. Interferongamma release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One. 2012;7(3):e32482. doi: 10.1371/journal.pone.0032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Machingaidze S, Verver S, Mulenga H, et al. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201206-1134OC. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 178▪.Mitchell JE, Chetty S, Govender P, et al. Prospective monitoring reveals dynamic levels of T cell immunity to Mycobacterium tuberculosis in HIV infected individuals. PLoS One. 2012;7(6):e37920. doi: 10.1371/journal.pone.0037920. Recent study demonstrating wide variability in ELISpot responses in a longitudinal study of chronic HIV-1 infection, demonstrating the complexity of M.tb.-specific antigen responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Evans TG, Bonnez W, Soucier HR, Fitzgerald T, Gibbons DC, Reichman RC. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res. 1998;39(3):163–173. doi: 10.1016/s0166-3542(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 180.Mezzaroma I, Carlesimo M, Pinter E, et al. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. AIDS. 1999;13(10):1187–1193. doi: 10.1097/00002030-199907090-00006. [DOI] [PubMed] [Google Scholar]
- 181.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277(5322):112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 182.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blanc F-X, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wendland T, Furrer H, Vernazza PL, et al. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS. 1999;13(14):1857–1862. doi: 10.1097/00002030-199910010-00007. [DOI] [PubMed] [Google Scholar]
- 186.Sutherland R, Yang H, Scriba TJ, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite longterm HAART. AIDS. 2006;20(6):821–829. doi: 10.1097/01.aids.0000218545.31716.a4. [DOI] [PubMed] [Google Scholar]
- 187.Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122(2):597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- 188.Mendonca M, Tanji MM, Silva LC, et al. Deficient in vitro anti-mycobacterial immunity despite successful long-term highly active antiretroviral therapy in HIV-infected patients with past history of tuberculosis infection or disease. Clin Immunol. 2007;125(1):60–66. doi: 10.1016/j.clim.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 189.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204(1):154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antiviral Chem Chemother. 2009;20(2):63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hadad N, Levy R, Schlaeffer F, Riesenberg K. Direct effect of human immunodeficiency virus protease inhibitors on neutrophil function and apoptosis via calpain inhibition. Clin Vaccine Immunol. 2007;14(11):1515–1521. doi: 10.1128/CVI.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cobos-Jimenez V, Booiman T, Hamann J, Kootstra NA. Macrophages and HIV-1. Curr Opin HIV AIDS. 2011;6(5):385–390. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- 193.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5(6):361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 196.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–e107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 197.Salliot C, Guichard I, Daugas E, Lagrange M, Verine J, Molina JM. Acute kidney disease due to immune reconstitution inflammatory syndrome in an HIV-infected patient with tuberculosis. J Int Assoc Physicians AIDS Care (Chic) 2008;7(4):178–181. doi: 10.1177/1545109708320683. [DOI] [PubMed] [Google Scholar]
- 198.Breton G, Duval X, Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39(11):1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 199.Wang SH, Menon A, Hyslop NE. Cardiac tamponade: an unusual complication of simultaneous treatment of tuberculosis and HIV. South Med J. 2008;101(5):558–560. doi: 10.1097/SMJ.0b013e3181684b92. [DOI] [PubMed] [Google Scholar]
- 200.Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012 doi: 10.1093/cid/cis899. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11(12):1282–1289. [PubMed] [Google Scholar]
- 202.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meintjes G, Scriven J, Marais S. Management of the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2012;9(3):238–250. doi: 10.1007/s11904-012-0129-5. [DOI] [PubMed] [Google Scholar]
- 204.Naidoo K, Yende-Zuma N, Padayatchi N, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157(5):313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Smith CJ, Sabin CA, Lampe FC, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS. 2003;17(7):963–969. doi: 10.1097/00002030-200305020-00004. [DOI] [PubMed] [Google Scholar]
- 206.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20(2):F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 207.Tan DB, Yong YK, Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9(5):307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 208.Bourgarit A, Carcelain G, Samri A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183(6):3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 209▪.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178(10):1083–1089. doi: 10.1164/rccm.200806-858OC. Important study demonstrating heterogeneous IFN-γ responses to M.tb. antigens in both TB-associated immune reconstitution inflammatory syndrome (IRIS) and non-IRIS patients, disputing a causal relationship between T-cell expansions and paradoxical TB-associated IRIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25(11):1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200(11):1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 212.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23(1):143–145. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- 213.Tan DB, Lim A, Yong YK, et al. TLR2-induced cytokine responses may characterize HIV-infected patients experiencing mycobacterial immune restoration disease. AIDS. 2011;25(12):1455–1460. doi: 10.1097/QAD.0b013e328348fb18. [DOI] [PubMed] [Google Scholar]
- 214.Ruhwald M, Ravn P. Immune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm? AIDS. 2007;21(7):882–884. doi: 10.1097/QAD.0b013e3280b079c8. [DOI] [PubMed] [Google Scholar]
- 215.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2010;37(5):1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216▪▪.Meintjes G, Skolimowska KH, Wilkinson KA, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2012;186(4):369–377. doi: 10.1164/rccm.201201-0094OC. Immunological analysis of effect of corticosteroids on the inflammatory response during TB-IRIS, implicating innate immune dysfunction in TB-IRIS pathophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oliver BG, Elliott JH, Price P, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis. 2010;202(11):1728–1737. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 218.Oliver BG, Elliott JH, Price P, Phillips M, Cooper DA, French MA. Tuberculosis after commencing antiretroviral therapy for HIV infection is associated with elevated CXCL9 and CXCL10 responses to Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr. 2012;61(3):287–292. doi: 10.1097/QAI.0b013e31826445ef. [DOI] [PubMed] [Google Scholar]
- 219.Barber DL, Mayer-Barber KD, Antonelli LR, et al. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116(18):3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol. 2012;10(2):150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zaidi I, Peterson K, Jeffries D, et al. Immune reconstitution inflammatory syndrome and the influence of T regulatory cells: a cohort study in The Gambia. PLoS One. 2012;7(6):e39213. doi: 10.1371/journal.pone.0039213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Seddiki N, Sasson SC, Santner-Nanan B, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39(2):391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 223.Lim A, D’Orsogna L, Price P, French MA. Imbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration disease. AIDS Res Ther. 2008;5:9. doi: 10.1186/1742-6405-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bourgarit A, Carcelain G, Samri A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183(6):3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 225.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10(5):645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.WHO. Global Advisory Committee on Vaccine Safety, 3–4 December 2009 Use of BCG vaccine in HIV-infected infants. Weekly Epidemiol Rec. 2010;85:29–36. [PubMed] [Google Scholar]
- 227.Schoeman JF, Fieggen G, Seller N, Mendelson M, Hartzenberg B. Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J Child Neurol. 2006;21(4):301–308. doi: 10.1177/08830738060210040801. [DOI] [PubMed] [Google Scholar]
- 228.Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. Management of patients with the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2009;6(3):162–171. doi: 10.1007/s11904-009-0022-z. [DOI] [PubMed] [Google Scholar]
- 229.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 230.Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart. 2000;84(2):183–188. doi: 10.1136/heart.84.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]